Myocarditis, inflammation of the myocardium, is usually due to viral infection. Diagnostic confirmation in ordinary clinical practice is difficult because the findings on the clinical history, physical examination, electrocardiogram, and laboratory tests offer scant diagnostic accuracy, and the differential diagnosis is often done with acute myocardial infarction. Cardiac magnetic resonance imaging (CMR) has become the method of choice for the diagnosis of myocarditis. In this article, we describe the CMR findings at diagnosis and during the follow-up of patients with myocarditis, the differential diagnosis with other acute processes like myocardial infarction, and the prognostic factors studied with CMR.
La miocarditis consiste en la inflamación del miocardio producida la mayoría de las veces por una infección viral. La confirmación diagnóstica en la práctica clínica habitual es difícil porque la historia clínica y la exploración física, las alteraciones en el electrocardiograma, la determinación de las enzimas cardiacas y el ecocardiograma ofrecen escasa precisión diagnóstica, y no es infrecuente que se plantee el diagnóstico diferencial con el infarto agudo de miocardio. La resonancia magnética (RM) cardiaca se ha convertido en el método de imagen de elección para el diagnóstico de la miocarditis. En este trabajo se describen los hallazgos de imagen en la RM en el momento del diagnóstico y en el seguimiento de los pacientes con miocarditis, el diagnóstico diferencial con otros procesos agudos como el infarto de miocardio, y los factores pronósticos estudiados mediante RM.
Myocarditis is defined as acute or chronic inflammation of myocardial tissue, whether focal or diffuse, which affects any heart chamber. It was included among acquired-cause primary myocardiopathies in the classification made by the American Heart Association in the year 2006.1 Later on, the European Society of Cardiology classified myocardiopathies in 2008 with a more clinical point of view grouping myocardial diseases on the basis of ventricular form and function. Chronic myocardial inflammation and/or infection may trigger a dilated myocardiopathy.2
If the pericardium is affected, a myopericarditis occurs, often with associated pleural and pericardial effusion. Up to 15% of acute pericarditis has important myocardial affection.3
It is difficult to establish its actual incidence because it is not easy to confirm diagnosis in usual clinical practice. It has been found in 5–12% of the autopsies in adults with sudden death4,5 and in 1–9% of autopsies in general.6 Myocarditis is the cause of 6–8% of sudden death in athletes.7 Diagnostic confirmation is difficult because health history and physical examination, electrocardiogram (ECG) alterations, heart enzyme determination and echocardiogram are not very accurate, and it is not infrequent that differential diagnosis with acute myocardial infarction (AMI) is considered. Even though endomyocardial biopsy (EMB) is considered as the reference for myocarditis diagnosis, it is not justified in most patients. Under these circumstances, cardiac magnetic resonance (MR) imaging has become the imaging method of choice to diagnose myocarditis, especially due to its capacity for tissue characterization which enables to detect areas of edema and myocardial fibrosis.
This article describes the MR sequences used for diagnosis, the MR image findings at the moment of diagnosis and the follow-up of myocarditis patients, differential diagnosis with other acute processes such as acute myocardial infarction and the possible prognostic factors in MR studies.
