Dementia is a syndrome characterised by chronic, multi-domain, acquired cognitive impairment that causes significant functional limitations. MRI is the standard imaging study for these cases, since it enables detection of the atrophy patterns of the various neurodegenerative diseases (Alzheimer's disease, frontotemporal degeneration, Lewy body dementia), the vascular lesions associated with vascular dementia, and various potentially reversible diseases (for example, tumours, hydrocephaly) or diseases that require special management measures (for example, prion diseases). In certain cases other imaging methods can be used, such as CT, functional MRI, HMPAO SPECT or dopaminergic markers and FDG PET, amyloid markers or dopaminergic markers. The indications for these methods have not yet been clearly established, and therefore should be used in multidisciplinary dementia units.
La demencia es un síndrome caracterizado por un deterioro cognitivo crónico, adquirido, multidominio y que produce limitaciones funcionales significativas. La RM estructural es el estudio de imagen de elección en estos casos, dado que permite detectar los patrones de atrofia propios de las distintas enfermedades neurodegenerativas (enfermedad de Alzheimer, degeneración frontotemporal, demencia con cuerpos de Lewy), las lesiones vasculares asociadas a las demencias vasculares y varias patologías potencialmente reversibles (p.ej. tumores, hidrocefalia) o que requieren medidas de manejo especiales (p.ej. enfermedades priónicas). En casos seleccionados pueden utilizarse otros métodos de imagen, tales como la TC, la RM funcional, el SPECT con HMPAO o marcadores dopaminérgicos y el PET con FDG, marcadores de amiloide o marcadores dopaminérgicos. Las indicaciones de estos métodos no están aún bien establecidas, con lo que conviene utilizarlos en el contexto de unidades de demencia multidisciplinares.
Dementia is a clinical syndrome that consists of a multi-domain, acquired, chronic cognitive impairment that significantly interferes with daily activities1,2:
- •
Previous definitions of dementia required the involvement of recent memory plus at least one other cognitive function. Currently, we know that the involvement of memory is a secondary or delayed element in some dementias, so it is no longer considered essential. The requirement of a multi-domain involvement distinguishes dementia from focal syndromes.
- •
The acquired origin of dementia differs from mental retardation.
- •
The chronic course, arbitrarily defined as longer than six months, makes it possible to differentiate dementia from acute confusional syndrome. However, the limit between these two syndromes is imprecise, as well as with the rapidly evolving forms of dementia (subacute dementia).3
- •
The presence of significant functional alterations differentiates dementia from mild cognitive impairment.
The incidence and prevalence of dementia increase exponentially with age. The prevalence of dementia in those under 65 years of age is less than 5%, while in those over 85 years of age it reaches 30–60%. Taking into account the progressive ageing of the population and the expense associated with these pathologies, dementia is one of the main public health challenges in Western countries.4
The aetiology of dementia also varies greatly depending on age. In cases of early onset, genetic and secondary causes predominate (e.g. trauma, infections, cerebrovascular diseases). In those older than 65 years of age, most cases correspond to sporadic degenerative dementias, vascular dementias or a combination of both.
Most patients with dementia have subtle clinical manifestations for years. The first symptoms usually correspond to subjective memory complaints. Subsequently, they develop a mild cognitive impairment, characterised by deficits in one or more cognitive spheres without significant interference in social and work activities. Dementia would represent the final phase of this continuum, and in turn includes different phases of severity until complete dependence.
Clinical–anatomical classificationThe main neural networks involved in cognitive functions are the following5:
- •
The frontal network, on which the executive functions and the control of social behaviour depend.
- •
The temporolimbic network, dedicated to memory fixation and emotions.
- •
The occipitotemporal network, responsible for the visual recognition of objects.
- •
The right parietofrontal network, dedicated to the spatial functions of location and movement.
- •
The left perisylvian network, which deals with language and other related functions.
In addition to these classic neural networks, in recent years a neural network has been described by default, which is active with the subject at rest, and which consists of several structures close to the midline (medial prefrontal cortex, posterior cingulate, precuneus) and the associative regions of the parieto-temporo-occipital confluence.6
The anatomical distribution of neural networks and their selective involvement in different entities explain the great variability in the clinical manifestations of dementias. However, in clinical practice we observed two characteristic Pictures7,8:
- •
Cortical dementias, for which the prototype is Alzheimer's disease (AD), are characterised by an early involvement of memory fixation and the development of classic cognitive syndromes, such as aphasia, apraxia and agnosia. In the initial phases, these patients do not usually have prominent motor manifestations.
- •
Frontal-subcortical dementias, typical of parkinsonism and ischaemic cerebral small vessel disease, are characterised by bradypsychia, executive dysfunction and the presence of prominent motor alterations from the initial phases.
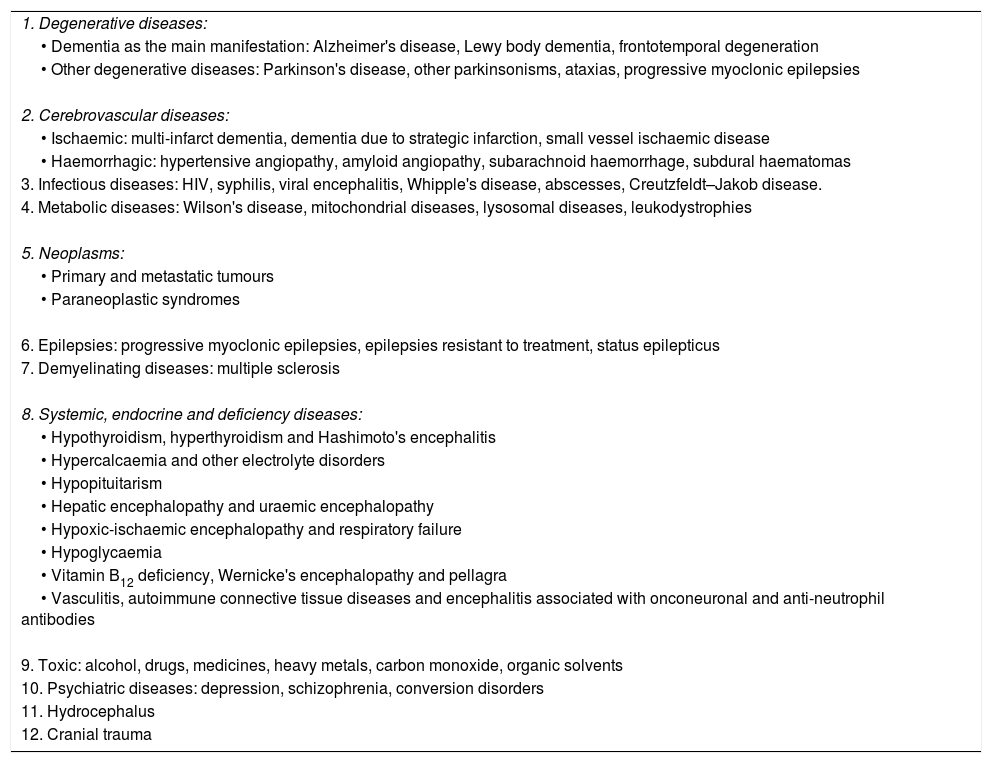
The main causes of dementia can be classified into two large groups (Table 1):
- •
Primary, idiopathic or degenerative dementias represent the dominant group in our setting. These processes are characterised by a slowly progressive course and the presence of brain deposits of abnormally folded proteins. Most cases are sporadic, but may have an autosomal dominant genetic origin. The most frequent degenerative dementias are AD, Lewy body dementia (LBD) and frontotemporal degeneration.
- •
Secondary dementias occur as a consequence of structural neurological pathologies or specific systemic diseases. The most frequent entity in this group is vascular dementia. Other important causes include normal pressure hydrocephalus, hypothyroidism and vitamin B12 deficiency. The identification of these processes makes it possible to initiate a specific treatment and, in some cases, to stabilise or reverse the cognitive deterioration.
