To describe the neuroradiological characteristics of a series of high grade cortical astrocytomas in the initial phase of development and their pattern of growth during a short time period.
Material and methodsThis was a retrospective observational study of the neuroradiological findings in six patients diagnosed with high grade astrocytoma. All presented with a new onset epileptic seizure and focal supratentorial cortical lesions. The diagnosis was established after a short period of clinical and radiological follow-up.
ResultsMagnetic resonance imaging (MRI) detected a small cortical lesion that was hyperintense in T2-weighted sequences in all six patients. This lesion showed slight or moderate enhancement after the administration of a contrast agent in five patients. All six patients underwent follow-up MRI within six months of the initial MRI examination (mean 79 days). Follow-up MRI showed marked growth of the lesions in all cases, and the histological diagnosis of high grade astrocytoma (glioblastoma in five patients and anaplastic oligoastrocytoma in one) was established after surgical debulking.
ConclusionHigh grade astrocytomas originating in the supratentorial cortex can have an atypical neuroradiological presentation in the early stages. High grade astrocytoma should be included in the differential diagnosis of a cortical lesion that enhances slightly or moderately after the administration of contrast material in a patient with a new onset epileptic seizure.
Describir las características neurorradiológicas de una serie de astrocitomas corticales de alto grado de malignidad en su fase inicial de desarrollo y su patrón de crecimiento durante un corto período de tiempo.
Material y métodosEstudio observacional retrospectivo de los hallazgos neurorradiológicos de 6 pacientes que, tras una primera crisis epiléptica, mostraron lesiones focales corticales supratentoriales que fueron seguidas clínico-radiológicamente durante un corto período de tiempo, tras el cual se estableció el diagnóstico de astrocitoma de alto grado de malignidad.
ResultadosEn los estudios de resonancia magnética (RM) de todos los pacientes se constató una pequeña lesión cortical hiperintensa en secuencias T2 con realce leve o moderado con la administración de contraste (en 5 de ellos). En los 6 pacientes se llevó a cabo un seguimiento radiológico en los 6 primeros meses tras el examen inicial (media: 79 días), que reveló un marcado crecimiento de las lesiones. Tras esta segunda RM, los pacientes fueron sometidos a cirugía citorreductora, y se estableció el diagnóstico histológico de astrocitomas de alto grado (glioblastoma en 5 pacientes y oligoastrocitoma anaplásico en uno).
ConclusiónLos astrocitomas de alto grado de malignidad de origen cortical supratentorial en estadios iniciales pueden presentarse en los estudios neurorradiológicos de forma atípica. La presencia de una lesión cortical con realce leve o moderado tras la administración de contraste en un paciente que ha presentado una primera crisis epiléptica debería incluir en su diagnóstico diferencial los astrocitomas de alto grado de malignidad.
Primary central nervous system tumors account for 2–3% of all neoplasms, with an annual incidence of 15 cases per 100000 population.1,2 Among these tumor, high-grade astrocytic (HGA) tumors and meningiomas are the most common neoplasms.3
The prognosis of HGAs remains dismal, with a median survival of 12.2–18.2 months for glioblastomas4 and a mean survival of 41 months for anaplastic astrocytomas.5 Radiologic features of HGAs are relatively typical, but in the early stages imaging findings may be normal or show atypical changes,6–8 making the differential diagnosis with non-tumor lesions or low-grade astrocytomas (LGAs) difficult.
The objective of this study is to examine the radiologic features of a series of high-grade cortical astrocytomas in their initial phase of development and their growth pattern during a short period of time.
Material and methodsWe reviewed the database of the educational archive (period 2001–2010) of our institution and identified six patients with histologic diagnosis of HGA whose MRI studies, performed a few days after the onset of the first symptom attributable to HGA, showed cortical lesions that were atypical for this type of tumors. The six patients (4 males), with a mean age of 51 years (45–79 years), were admitted to the hospital because of a first focal epileptic seizure, which was simple partial in three cases, complex partial in one case, and complex partial with secondary generalization in the remaining two cases. All patients underwent an unenhanced computed tomography (CT) scan of the brain (within 24h after the onset of symptoms) that showed no abnormalities, and a subsequent MRI (within 6 days after the onset of symptoms). The imaging protocol included fast spin echo and/or FLAIR T2-weighted sequences, and diffusion sequences with a b value of 0 and 1000mm2/s. Apparent diffusion coefficient maps were obtained from all patients. In addition, T1-weighted sequences were obtained from five patients after the administration of a paramagnetic contrast agent (0.1mmol/kg). Three patients underwent a perfusion study of the brain using the first-pass technique (one patient) or the arterial spin labeling technique (two patients). One patient underwent single-voxel proton MR spectroscopy with long echo time.
The average time to follow-up MRI was 79 days (range 28–179 days) after the first study. During this time interval, patients only received symptomatic treatment. The follow-up examination included fast spin echo and/or FLAIR T2-weighted sequences, and T1-weighted sequences obtained after gadolinium administration. After the follow-up MRI, the patients underwent cytoreductive surgery, which provided the histologic diagnosis of HGA.