EtiopathogenesisIn developed countries, viral infection is the most frequent cause. Even though the most frequent viruses during the 1980's and the 1990's were the enteroviruses (coxsackie B), in the last 10 years, other viruses such as parvovirus B19, human herpesvirus 6, adenovirus, the hepatitis C virus, echovirus, human immunodeficiency virus, the Epstein–Barr virus and cytomegalovirus, have gained importance. Other infectious agents such as bacteria, fungi, protozoan (Trypanosoma cruzi [T. cruzi], toxoplasm) and helminths may cause myocarditis. Other possible etiologies are the toxic one (anthraciclins, cyclophosphamide, traztuzumab, catecholamines), alcohol, cocaine or radiation, hypersensitivity reactions (antibiotics, diuretics such as thiazides, dobutamin, mesalazine, insect and snake bites), systemic diseases such as collagenosis, sarcoidosis, celiac disease, Wegener's disease and thyrotoxicosis.6,8–12
From the physiopathological point of view, myocarditis may be divided into three phases: (1) acute viral, (2) subacute immune, and (3) chronic myocardial. In the acute phase, the virus reaches the myocardium either by lymphatic or hematogenous dissemination or both in a susceptible patient and it enters the myocite. It is a short phase and it often goes unnoticed. Viral proliferation in the myocite may cause direct tissue damage, but most of the tissue damage in myocarditis occurs due to the immune system response to the virus when it reaches the myocite. The patient's evolution depends on the immune response which, in extreme cases, leads to an adverse response with autoantibody formation (antibodies anti-myosin). Viral persistence may activate the immune system continuously resulting in chronic myocardial inflammation, cardiac remodeling and function alteration, which develop a dilated myocardiopathy.13
In the disease's acute phase there is edema, cellular infiltrates with histiocytes and mononuclear cells, with or without myocardial cell damage. In the subacute and chronic phases, fibrosis replaces myocardial cells.14,15
Clinical presentation is very variable. The patient may be asymptomatic or have unspecific symptoms, suffer from chest pains, heart failure, arrhythmias, conduction alterations, cardiogenic shock or sudden death. Patients with myocarditis may present symptoms similar to those of AMI, above all the young subjects.16 In up to 89% of myocarditis, symptoms similar to those of the influenza, gastrointestinal or urinary tract infection have been described days or weeks before. In most cases the clinical course of myocarditis is favorable and it heals without secuelae, but 5–10% of the patients present an unfavorable evolution to a dilated myocardiopathy or sudden death. In a review of 1,230 patients with myocardiopathy of initially unknown cause, 9% had myocarditis.17 In 12% of the young patients who suffered sudden death, the cause was myocarditis.4
For Mahrholdt et al.6 clinical presentation is different depending on the type of virus present in the EMB. The symptoms similar to those of an AMI are more typical in acute myocarditis by parvovirus B19. These patients often have normal systolic function and left ventricular volume and a favorable clinical course. Infection by human herpesvirus 6 and the combination parvovirus B19-herpesvirus 6 usually occurs with symptoms of recent heart failure, often with malaise and conduction alteration (bundle-branch block). It is a more insidious clinical manifestation, one with a more prolonged course and it often occurs with left ventricle systolic function alteration. Given that human herpesvirus 6 tends to remain latent after primo-infection (which usually occurs in childhood), every infected individual may suffer from reactivations throughout their lives.
DiagnosisThe health history, physical examination, the laboratory data, including heart enzymes that may rise slightly or moderately in myocarditis patients (creatinephosphokinase [CPK], MB isoenzyme of CPK, and above all, troponins T and I), and the ECG help diagnose myocarditis even though diagnostic accuracy is low. ECG may be normal or show ST alterations and T wave and Q wave alterations, auricular–ventricular blockage and branch blockage and arrhythmias such as tachycardia or ventricular fibrillation. These findings have limited value and low specificity.
Acute myocarditis is to be suspected when a patient, especially a young male, presents recently started inexplicable heart anomalies, such as heart failure, angina, arrhythmias or conduction alterations. The patients very often refer a viral infection days or week before. In many cases, differential diagnosis arises with AMI due to chest pains, a rise in heart enzymes and ECG alterations.18,19
EMB with immunohistochemical analysis and molecular techniques is the reference to diagnose myocarditis and other non-ischemic myocardiopathies, but it is not recommended in most myocarditis patients. It is indicated for symptoms of rapidly progressive myocardiopathy refractory to treatment or a myocardiopathy of unknown causes with life-threatening arrhythmias. EMB serious complications occur 0.1–0.5% of the cases (myocardial perforation and heart plugging, permanent A–V blockage, AMI, ischemia or cerebral infarction, serious valvular damage and death). The global rate of complications is 6%.20 EMB has limited sensitivity and specificity (60 and 80% respectively) when the autopsy is taken as referent.21 The negative false rate is high due to the great interobserver variability in histological interpretation and also due to the fact that the samples may be insufficient, since inflammatory infiltrates are usually focal and transitory and they do not often affect the right ventricle (EMB is usually performed on the septal side of the right ventricle). For Yilmaz et al.,22 diagnostic performance is greater when the biopsy is bi-ventricular (79 as opposed to 67%), and if a choice must be made to choose one ventricle, it is preferable to perform it on the free left ventricle wall. Other authors believe that the tests’ profitability increases if the EMB is obtained from the areas affected in the MR or the echocardiogram.14,23 An additional problem is that viral genome may be found in the myocardium without there being sufficient histological changes that comply with Dallas’ criteria21 (Table 1). These criteria have limited sensitivity that may increase if, in addition, an immunohistochemical study and molecular pathology techniques are performed.23
Dallas criteria for anatomopatological diagnosis of myocarditis.