Aetiological classification of dementias. The main causes of each category are shown.
| 1. Degenerative diseases: |
| • Dementia as the main manifestation: Alzheimer's disease, Lewy body dementia, frontotemporal degeneration |
| • Other degenerative diseases: Parkinson's disease, other parkinsonisms, ataxias, progressive myoclonic epilepsies |
| 2. Cerebrovascular diseases: |
| • Ischaemic: multi-infarct dementia, dementia due to strategic infarction, small vessel ischaemic disease |
| • Haemorrhagic: hypertensive angiopathy, amyloid angiopathy, subarachnoid haemorrhage, subdural haematomas |
| 3. Infectious diseases: HIV, syphilis, viral encephalitis, Whipple's disease, abscesses, Creutzfeldt–Jakob disease. |
| 4. Metabolic diseases: Wilson's disease, mitochondrial diseases, lysosomal diseases, leukodystrophies |
| 5. Neoplasms: |
| • Primary and metastatic tumours |
| • Paraneoplastic syndromes |
| 6. Epilepsies: progressive myoclonic epilepsies, epilepsies resistant to treatment, status epilepticus |
| 7. Demyelinating diseases: multiple sclerosis |
| 8. Systemic, endocrine and deficiency diseases: |
| • Hypothyroidism, hyperthyroidism and Hashimoto's encephalitis |
| • Hypercalcaemia and other electrolyte disorders |
| • Hypopituitarism |
| • Hepatic encephalopathy and uraemic encephalopathy |
| • Hypoxic-ischaemic encephalopathy and respiratory failure |
| • Hypoglycaemia |
| • Vitamin B12 deficiency, Wernicke's encephalopathy and pellagra |
| • Vasculitis, autoimmune connective tissue diseases and encephalitis associated with onconeuronal and anti-neutrophil antibodies |
| 9. Toxic: alcohol, drugs, medicines, heavy metals, carbon monoxide, organic solvents |
| 10. Psychiatric diseases: depression, schizophrenia, conversion disorders |
| 11. Hydrocephalus |
| 12. Cranial trauma |
HIV: human immunodeficiency virus.
Although the causes of dementia are usually described independently, in the series of autopsies it was observed that most patients present a combination of pathologies, including degenerative lesions such as Alzheimer's and vascular lesions.
General assessment of patients with dementiaThe assessment of a patient with cognitive or behavioural symptoms begins with the medical history. In these cases, it is essential to obtain additional information from a family member. We must collect the list of symptoms and pay special attention to their order of onset and their speed of development.
The neurological examination should include, apart from the usual components, a detailed analysis of the mental state.9 The level of consciousness, attention, orientation, recent and remote memory, language, visual recognition, execution of motor acts, the capacity for abstraction, constructive skills and executive functions should be examined in an orderly manner. We must also investigate in all cases the patients’ mood and functional capacity. In order to facilitate the systematic collection of this information, it is convenient to use validated scales