The MRI features of the lesions (location, contrast enhancement, diffusion signal) as well as the changes in cerebral blood flow or volume on perfusion sequences were examined. In addition, the tumor growth rate (TGR) was calculated by dividing the changes in the maximum diameter (in millimeters) of the lesion (excluding perilesional edema) by the time interval (in weeks) elapsed between the initial and the follow-up MRI studies (TGR=difference in maximum diameters between initial and follow-up MRI/time interval). The percentage of tumor growth was also calculated for each lesion according to the following equation: B−A/A×100 (A=maximum diameter at initial imaging; B=maximum diameter at follow-up imaging).
The study was approved by our Institutional Ethics Committee and all the patients gave informed consent for the imaging studies.
ResultsInitial demographic, clinical and radiologic findings of the patients reported in this study are summarized in Table 1.
Demographic, clinical and radiologic findings.
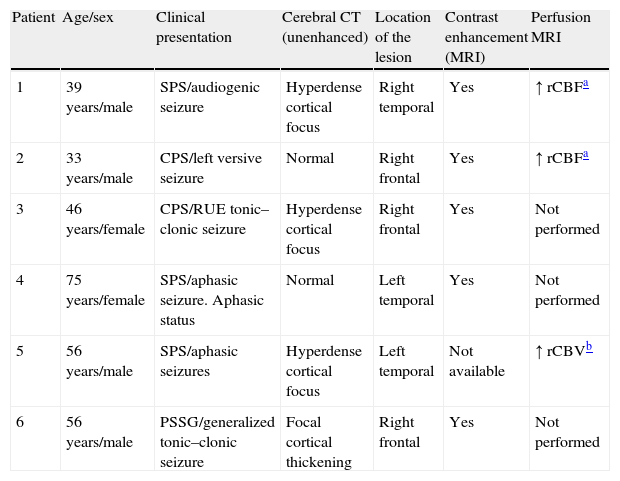
| Patient | Age/sex | Clinical presentation | Cerebral CT (unenhanced) | Location of the lesion | Contrast enhancement (MRI) | Perfusion MRI |
| 1 | 39 years/male | SPS/audiogenic seizure | Hyperdense cortical focus | Right temporal | Yes | ↑ rCBFa |
| 2 | 33 years/male | CPS/left versive seizure | Normal | Right frontal | Yes | ↑ rCBFa |
| 3 | 46 years/female | CPS/RUE tonic–clonic seizure | Hyperdense cortical focus | Right frontal | Yes | Not performed |
| 4 | 75 years/female | SPS/aphasic seizure. Aphasic status | Normal | Left temporal | Yes | Not performed |
| 5 | 56 years/male | SPS/aphasic seizures | Hyperdense cortical focus | Left temporal | Not available | ↑ rCBVb |
| 6 | 56 years/male | PSSG/generalized tonic–clonic seizure | Focal cortical thickening | Right frontal | Yes | Not performed |
SPS: simple partial seizure; CPS: complex partial seizure; PSSG: partial seizure with secondary generalization; RUE: right upper extremity; rCBF: relative cerebral blood flow; MR: magnetic resonance; rCBV: relative cerebral blood volume.
Although the initial CT scan was interpreted as normal in all the patients, a retrospective analysis revealed the presence of abnormalities in four of them: focal areas of slight hyperattenuation in the cortex were seen in three patients (Figs. 1A, 3A and 4A), and mild focal thickening of the cortex was seen in one.