| - Acute myocarditis is defined as an inflammatory infiltrate of the myocardium with necrosis and/or degeneration of adjacent myocites not typical of ischemic damage associated with coronary disease. These infiltrates are usually mononuclear cells, but there may be neutrophils or occasionally eosinophils |
| - Borderline myocarditis, when the inflammatory infiltrate is very scarce or there is no myocite damage |
Source: taken from Aretz et al.15
For Kindermann et al.23 the EMB may predict the evolution of myocarditis. The authors consider the myocardial inflammation diagnosed with immunohistochemical techniques to be an adverse prognostic factor, whereas the histopathology with Dallas’ criteria and viral genome detection are not independent factors of bad prognosis. For them, and in view of future treatment strategies with antivirals or immunosuppressors, it is fundamental to distinguish between an active myocarditis (inflammation and viral infection), an inflammation without viral genome (post-viral autoimmunity) and the persistence of a viral genome without inflammation. Due to the importance of immunohistochemical, the authors defend performing an early EMB for prognostic purposes.
Image- -
Echocardiogram: It is usually the first imaging technique when myocarditis is suspected. It may show a global or segmentary alteration of contractility and a left ventricle systolic dysfunction, which are unspecific. Pericardial effusion occurs in 32–57% of the patients and it indicates active inflammation. In patients with less serious symptoms, the echocardiogram may be normal.24,25
- -
Isotopic studies (antimyosin antibodies marked with Indium-111 and Galium-67): They are seldom used in clinical practice due to their scarce spatial resolution, the radiation doses they imply and their limited availability. In addition, specificity and positive predictive value are low.23
- -
Coronary angiography: It is valuable to rule out coronary disease. It is not performed routinely in all the patients with clinical suspicion of myocarditis, only in those where there is diagnostic doubt with AMI.
- -
Magnetic resonance imaging: From the first series of cases studied with sequences potentiated in T2 in the year 199126 and the first clinical studies with contrast in the year 1998,27 many works have shown the diagnostic usefulness of MR in myocarditis patients. The great advantage of MR is that it offers triple information: morphological, functional and tissue characterization.
MR is indicated in patients with persistent or newly appeared symptoms (dypsnea, orthopnea, palpitations, intolerance to exercise and chest pains), who present myocardial lesion data (ventricular dysfunction, ECG alterations and troponin increase) and in those suspicious of a viral etiology (a recent viral infection, absence of coronary risk factors and aged under 35 years). It may also be indicated for patients with ECG alterations unexplained by other causes, even in the absence of myocarditis symptoms. If an EMB is to be performed, MR works as a guide showing signal hyperintensity areas in the potentiated sequences in T2 and those of enhancement after the gadolinium injection.14 Some studies suggest that it is better to perform cardiac MR imaging after the seventh day from the beginning of the clinical examination, since it may be less sensitive in the first few days. This seems to be due to the focal affectation in the first days of the disease, which becomes more diffuse from the seventh day on and it persists into the third or fourth weeks.24
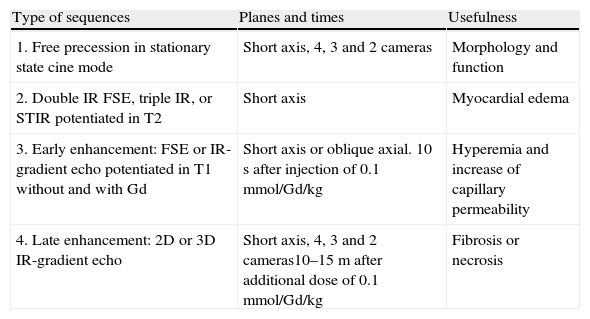
The MR diagnosis of acute myocarditis is based on several sequences (Table 2):
- (1)
Gradient echo sequence in cine mode (Stationary State Free Precession [SSFP]). It assesses the heart's shape and function. In acute myocarditis patients, a transitory augmentation of myocardial thickness and mass has been described, as well as an increase in volume and a decrease of left ventricle ejection fraction.28 Unspecific alterations of segmentary or global contractility have also been described in the most serious cases.28,29 Furthermore, pericardial effusion may occur.