and questionnaires. The specific tools will depend to a great extent on the clinical setting (e.g. primary care, memory unit) and the reference population (e.g. low educational level). The most widely used tests, in different combinations, include: Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), selective reminding test (Buschke), Wechsler memory scale (WMS-III), categorical (animals) and phonological (letter “p”) fluency, Trail Making Test (parts A and B), clock test, visual screening test (Mesulam), geriatric depression scale (GDS-15), informant test (SS-IQCODE) and functional activities questionnaire (FAQ).
Regarding the additional examinations, the essential studies include1,2:
- •
Blood tests: blood count, erythrocyte sedimentation rate, general biochemistry and levels of vitamin B12, folic acid and TSH.
- •
A brain imaging test, preferably magnetic resonance imaging (MRI).
In the initial study of a patient with dementia, the classic role of neuroimaging is to detect secondary forms of dementia, especially potentially treatable causes. The most frequent cases include adult hydrocephalus (“normal pressure hydrocephalus”), chronic subdural haematoma and some tumours, especially slow-growing ones, such as meningiomas.
The rest of the examinations will be performed in selected cases, depending on the clinical suspicion and their availability: immunological and microbiological tests in blood and cerebrospinal fluid (CSF), quantification of Aβ-42, tau and phosphorylated tau in CSF, electroencephalogram, genetic analysis, cerebral positron emission tomography (PET) scan (fluorodeoxyglucose [FDG], ioflupane, amyloid markers) and other studies aimed at ruling out systemic processes (e.g. hidden neoplasms).
Image findings in the main causes of dementiaAlzheimer's diseaseAD is the most frequent cause of dementia in Western countries, where it accounts for 60% of all cases.10,11 The characteristic anatomopathological lesions include senile plaques, composed of beta-amyloid peptide (Aβ), and the neurofibrillary tangles, made up of phosphorylated tau protein. Most cases correspond to sporadic forms of late onset (over 65 years of age). Allele 4 of apolipoprotein E is a risk factor for these late forms. Less than 5% of cases correspond to genetic forms associated with dominant mutations in the genes of the amyloid precursor protein (APP), presenilin-1 or presenilin-2.
AD is the prototype of cortical dementia. Typical cases present with a serious affectation of recent episodic memory. Atypical cases may begin with frontal manifestations, visuospatial abnormalities (posterior variant) or language alterations (logopenic aphasia).
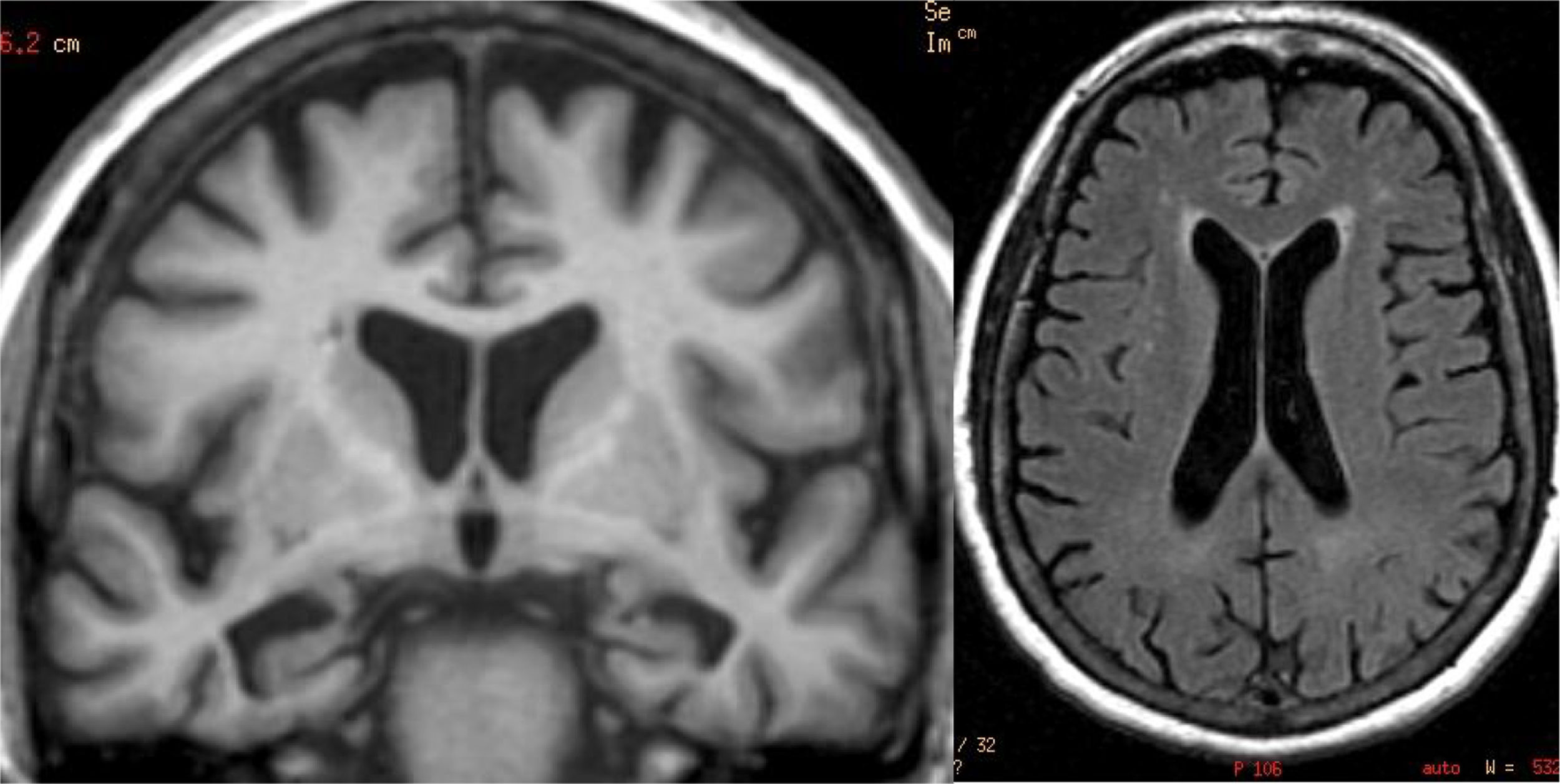
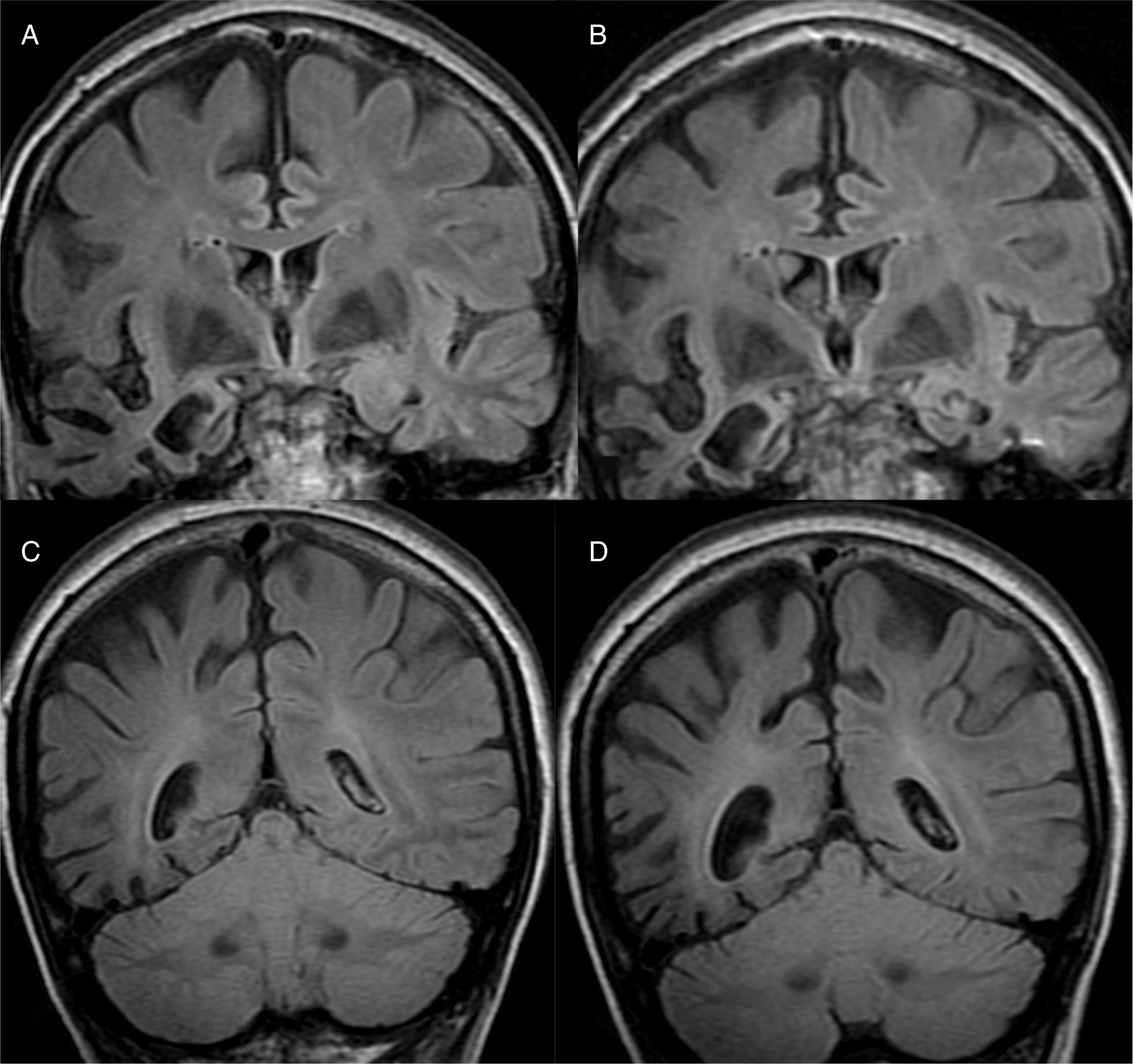
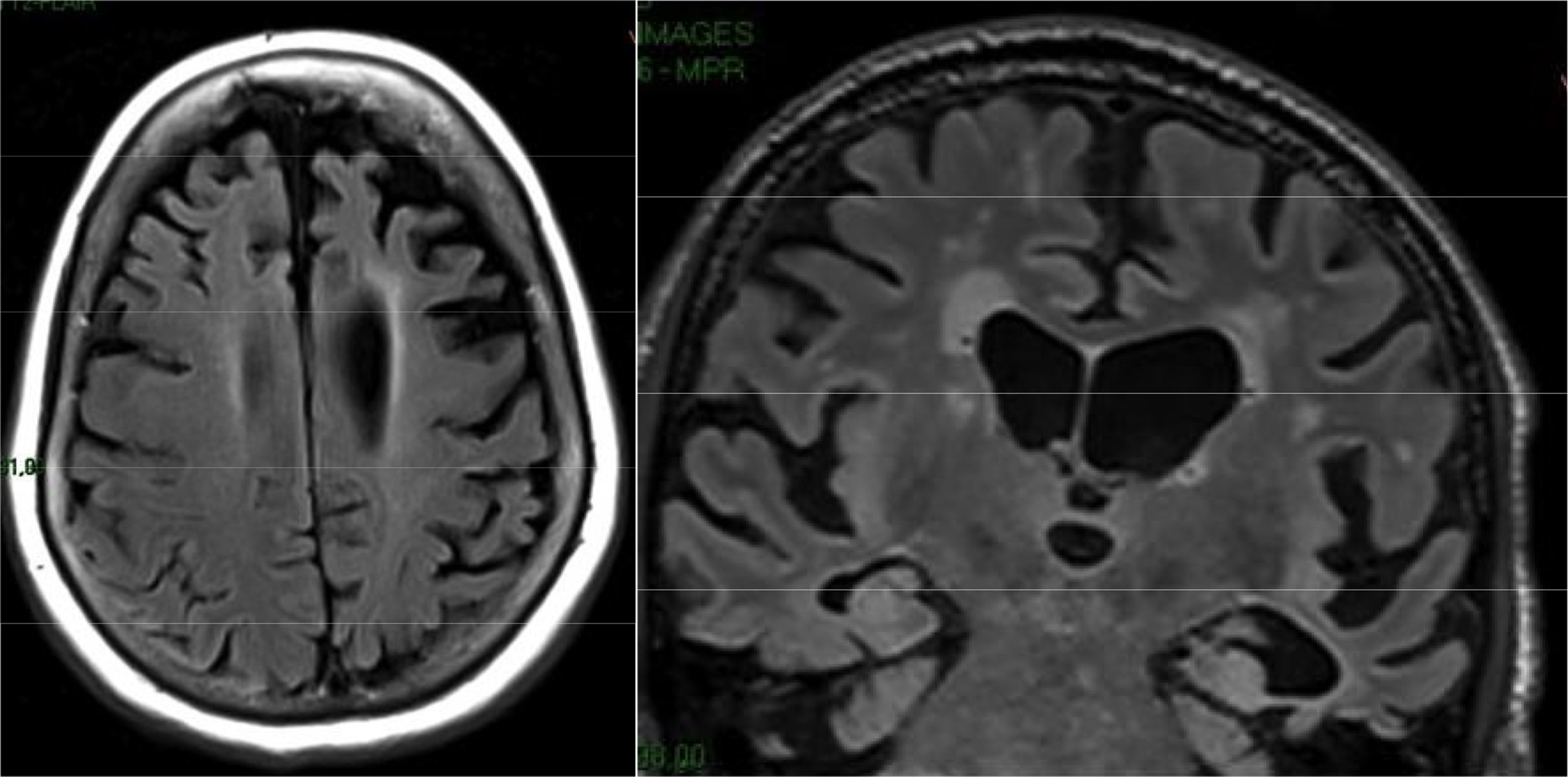
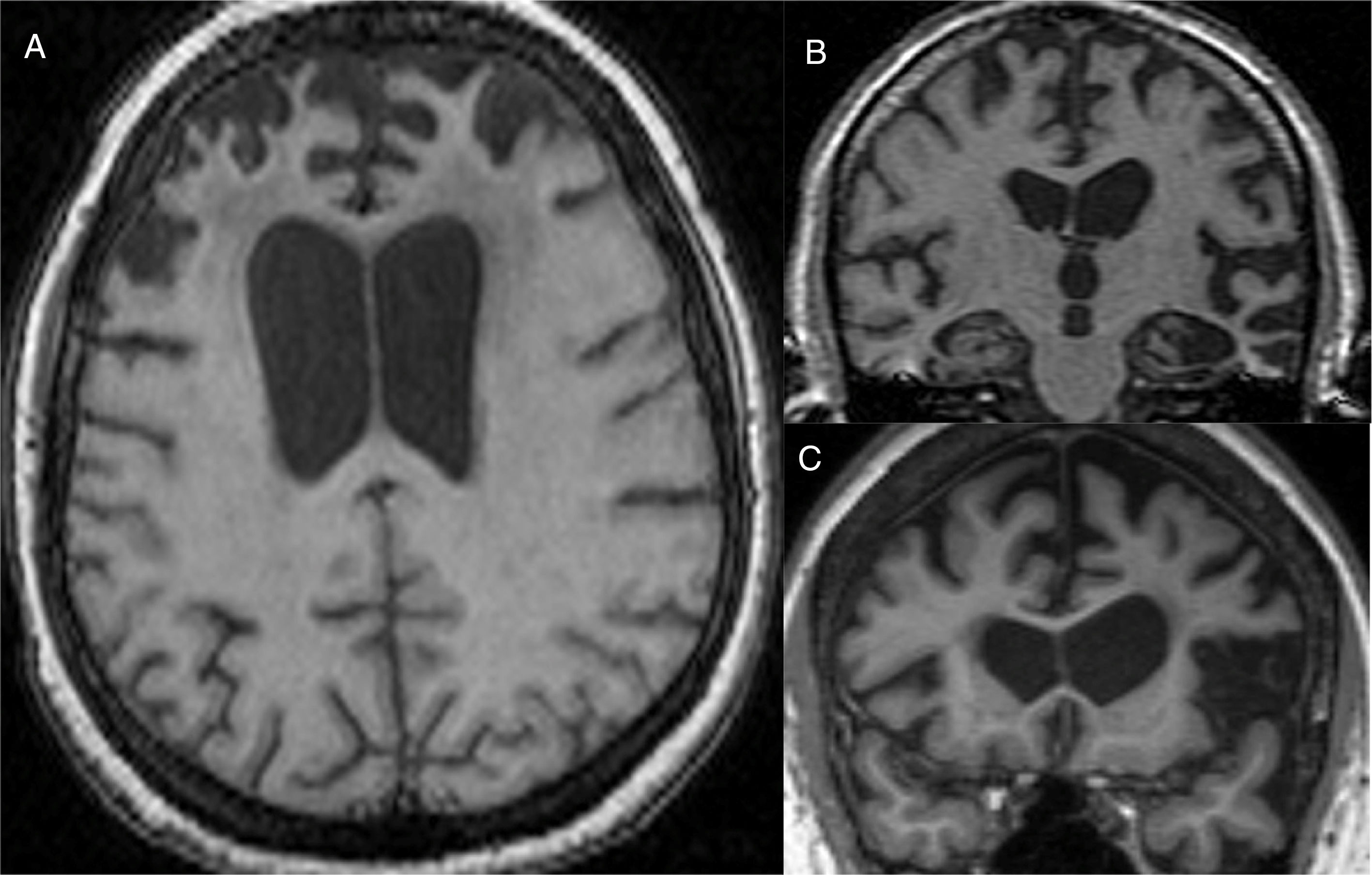
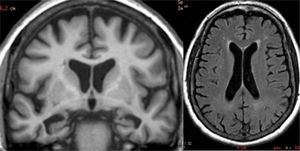
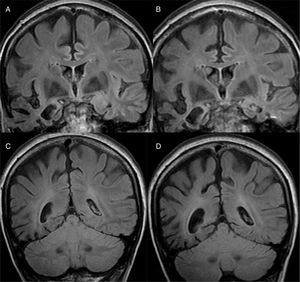
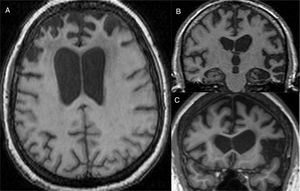