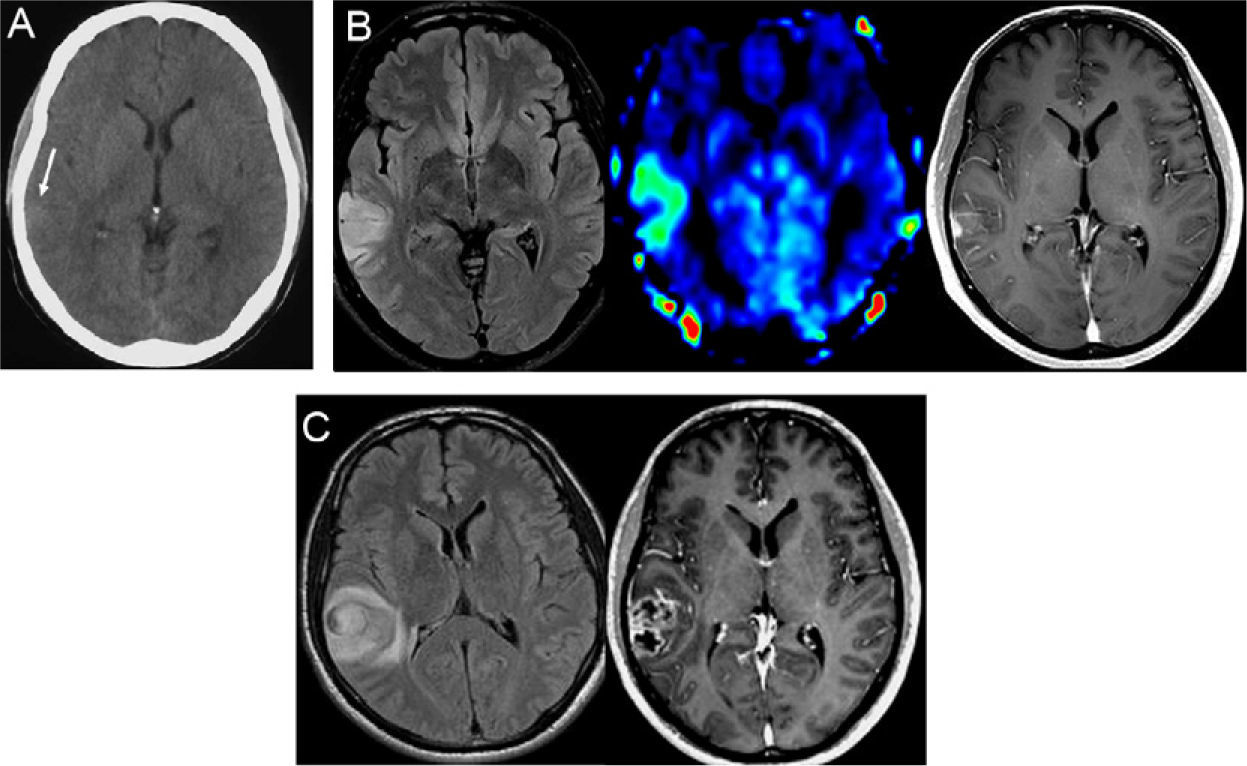
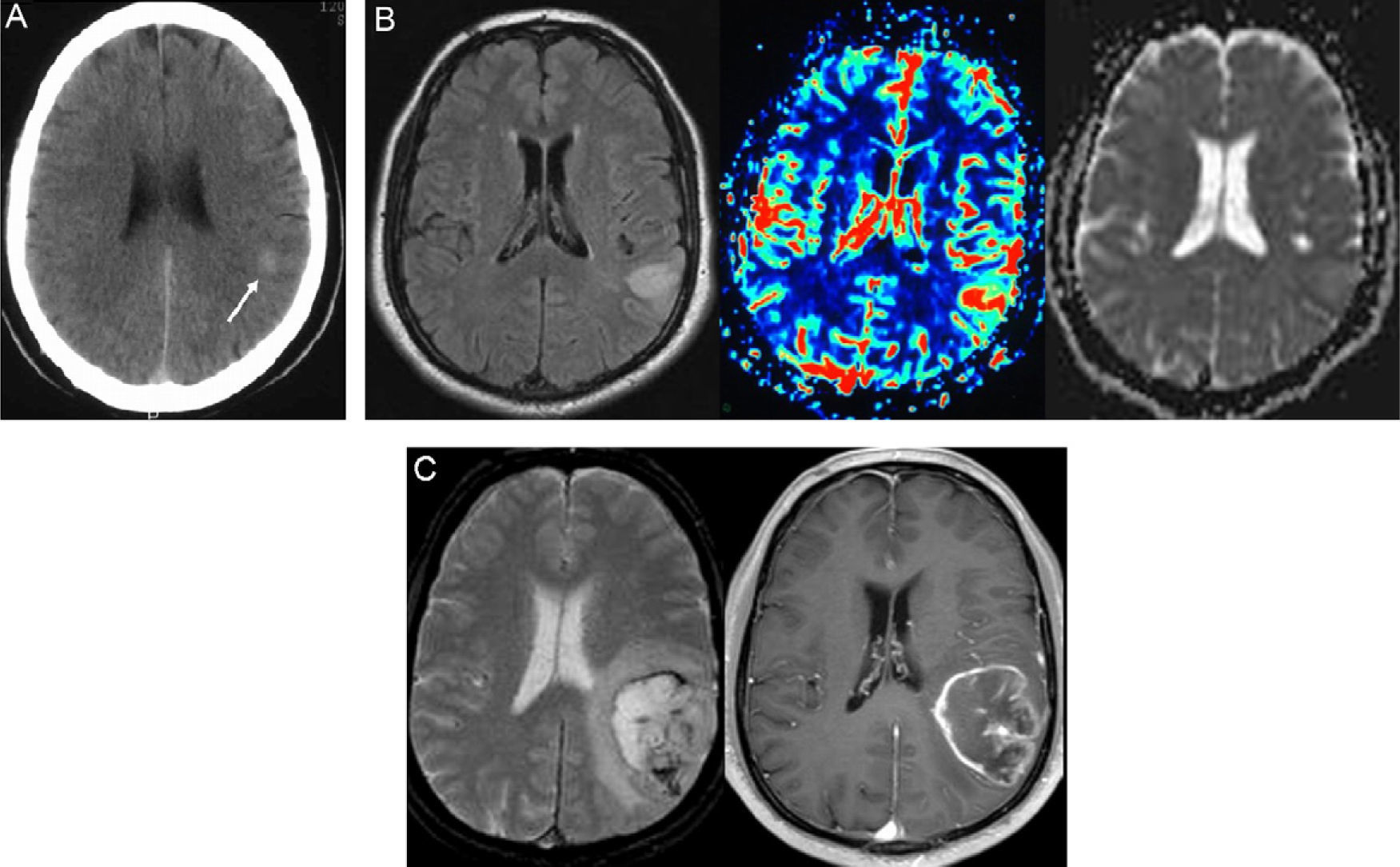
Patient No. 1. A 39-year-old man presents to the hospital because of an audiogenic, simple partial seizure. (A) The emergency CT of the brain performed without administration of contrast agent was initially interpreted as normal, but a retrospective examination of the CT scan reveals a very subtle hyperattenuating area in the right temporal cortex (arrow). (B) MRI sequences performed 48h after the ictal episode (FLAIR T2-weighted [left], perfusion MRI using arterial spin labeling [center] and contrast enhanced T1-weighted [right]) show a focal lesion located in the cortex of the right middle temporal gyrus, with increased cerebral blood flow in the perfusion sequence and partial enhancement after intravenous administration of contrast. (C) FLAIR T2-weighted (left) and contrast enhanced T1-weighted (right) MRI sequences performed 81 days later demonstrate marked tumor growth (TGR=2.3mm/week) and typical features of high-grade glioma, with perilesional edema and central necrosis. The histologic diagnosis was glioblastoma.
Initial MRI showed small cortical lesions that appeared hyperintense in relation to the adjacent cortex on T2-weighted sequences. In three cases the lesions were located in the frontal cortex (Figs. 2 and 4) and in the other three cases in the temporal cortex (Figs. 1 and 3), with a mean maximum diameter of 11mm (range 9–15mm) and without restricted diffusion. A mild to moderate homogeneous enhancement within the lesion was seen in five patients in whom T1-weighted images were