- (2)
Black blood Fast Spin Echo sequences (FSE) potentiated in T2 (double inversion-recovery, triple inversion-recovery, or short-tau inversion recovery [STIR]) in the short ventricular axis. They assess myocardial edema, which is always convenient to confirm with at least an additional orthogonal plane. The cut's thickness must be 10mm to have greater signal/noise relation. Myocardial edema occurs due to an increase of cell membrane permeability with output of intracellular water. It manifests as signal intensity areas augmented in the potentiated sequences in T2, of subepicardial or transmural location, either patched or diffuse. The sequence may have less sensitivity in the cases of mild myocardial inflammation and due to the annulment of slow endocavitary flow which appears as signal hyperintensity near the myocardium. The analysis may be performed visually or quantitatively. It is preferable to use the body antenna instead of the heart surface antenna for quantitative assessment, since that way signal intensity is more homogeneous. When the edema is diffuse, it may go unnoticed if a signal intensity quantitative analysis is not conducted calculating the edema quotient, or T2 quotient between the signal intensity of the damaged myocardium and the signal intensity of skeletal muscle, generally a paraspinal muscle (measured in the same image). A T2>2 quotient suggests active inflammation. With that cut value, the T2 quotient has sensitivity, specificity and diagnostic accuracy of 84, 74 and 79% respectively.30
Edema in the acute phase of myocarditis and the increase of capillary permeability are reversible, transitory processes and its diminishment is eventually associated with an improvement of heart function parameters.31,32 Other authors believe that focal edema without associated areas of late enhancement may indicate a less serious form of lesion that does not progress into necrosis.28,31,33,34 Another possible explanation is that the evolution/resolution patterns of the myocardial edema in the sequences potentiated in T2 have a time window different from those of the late enhancement areas (Fig. 1).
Figure 1.Acute myocarditis with myocardial edema. 32-year-old male, who after catarrhal symptoms with fever, presents pain in the middle of his chest with elevation of ST in the ECG and elevation of serum troponins and CPK, normal echocardiography. (A) Double-inversion-recovery FSE Sequence potentiated in T2 on short axis. Inferolateral subepicardial and intramyocardial signal hyperintensity is observed (arrow) due to myocardial edema. (B) Late enhancement sequence after gadolinium injection. Mild to moderate inferolateral subepicardial and intramyocardial intensity enhancement is observed, which is indicative of a certain degree of myocardial necrosis (arrow).
(0.11MB). - (3)
Sequence potentiated in T1 without and with gadolinium to study early enhancement. It assesses the degree of hyperemia and the increase of capillary permeability, which in acute myocarditis are due to vasodilatation in inflamed tissue, with greater blood volume and accumulation of greater amount of contrast in the early vascular phase. As gadolinium passes quickly into the extracellular space, this phase lasts only a few minutes after the contrast is injected. That is why this sequence is performed during the first three minutes after injection. Most of the times a black blood FSE sequence is used, but it may also be done with a gradient echo sequence with an inversion-recovery pulse, before and after the gadolinium introduction, in the same cuts and with the same parameters.
Although it may sometimes be done visually, early enhancement must be assessed quantitatively; therefore it is preferable to use the body antenna so that the signal is more homogeneous. The myocardium relative global enhancement in relation to that of the skeletal muscle or else the myocardium absolute global enhancement in respect of the basal myocardial signal is calculated quantitatively (greater than or equal to 45%).35 To calculate the relative global enhancement a myocardial signal intensity ratio is used in respect of the skeletal muscle >4 to indicate active inflammation, with diagnostic sensitivity, specificity and accuracy of 80, 68 and 74% respectively. In case skeletal muscle affection (myositis) coexists, it is preferable to use myocardial absolute global enhancement quantification.
Late enhancement sequence. When necrosis and/or myocardial fibrosis have occurred, extracellular space increases and more gadolinium concentrates in these areas. After performing the early enhancement sequence an additional dose of gadolinium is administered and a 2D/3D gradient echo sequence is conducted with a previous inversion-recovery pulse 10–15min after the injection (late enhancement sequence) in ventricular short axes and long axes. The inversion time is adjusted individually to annul the signal from the healthy myocardium so that signal hyperintensity areas indicate irreversible myocardial damage by necrosis or fibrosis. The spatial extension of late enhancement may be calculated (myocardium volume with signal intensity greater than normal myocardium signal intensity plus two standard deviations) and it is expressed in grams (myocardium volume that enhances with gadolinium multiplied by 1.05g/ml, which is the myocardium's density). The software dedicated to cardiac MR imaging of the MR work stations usually includes calculation of this parameter.