The main finding in structural imaging in AD is cerebral atrophy with medial temporal predominance (Fig. 1). The entorhinal cortex and the hippocampus are affected early; as the hippocampus is an easily identifiable structure in MRI, its atrophy is the main radiological sign in AD.12 Temporal involvement in AD is typically symmetric, although occasionally there may be asymmetric involvement, which is more typical in frontotemporal dementia (FTD). In these cases, it is very useful to assess the so-called anteroposterior gradient: in AD, the loss of volume is greater in the parietal lobe than in the frontal lobe (posterior gradient), while in FTD the loss of volume predominates in the frontal lobe (anterior predominant gradient) (Fig. 2). However, the identification of significant atrophy in elderly patients has important limitations due to the frequent onset of loss of brain volume in asymptomatic individuals in this age group, including the medial temporal region.13 On the other hand, although the cross-sectional studies of groups indicate that there are significant differences in the degree of atrophy between normal subjects and patients with AD, the estimation of atrophy in isolated individuals has a limited value due to the overlap with healthy individuals, especially the elderly. Longitudinal studies increase the specificity, since the speed with which loss of volume occurs (especially in the hippocampus) is significantly higher in patients with AD, which can be useful when studying a single individual, since the annual hippocampal atrophy rate is better at separating healthy individuals and patients with AD.14
Alzheimer's disease with atypical phenotype (A and C), which presents asymmetric involvement, although with medial temporal predominance and greater parietal than frontal involvement (gradient of posterior predominance). In the annual check-up (B and D), the posterior predominance is more evident.
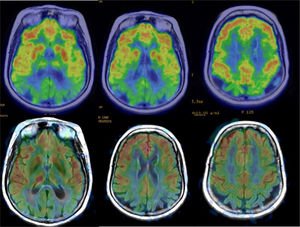
Functional studies, obtained by single-photon emission computed tomography (SPECT) or PET, and more recently by MRI, with arterial spin-labelling (ASL) perfusion sequences, can demonstrate cortical hypofunction in patients with neurodegeneration. In AD, functional alterations can be observed in early stages, whereas healthy individuals almost always show a normal cortical function pattern; therefore, in a patient with dementia, a negative functional study makes a neurodegenerative disease very unlikely and other causes of dementia must be considered. In addition, new radiopharmaceuticals for PET can detect pathological deposits of amyloid in the cerebral cortex; healthy subjects under 65 years of age present PET studies with a negative amyloid marker in more than 80% of cases, while a negative study in patients with AD is exceptional. The main limitation of these studies is that subjects older than 85 years of age have up to 50% positive amyloid PET scans, so the greatest potential of this test in older ages is the negative predictive value.