obtained after administration of a paramagnetic contrast agent. Minimal perilesional edema was seen only in one patient (patient 3) (Fig. 2B), but none of the lesions had necrosis. Increased relative cerebral blood flow (Figs. 1B and 4A) or volume (Fig. 3B) was seen in the three patients who underwent perfusion studies.
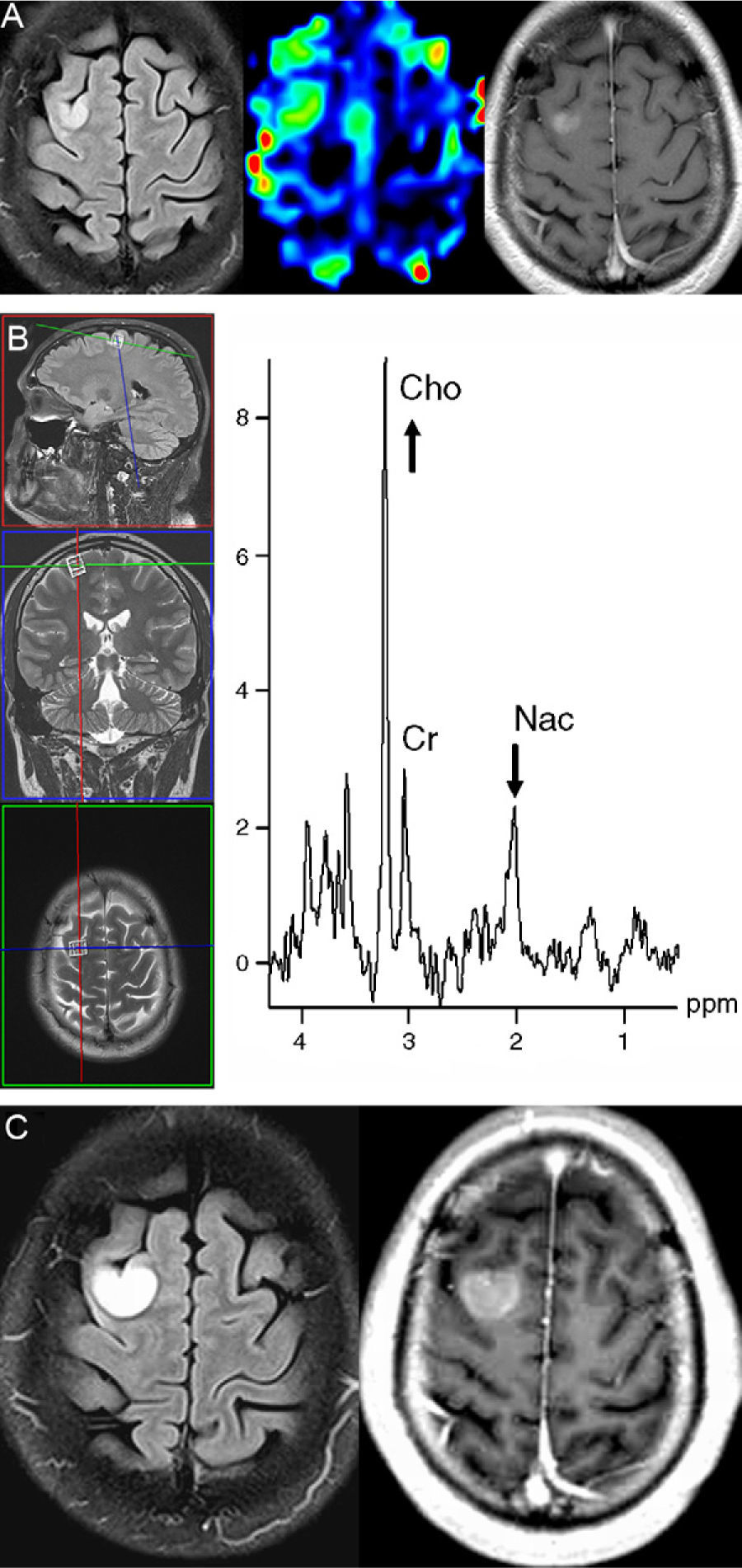
Patient No. 2. A 33-year-old male presents to the emergency room because of a partial seizure with secondary generalization. (A) The MRI sequences (FLAIR T2-weighted [left], perfusion MRI using arterial spin labeling [center] and contrast enhanced T1-weighted [right]) performed 48h after the seizure show a focal lesion located in the cortex adjacent to the right superior frontal sulcum, with increased cerebral blood flow in the perfusion sequence and mild homogeneous enhancement after intravenous administration of contrast. (B) Proton MR spectroscopy (single-voxel technique with long echo time) reveals a marked increase in choline (Cho) associated with decreased N-acetyl-containing compounds (Nac) (Cr: creatine). (C) FLAIR T2-weighted (left) and contrast enhanced T1-weighted (right) MRI sequences of the brain performed 28 days later demonstrate marked tumor growth (TGR=2.0mm/week), but without the presence of necrosis or perilesional edema. The histologic diagnosis was anaplastic oligoastrocytoma.
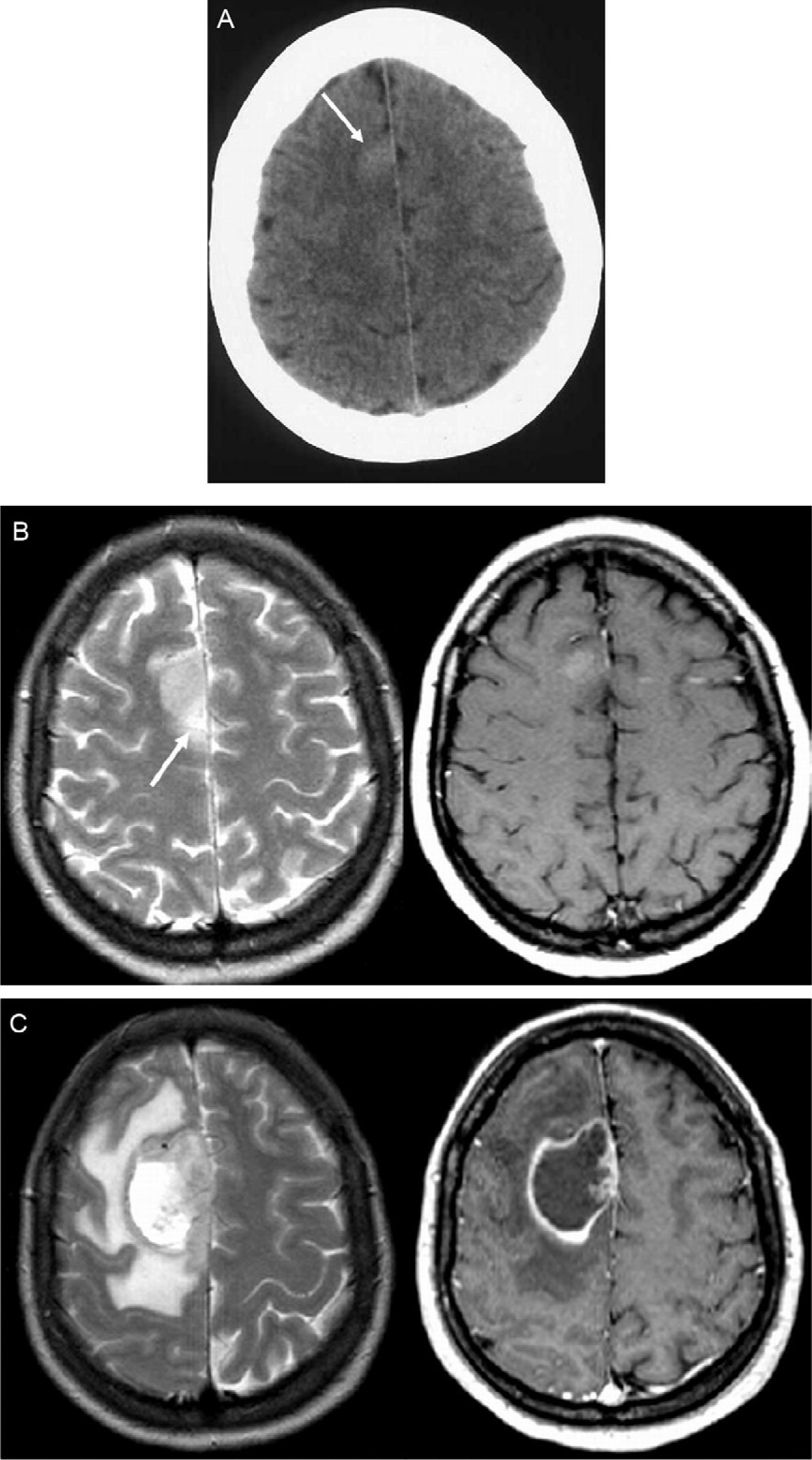