Late enhancement in acute myocarditis is present in 44–88% of the cases, it is typically subepicardial with variable extension to the rest of the myocardial thickness (Fig. 2), but it does not affect the subendocardium in an isolated manner. In myocarditis, it is very often located on the inferolateral side of the left ventricle, but also in the anteroseptal or septal segments, or in a multifocal or diffuse manner.14,30,36–39 Parvovirus B19 affects more often the left ventricle lateral side and the human herpesvirus 6, the interventricular septum.6 In Chagas disease, late enhancement is often located in the basal and apical inferolateral segments12 (Fig. 3). Sometimes, in active myocarditis the number of myocites destroyed is not enough to produce necrosis detectable with this sequence.40,41
Figure 2.Typical late enhancement in acute myocarditis. 19-year-old male with upper airway infection symptoms, who presents precordial oppression, with alterations of ECG repolarization, elevation of serum troponins and CPK and normal echocardiography. (A) and (B) Late enhancement sequence after the gadolinium injection. Subepicardial and intramyocardial enhancement is observed, which does not follow a vascular distribution pattern and only respects midoinferior and basal-anterior segments (arrows).
(0.14MB).AMI patients have myocardial edema in the affected coronary region, visible in the black blood sequences potentiated in T2. Moreover, they present alterations of segmentary contractility in the echocardiogram and in the cine mode gradient echo sequences, with normal or diminished myocardial thickness. In late enhancement sequences the subendocardium is always affected34,38,41 (Fig. 4). The enhancement is usually more intense than those of myocarditis because in them, the tissue necrosis areas within the inflammation focuses are smaller than in AMI.37,38
Figure 4.Acute Myocardial Infarction. Differential diagnosis with myocarditis. 47-year-old male with chest pains, elevation of CPK and serum troponins and ST elevation in the ECG. (A) Double-inversion-recovery FSE Sequence potentiated in T2 potentiated on the short axis. A lateral signal hyperintensity (arrow) indicative of myocardial edema is observed. There is also a mild lower pericardial effusion. (B) Late enhancement sequence after gadolinium injection on the short axis. Transmural enhancement is observed on the lateral side (arrow) with several intramyocardial focuses of enhancement absence indicative of microcirculation obstruction. (C) Coronary angiography showing circumflex artery obstruction (arrow). (D) Cardiac MR imaging performed two months after. Late enhancement sequence on the short axis after gadolinium injection. Intense transmural enhancement is observed on the lateral side compatible with myocardial fibrosis (arrow).
(0.2MB).In patients with an acute coronary syndrome, a serum increase of troponins and a cardiac catheterization that do not show significant lesions, late enhancement sequences may help to differentiate among a myocarditis (subepicardial enhancement), an emboli AMI, recannulated thrombus or coronary spasm (subendocardial enhancement in a coronary vascular region) and a Tako-Tsubo's disease, which typically does not show late enhancement.18,37,41,42
For Baccouche et al.43 the extension of the late enhancement is greater in patients with an active myocarditis EMB, than in those that only have a borderline myocarditis histological diagnosis or viral genome without inflammation. The diagnosis of myocarditis was established in a significantly greater percentage with EMB than with MR, probably due to the fact that positive EMB includes more “subtle” forms (borderline and viral genome) that go unnoticed in the MR. It is reasonable for the authors to begin by an MR which, if it is diagnostic, avoids performing the EMB.