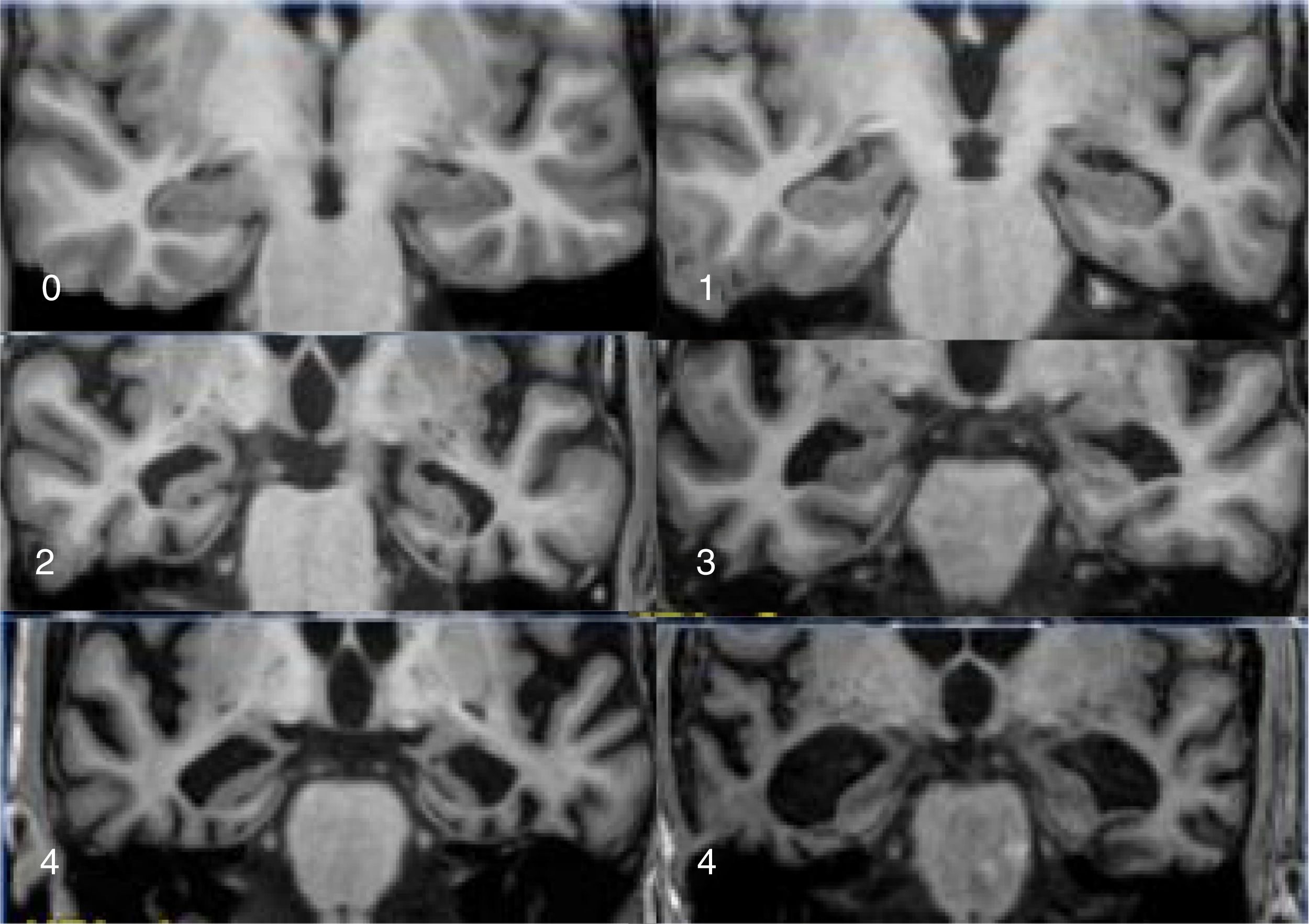
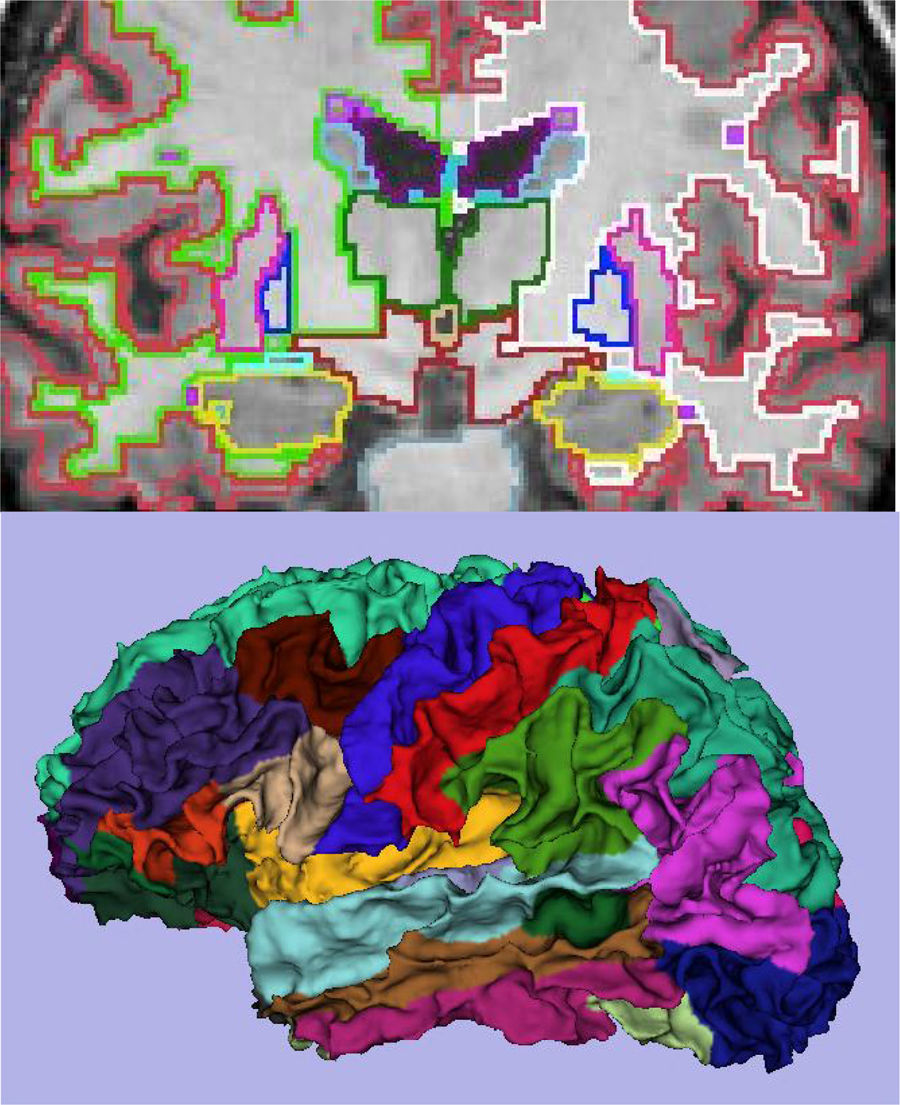
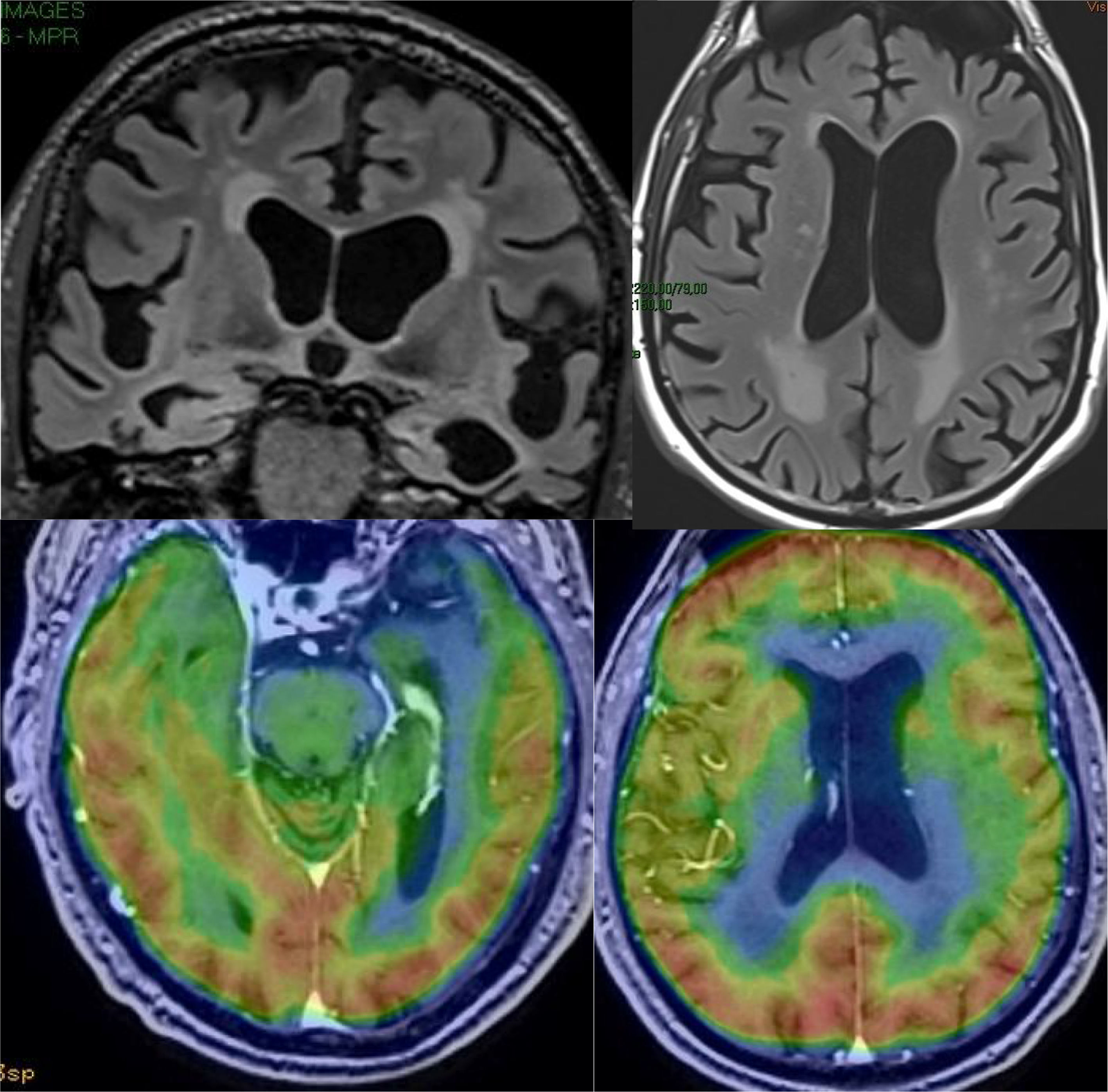
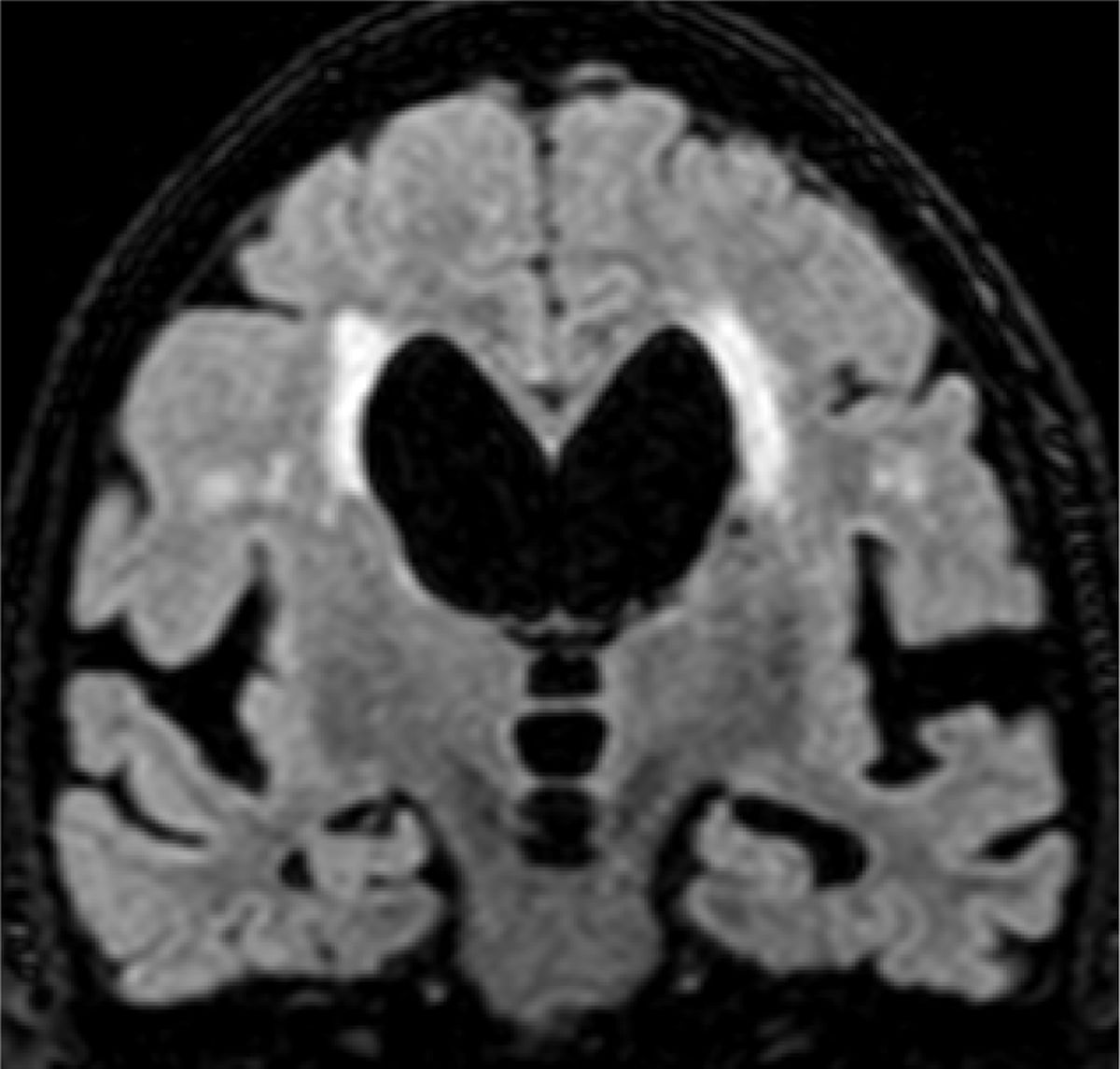
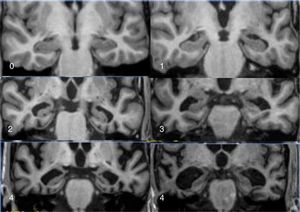
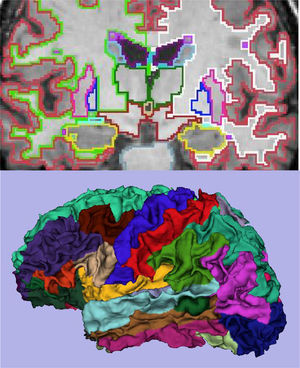
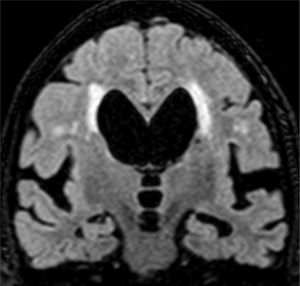
The main marker of neurodegeneration with MRI is atrophy of the hippocampus, which is usually 20–25% in subjects with AD and 10–15% in subjects with mild cognitive impairment.13 For the qualitative estimation of hippocampal atrophy, the most-used scale is the Scheltens scale, which assesses the morphology of the choroidal fissure, the temporal horn and the hippocampus itself and develops a scoring system on both sides from 0 to 4 (Fig. 3).15 Volumetry with MRI allows for calculation of volumes of cortical and subcortical structures, as well as cortical thickness (Fig. 4),16 which improves the estimation of atrophy, especially of the hippocampus, and, in addition, the annual rate of atrophy can be accurately measured. Atrophy of the hippocampus is also observed in other diseases, including dementia related to vascular lesions. Atrophy of other structures, such as the precuneus and the posterior region of the cingulate gyrus, is more specific to AD; therefore, it may be useful to add these measurements to that of the hippocampus itself.17 Cortical thickness measurements are very sensitive to small changes, but are used more in group studies, so their use in the clinic has less potential than volume calculations. Currently, there are validated programs that automatically calculate the volume of the hippocampus and estimate the deviation from the normal measurements of each age range.