Patient No. 3. A 46-year-old woman presents to the emergency room because of a simple partial seizure with motor symptoms. (A) The emergency CT of the brain performed without administration of contrast agent was initially interpreted as normal, but a retrospective examination of the CT scan reveals a very subtle hyperattenuating area in the right parasagittal frontal cortex (arrow). (B) Contrast enhanced T2-weighted (left) and T1-weighted (right) sequences performed six days after the ictal episode show a focal lesion located in the right parasagittal frontal cortex with mild enhancement after intravenous contrast administration. Note the slight perilesional edema on the T2-weighted image (arrow). (C) Follow-up MRI study, including contrast-enhanced T2- and T1-weighted sequences performed 36 days later, shows marked tumor growth (TGR=7.6mm/week) and characteristic signs of high-grade glioma, with extensive perilesional edema and central necrosis. The histologic diagnosis was glioblastoma.
Patient No. 5. A 56-year-old man presents to the emergency room because of an aphasic seizure. (A) The unenhanced CT of the brain performed 4h after the onset of the symptoms was interpreted as normal, but a retrospective examination of the CT scan reveals mild hyperattenuation in the left posterior temporal cortex (arrow). (B) FLAIR T2-weighted (left), first pass perfusion (center), and diffusion (apparent diffusion coefficient map) (right) MRI sequences performed 1h after the CT study show a focal lesion located in the left temporal cortex, with increased cerebral blood volume, and hypersignal in the apparent diffusion coefficient map, indicative of increased diffusion in the lesion. (C) Follow-up MRI study, including contrast-enhanced T2- and T1-weighted sequences performed 102 days later, shows marked tumor growth (TGR=2.5mm/week) and characteristic signs of high-grade glioma, with extensive perilesional edema, hemorrhagic foci and central necrosis. The histologic diagnosis was glioblastoma.