In general, there is no clear relation between the myocardial extension of the late enhancement in the MR and the serum levels of the myocardial damage markers (CPK, troponin) or the ECG alterations.14,24,32 Nor is there any clear relation with the left ventricular function parameters (ejection fraction, telediastolic volume, telesystolic volume).18,19 This may be due to the fact that when the subepicardium is damaged in a predominant manner and the subendocardium is spared, repercussion on systolic function is less.14,44
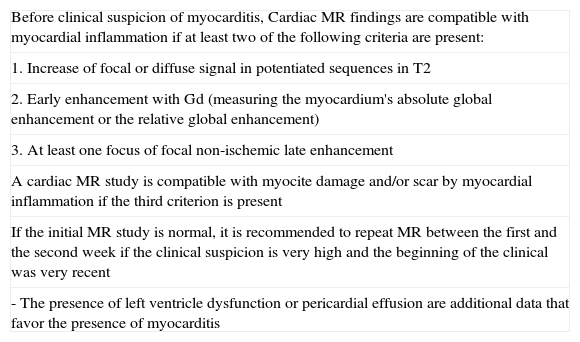
The combination of Sequences 2, 3 and 4 increases diagnostic accuracy. If two or more are positive, diagnostic accuracy is 78%, sensitivity 76% and specificity 95.5%; if only the late enhancement sequence is used, diagnostic accuracy is 68–71% with sensitivity of 44–54% and specificity of 64–100% (Table 3).45
Table 3.Criteria in cardiac MR for myocarditis diagnosis (Lake Louise consensus criteria).
Before clinical suspicion of myocarditis, Cardiac MR findings are compatible with myocardial inflammation if at least two of the following criteria are present: 1. Increase of focal or diffuse signal in potentiated sequences in T2 2. Early enhancement with Gd (measuring the myocardium's absolute global enhancement or the relative global enhancement) 3. At least one focus of focal non-ischemic late enhancement A cardiac MR study is compatible with myocite damage and/or scar by myocardial inflammation if the third criterion is present If the initial MR study is normal, it is recommended to repeat MR between the first and the second week if the clinical suspicion is very high and the beginning of the clinical was very recent - The presence of left ventricle dysfunction or pericardial effusion are additional data that favor the presence of myocarditis Source: taken from Friedrich et al.45
MR sequences in the study of myocarditis.
| Type of sequences | Planes and times | Usefulness |
| 1. Free precession in stationary state cine mode | Short axis, 4, 3 and 2 cameras | Morphology and function |
| 2. Double IR FSE, triple IR, or STIR potentiated in T2 | Short axis | Myocardial edema |
| 3. Early enhancement: FSE or IR-gradient echo potentiated in T1 without and with Gd | Short axis or oblique axial. 10s after injection of 0.1mmol/Gd/kg | Hyperemia and increase of capillary permeability |
| 4. Late enhancement: 2D or 3D IR-gradient echo | Short axis, 4, 3 and 2 cameras10–15m after additional dose of 0.1mmol/Gd/kg | Fibrosis or necrosis |
FSE: fast spin echo sequence; Gd: gadolinium; IR: inversion-recovery; STIR: short-tau inversion recovery.
Several studies have described the decrease of the late enhancement areas in the follow-up studies with MR in myocarditis patients.6,14,32,40,46,47 This might explain why as healing progresses, the edema is resolved, the scar contracts and the myocardium remodels and, therefore; the extension of the late enhancement area diminishes (Fig. 5). In some cases microscars persist that are below the MR spatial resolution and the visual disappearance of the late enhancement regions occur. This fact augments due to the patched distribution of scars in myocarditis.48–50 On other occasions, the enhancement areas may persist without changes.32 The left ventricle systolic function and telediastolic volume may also improve.14
Evolution of late enhancement in myocarditis. 29-year-old male with acute manifestations of palpitations and chest pains, with elevation of serum troponins and CPK and ST alterations in the ECG. (A) Cardiac MR imaging performed during acute manifestations. Late enhancement sequence on the short axis after gadolinium injection. Subepicardial mid-anteroseptal and inferolateral enhancement is observed (arrow). B) Cardiac MR performed 3 months after. Late enhancement sequence on the short axis after gadolinium injection. The enhancement persists but it has diminished in signal intensity and extension (arrow).
Zagrosek et al.48 studied 36 patients with MR at the onset of myocarditis and between 8 and 18 months later. In the acute phase, the T2 quotient was high in 86% of the patients, the relative global enhancement in 80%, and there was late enhancement in 63% of the cases. In the follow-up, the left ventricle ejection fraction increased while the T2 quotient and the relative global enhancement diminished significantly. Late enhancement persisted in all the cases except in one where it disappeared completely. The extension of late enhancement diminished significantly between the acute and the chronic phases. Distinction between acute and chronic myocarditis is important because during the acute phase physical exercise is prohibited, since it may even cause sudden death.