The definitive diagnosis of AD requires the demonstration in pathological anatomy (PA) of neuritic plaques and neurofibrillary rings. However, the growing interest in early diagnosis has led to the development of biomarkers that increase the reliability of the diagnosis of probable AD. Currently, the diagnosis of probable AD is accepted in patients without dementia. Within the new diagnostic criteria, the prodromal stage of AD is considered when there is memory loss along with positive biomarkers. Validated biomarkers include those that detect amyloid deposit (by PET or CSF analysis), related to the pathophysiology of AD, and those related to neurodegeneration, detecting functional impairment (FDG-PET) or atrophy of the hippocampus (MRI). However, the main application of these new criteria is the research and development of new treatments, as long as therapies that modify the course of the disease are not available.
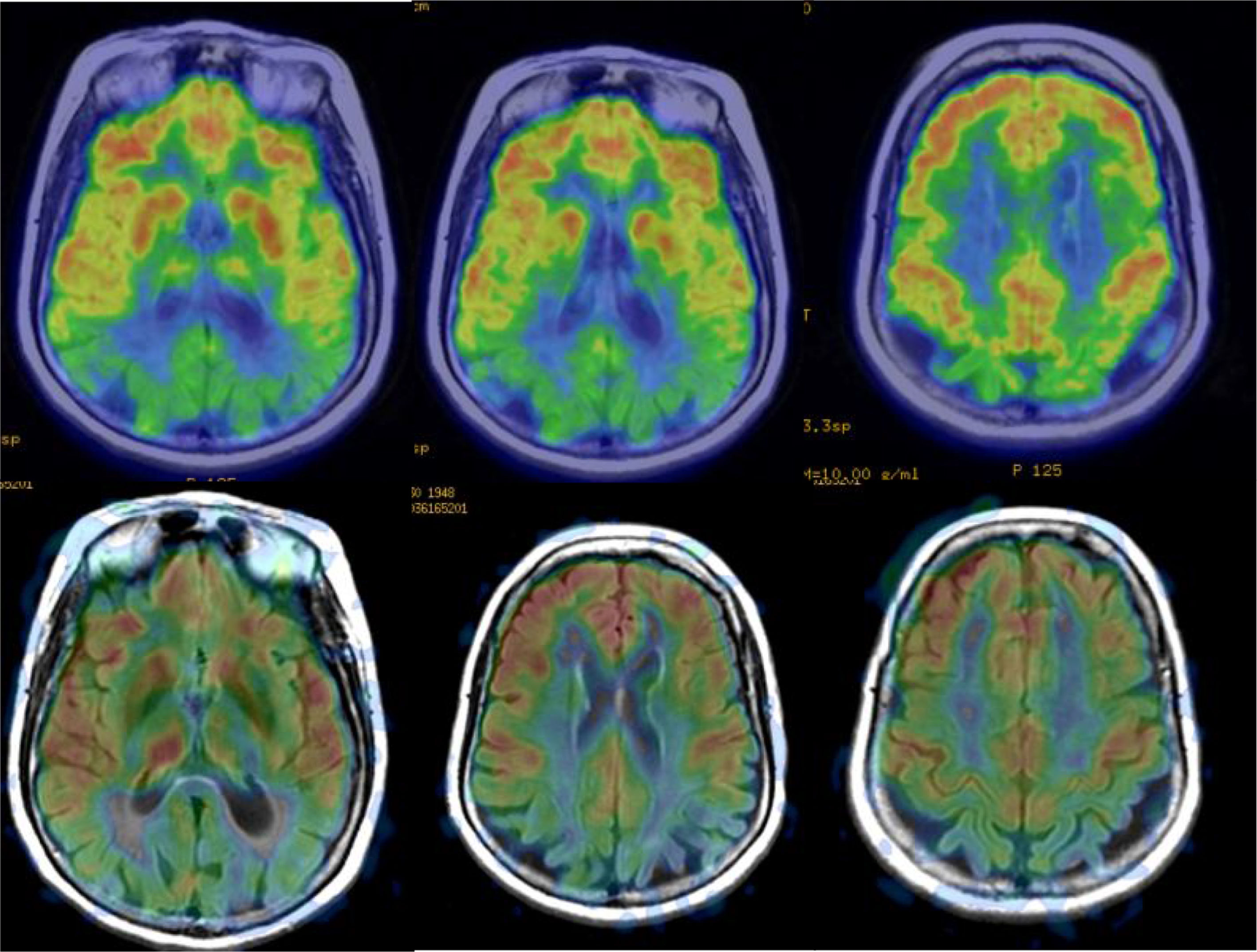
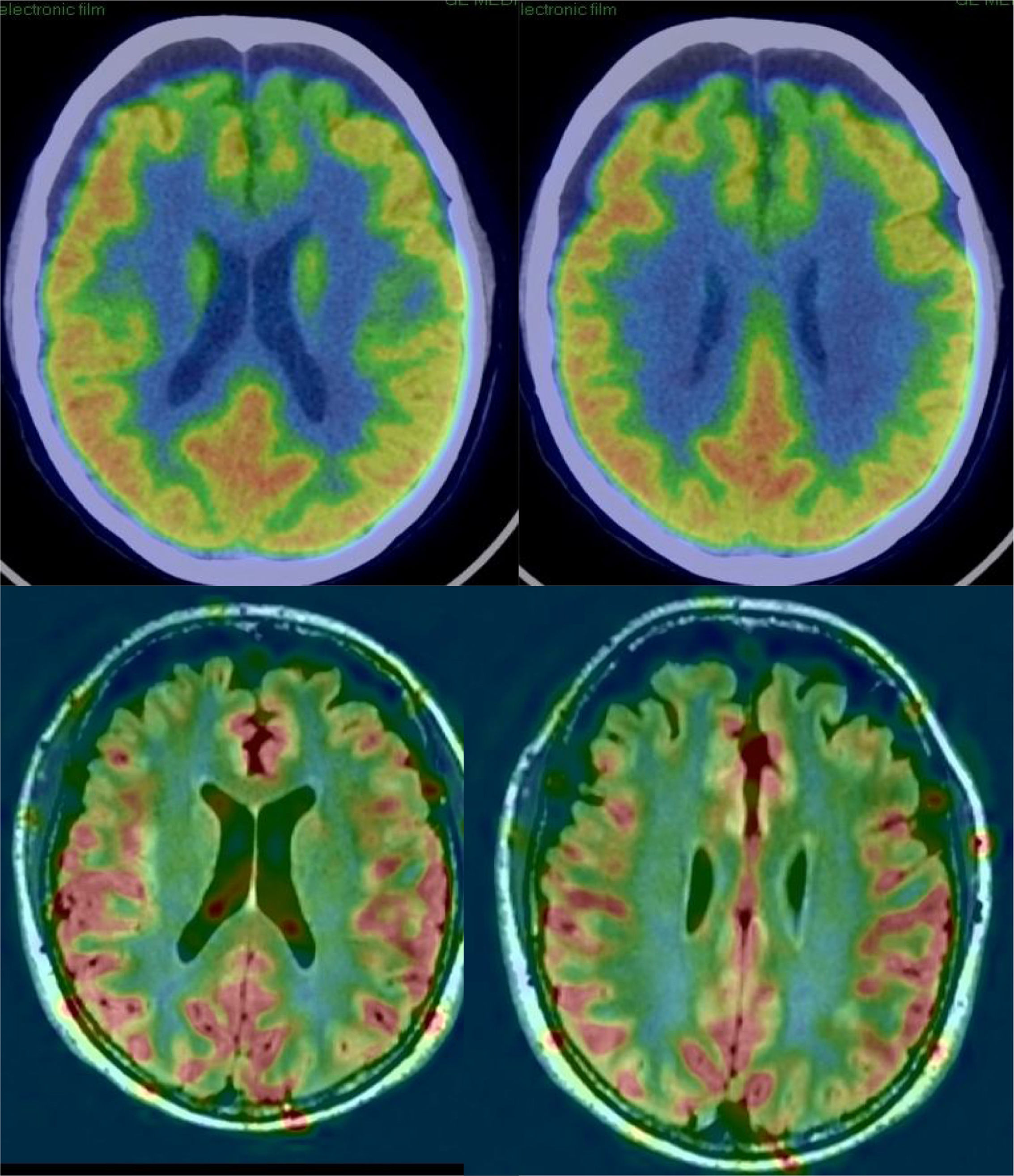
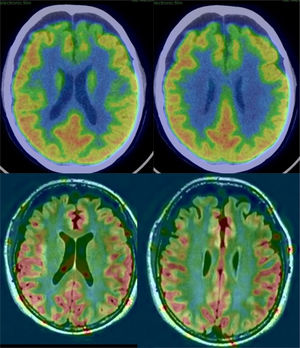
In AD, studies performed with FDG-PET frequently show a characteristic pattern of hypometabolism in the lateral temporoparietal cortex, precuneus and in the posterior region of the cingulate gyrus, as well as in the medial temporal region (Fig. 5). The frontal lobes are not involved until later stages of the disease, and there is no involvement of basal nuclei and primary cortex except in very late phases or atypical phenotypes, which are rare. In early stages, the most reliable sign is hypometabolism in the posterior region of the cingulate gyrus,18,19 although subtle changes are also observed in the other locations. It has been considered that these functional changes occur before the loss of volume, but recent studies show that both alterations occur simultaneously.20