An elevated choline peak associated with a decreased peak of N-acetyl-containing compounds, suggestive of high-grade tumor, was seen in the one patient who underwent single voxel spectroscopy (patient 2) using a 3.0 T scanner (Fig. 4B).
The average time to follow-up MRI was 79 days (range 28–179 days) after the first study. During this period, only one patient (patient 3) had recurrence of symptoms. Follow-up studies showed a mean tumor growth of 246% (range 61–380%; mean TGR of 3.3mm/week [range 0.9–7.6mm/week]), with a mean maximum diameter of 37.5mm (range 21–48mm). The characteristics of tumor progression are shown in Table 2.
Characteristics of tumor progression.
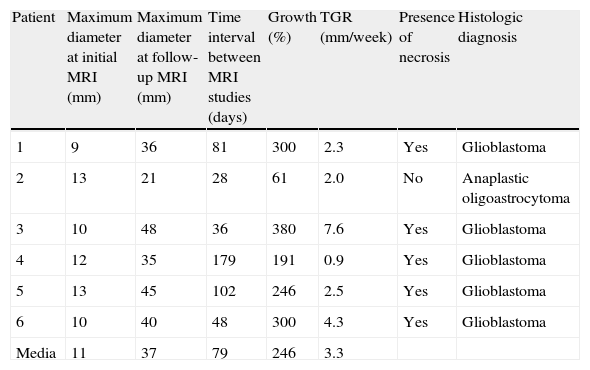
| Patient | Maximum diameter at initial MRI (mm) | Maximum diameter at follow-up MRI (mm) | Time interval between MRI studies (days) | Growth (%) | TGR (mm/week) | Presence of necrosis | Histologic diagnosis |
| 1 | 9 | 36 | 81 | 300 | 2.3 | Yes | Glioblastoma |
| 2 | 13 | 21 | 28 | 61 | 2.0 | No | Anaplastic oligoastrocytoma |
| 3 | 10 | 48 | 36 | 380 | 7.6 | Yes | Glioblastoma |
| 4 | 12 | 35 | 179 | 191 | 0.9 | Yes | Glioblastoma |
| 5 | 13 | 45 | 102 | 246 | 2.5 | Yes | Glioblastoma |
| 6 | 10 | 40 | 48 | 300 | 4.3 | Yes | Glioblastoma |
| Media | 11 | 37 | 79 | 246 | 3.3 |
TGR: tumor growth rate; MRI: magnetic resonance imaging.