Escher et al.51 observed that 10–20% of myocarditis patients evolved toward a dilated myocardiopathy related with chronic inflammation of the myocardium due to viral persistence and/or altered immune response (Fig. 6). The risk of cardiac-related death or the need for heart transplant was greater. 80–90% of myocarditis patients left the hospital with normal heart function. In the follow-up after 4–6 years, 50% of serious myocarditis patients developed heart failure due to diastolic dysfunction with normal ejection fraction. All the patients who evolved to heart failure who underwent cardiac MR imaging presented late myocardial enhancement. The authors concluded that treatment of myocarditis patients is necessary and strict clinical follow-up is a must. It was also concluded that myocardial fibrosis may contribute to the development of diastolic dysfunction.
Evolution from myocarditis to dilated myocardiopathy. 52-year-old male with dilated myocardiopathy. Antecedents of acute myocarditis. Late enhancement sequence after gadolinium injection, four-camera planes (A), short axis (B and D) and long axis (C). Extensive subepicardial/intramyocardial enhancement is observed, which does not follow a vascular distribution pattern.
An MR performed after 4 weeks after the onset of the clinical symptoms makes it possible to distinguish between an uncomplicated myocarditis that is resolved in 2–3 weeks and a complicated course due to viral persistence or autoimmune response (chronic myocarditis). For Wagner et al.,35 persistence of an early gadolinium uptake from the fourth week after the onset of the disease is associated with worse prognosis in terms of functional recovery and the persistence of symptoms in the control after 3 years.
For Kindermann et al.23 the 3 predicting factors associated with a worse evolution (cardiac-related death or the need for heart transplant) in acute myocarditis patients are the functional classes of heart failure iii and iv of the New York Heart Association (NYHA), data of inflammation in the EMB immunohistochemistry and absence of treatment with beta blockers. The efficaciousness of beta blockers may be due to the sympathetic activity that stimulates inflammation and apoptosis, it may be related to the progression of left ventricle dysfunction, with which beta blockers would cause inflammation to decrease.
Mahrholdt et al.6 described 3 independent prognostic factors of chronic ventricular dysfunction and left ventricle dilatation: left ventricle dilatation, septal late enhancement and the total amount of myocardium with late enhancement, in the acute phase of the disease. Moreover, they described a fourth factor that is combined parvovirus B19-human herpesvirus 6 viral etiology, because the myocardium viral disappearance is less frequent, and therefore; chronic ventricular failure is more frequent.
Right ventricle affectation with a decrease of ejection fraction in the echocardiography is an indicator of bad prognosis as to sudden death and the need for heart transplant.52
ConclusionsDiagnostic confirmation of myocarditis is difficult because health history and clinical examination, ECG and echocardiogram data and serology have low diagnostic accuracy. The EMB with immunohistochemical study continues to be the technique of choice, but it is not indicated in most patients, especially in those with mild myocarditis. Cardiac MR imaging has a fundamental role due to its triple morphological, functional and tissue characterization information allowing differential diagnostic with AMI and follow-up of symptomatic patients.
Ethical responsibilitiesProtection of people and animalsThe authors declare that no experiments on human beings or animals were conducted for this research.
Data confidentialityThe authors declare that they have followed the protocols of their work places concerning publication of patient data and that all the patients included in the study have received enough information and given their written consent to participate in said study.
Right to privacy and informed consentThe authors have obtained informed consent from the patients and/or subjects referred to in the article. This document is in the possession of the correspondence author.
Authors- (1)
Person responsible for the study's integrity: ABG, BCM, JFD, CGV and CFG.
- (2)
Conception of the study: ABG, BCM and CFG.
- (3)
Design of the study: ABG, BCM and CFG.
- (4)
Data acquisition: ABG, BCM and CFG.
- (5)
Data analysis and interpretation: ABG and BCM.
- (6)
Statistical treatment: ABG.
- (7)
Bibliographic search: ABG, BCM, JFD and CGV.
- (8)
Writing of the article: ABG, BCM, JFD and CGV.
- (9)
Critical revision of the manuscript with intellectually relevant contributions: JFD and CFG.
- (10)
Approval of the final version: ABG, BCM, JFD, CGV and CFG.
The authors declare that they have no conflict of interests.
Please cite this article as: Bustos García de Castro A, et al. Miocarditis: diagnóstico y seguimiento con resonancia magnética. Radiología. 2013;55:294–304.