In five patients the lesions showed the typical features of glioblastoma (central necrosis and perilesional edema) (Figs. 1C, 2C and 3C). In one patient (patient 2), the lesion exhibited similar initial characteristics but it had an increase in size of about 61% and a TGR of 2mm/week (Fig. 4C). All the patients underwent cytoreductive surgery after the follow-up MRI, with a histologic diagnosis of glioblastoma in five patients and of anaplastic oligoastrocytoma in one patient (patient 2).
DiscussionIn the early stages, the radiologic presentation of HGAs can be atypical. Based on our experience, and in agreement with other published series,7,8 HGA must be considered in the diagnosis of patients with focal lesions of minimal/moderate enhancement, especially in the setting of new-onset epileptic seizures. In three cases of this series, in addition to contrast enhancement, the perfusion MRI study showed an increase in cerebral blood flow/volume, a finding that also confirms the diagnosis of high-grade tumor.9 Nonetheless, this hyperperfusion could be secondary to the epileptic seizure itself, since the electric hyperactivity in the brain may induce hemodynamic changes that increase the cerebral blood flow.10
MRI findings in HGA are well known, and they include the presence of focal areas, usually solitary, of T1 iso- or hypointensity and T2 hyperintensity, with irregular and ill-defined margins, mass effect, perilesional vasogenic edema, and marked enhancement with intravenous contrast administration, as well as central necrosis in glioblastomas. The presence of these neuroimaging features is highly specific for the diagnosis of HGA. Nonetheless, in some cases these findings are not typically diagnostic. In this respect, 14–45% of nonenhancing supratentorial astrocytomas correspond to HGAs11,12 and 9% of HGAs (especially anaplastic astrocytomas) show no contrast enhancement.6 Similarly, HGAs may appear as small lesions without necrosis or perilesional edema,7,8 simulating less aggressive lesions.
In the patients reported in this article, the common presenting symptom was epileptic seizures, which are most commonly associated with the onset of LGAs, in contrast with HGAs, where the presenting symptom is normally intracranial hypertension or focal neurologic deficit.13 The cortical location of the lesions included in this article is responsible for this clinical presentation, facilitating their detection during the early stage of development using MRI.
In addition to HGAs, the differential diagnosis in these patients must include LGAs, cortical dysplasia, acute ischemia or cortical edema secondary to the epileptic seizure. The presence of contrast enhancement is an unusual finding in LGAs14 and in focal cortical dysplasias, but the latter may show enhancement when associated with certain tumors such as dysembryoplastic neuroepithelial tumor or ganglioglioma.15 Additionally, the absence of restricted diffusion is helpful to rule out acute stroke.16 In some cases, the postictal cortical edema may show reversible focal thickening, usually associated with moderately restricted diffusion; however, contrast enhancement of these lesion is uncommon.17
In our series, the key finding for making the radiologic diagnosis of HGA was the rapid growth of the lesion. Different studies in the literature that have analyzed the growth of infiltrating LGAs have demonstrated that these tumors grow slowly but continuously before progressing to high-grade gliomas, with a mean TGR of 4mm/year.18,19 Unfortunately, no factor can predict the moment when an infiltrating LGA will progress to HGA, with tumor growth within six months being the most sensitive radiologic finding to predict malignant transformation.18 There is little information regarding HGA growth in its natural history. In this respect, Woodward et al. followed for a year an untreated glioblastoma allowing them to establish a TGR of 30mm/year,19 but the data from our series indicate that TGR can be five times higher.
MR spectroscopy can help to better characterize these lesions, because a marked increase in choline associated with a decrease in N-acetyl-containing compounds is suggestive of HGA, as in one of our patients.20 Nonetheless, the small size and the cortical location of the lesions make it difficult to perform these studies.
The limitations of the study relate to its retrospective nature, which does not allow us to identify possible cortical lesions with similar clinical and radiological characteristics but with different progression and histopathological diagnosis. Therefore, the prevalence with which HGAs present in the form of atypical cortical lesion on neuroimaging studies cannot be determined nor can the specificity of the neuroimaging findings be evaluated. In any case, the conclusion that can be drawn from this study is that HGAs may appear as cortical lesions that do not show the typical features of HGAs, probably reflecting an early stage of their development.
HGA must be included in the differential diagnosis of any cortical lesion with mild or moderate enhancement on MRI, in patients with new-onset seizures. In case of adopting an expectant management, close follow-up with short interval (every few weeks) repeat neuroimaging studies should be performed, since the rapid growth rate of the lesion will be an indicator of tumor progression, a key feature suggestive of HGA, and therefore an indication for urgent cytoreductive surgery.
Authorship- 1.
Responsible for the integrity of the study: MS, ARC, MT.
- 2.
Conception of the study: ARC, CA, MS.
- 3.
Design of the study: ARC, MS.
- 4.
Acquisition of data: CA, SS, ARG.
- 5.
Analysis and interpretation of data: ARC, MS, MT.
- 6.
Bibliographic search: MS, ARC.
- 7.
Drafting of the paper: MS, ARC.
- 8.
Critical review with intellectually relevant contributions: ARG, SS, CA, MT.
- 9.
Approval of the final version: MS, ARG, ARC, SS, CA, MT.
- 10.
All the authors have read and approved the final manuscript.
The authors declare that they are not having any conflict interests.
Please cite this article as: Simonet Redondo M, et al. Hallazgos neurorradiológicos en la fase inicial del desarrollo de astrocitomas corticales de alto grado de malignidad. Radiología. 2012. doi:10.1016/j.rx.2011.04.003.




![Patient No. 1. A 39-year-old man presents to the hospital because of an audiogenic, simple partial seizure. (A) The emergency CT of the brain performed without administration of contrast agent was initially interpreted as normal, but a retrospective examination of the CT scan reveals a very subtle hyperattenuating area in the right temporal cortex (arrow). (B) MRI sequences performed 48h after the ictal episode (FLAIR T2-weighted [left], perfusion MRI using arterial spin labeling [center] and contrast enhanced T1-weighted [right]) show a focal lesion located in the cortex of the right middle temporal gyrus, with increased cerebral blood flow in the perfusion sequence and partial enhancement after intravenous administration of contrast. (C) FLAIR T2-weighted (left) and contrast enhanced T1-weighted (right) MRI sequences performed 81 days later demonstrate marked tumor growth (TGR=2.3mm/week) and typical features of high-grade glioma, with perilesional edema and central necrosis. The histologic diagnosis was glioblastoma. Patient No. 1. A 39-year-old man presents to the hospital because of an audiogenic, simple partial seizure. (A) The emergency CT of the brain performed without administration of contrast agent was initially interpreted as normal, but a retrospective examination of the CT scan reveals a very subtle hyperattenuating area in the right temporal cortex (arrow). (B) MRI sequences performed 48h after the ictal episode (FLAIR T2-weighted [left], perfusion MRI using arterial spin labeling [center] and contrast enhanced T1-weighted [right]) show a focal lesion located in the cortex of the right middle temporal gyrus, with increased cerebral blood flow in the perfusion sequence and partial enhancement after intravenous administration of contrast. (C) FLAIR T2-weighted (left) and contrast enhanced T1-weighted (right) MRI sequences performed 81 days later demonstrate marked tumor growth (TGR=2.3mm/week) and typical features of high-grade glioma, with perilesional edema and central necrosis. The histologic diagnosis was glioblastoma.](https://static.elsevier.es/multimedia/21735107/0000005400000005/v1_201305061506/S2173510712001048/v1_201305061506/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Patient No. 2. A 33-year-old male presents to the emergency room because of a partial seizure with secondary generalization. (A) The MRI sequences (FLAIR T2-weighted [left], perfusion MRI using arterial spin labeling [center] and contrast enhanced T1-weighted [right]) performed 48h after the seizure show a focal lesion located in the cortex adjacent to the right superior frontal sulcum, with increased cerebral blood flow in the perfusion sequence and mild homogeneous enhancement after intravenous administration of contrast. (B) Proton MR spectroscopy (single-voxel technique with long echo time) reveals a marked increase in choline (Cho) associated with decreased N-acetyl-containing compounds (Nac) (Cr: creatine). (C) FLAIR T2-weighted (left) and contrast enhanced T1-weighted (right) MRI sequences of the brain performed 28 days later demonstrate marked tumor growth (TGR=2.0mm/week), but without the presence of necrosis or perilesional edema. The histologic diagnosis was anaplastic oligoastrocytoma. Patient No. 2. A 33-year-old male presents to the emergency room because of a partial seizure with secondary generalization. (A) The MRI sequences (FLAIR T2-weighted [left], perfusion MRI using arterial spin labeling [center] and contrast enhanced T1-weighted [right]) performed 48h after the seizure show a focal lesion located in the cortex adjacent to the right superior frontal sulcum, with increased cerebral blood flow in the perfusion sequence and mild homogeneous enhancement after intravenous administration of contrast. (B) Proton MR spectroscopy (single-voxel technique with long echo time) reveals a marked increase in choline (Cho) associated with decreased N-acetyl-containing compounds (Nac) (Cr: creatine). (C) FLAIR T2-weighted (left) and contrast enhanced T1-weighted (right) MRI sequences of the brain performed 28 days later demonstrate marked tumor growth (TGR=2.0mm/week), but without the presence of necrosis or perilesional edema. The histologic diagnosis was anaplastic oligoastrocytoma.](https://static.elsevier.es/multimedia/21735107/0000005400000005/v1_201305061506/S2173510712001048/v1_201305061506/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)