Perfusion studies with MRI initially have not shown good results in neurodegenerative diseases, because the usual techniques (dynamic susceptibility contrast [DSC] and dynamic contrast enhanced [DCE]) that use a contrast injection are not very sensitive to changes in flow related to brain function. More recently, the ASL sequence, which does not require injection of a contrast and can quantify regional blood flow, has shown much greater sensitivity (Fig. 5), with results similar to nuclear medicine techniques.21 The ASL sequence has the drawback of being very sensitive to movement artefacts, but it can be a very useful complement to structural MRI, although it is necessary to improve the standardisation of the measurements and to clinically validate the results in series with a greater number of subjects.
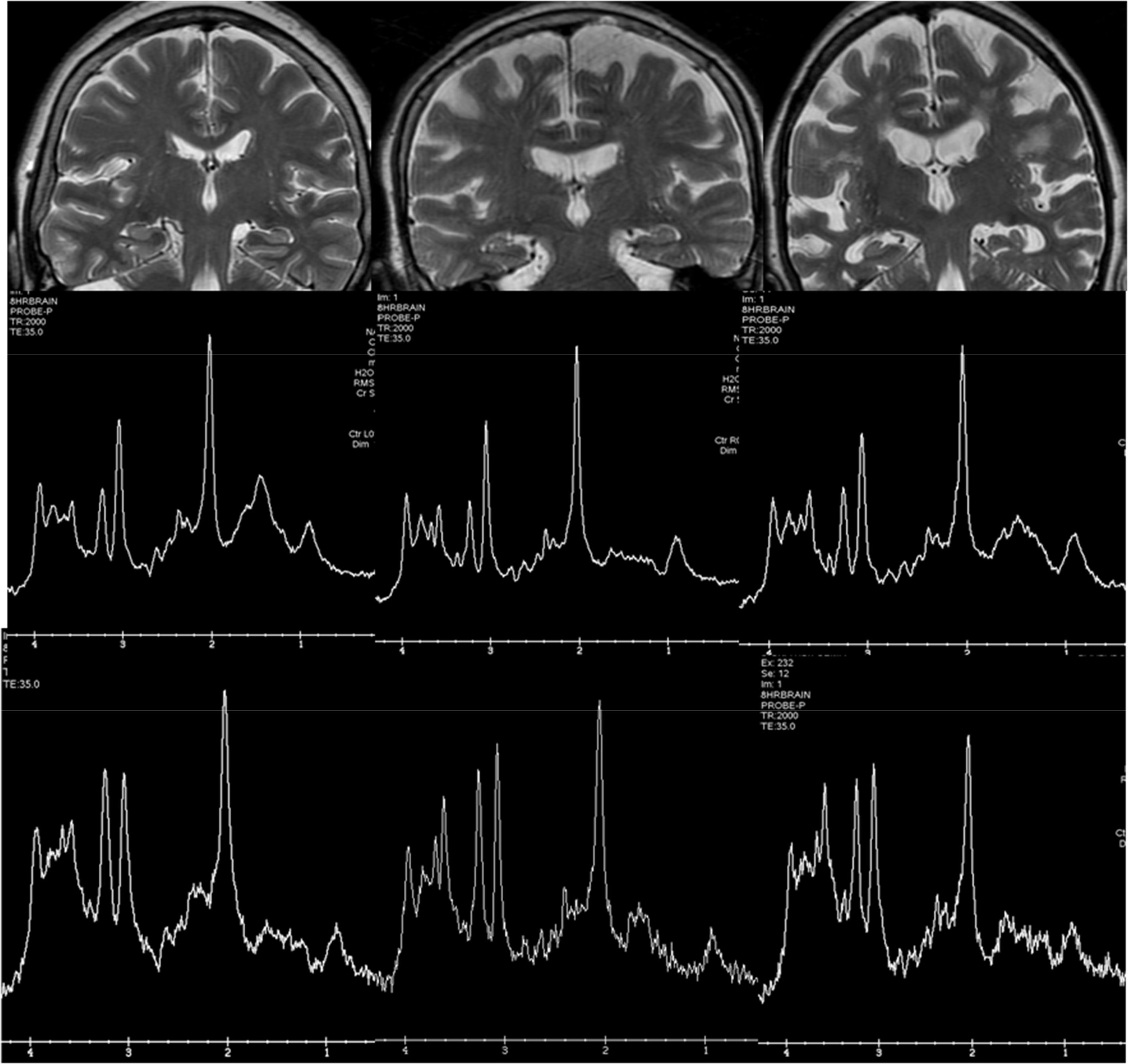
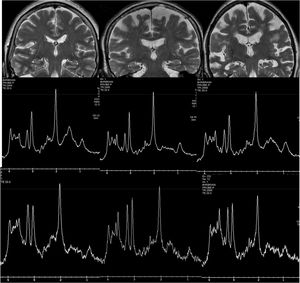
Spectroscopy has been widely used in research before the development of other MRI techniques, since AD presents a characteristic pattern, with a decrease in the peak of N-acetyl-aspartate (NAA) and an increase in the peak of myo-inositol (Fig. 6). However, the spectroscopic measurements show some variability, which in the medial temporal region is much greater due to the presence of the bone of the base of the skull and the air of the paranasal sinuses, which is why its clinical use in dementia is scarce.
Examples of hydrogen spectroscopy located in the precuneus (central row) and the hippocampus (lower row). The left column shows a normal subject. The central column corresponds to a patient with Lewy Body Dementia and the right column to a patient with Alzheimer's disease. The increase in Mi and the decrease in NAA are more prominent in the hippocampus, although the spectrum quality is lower.
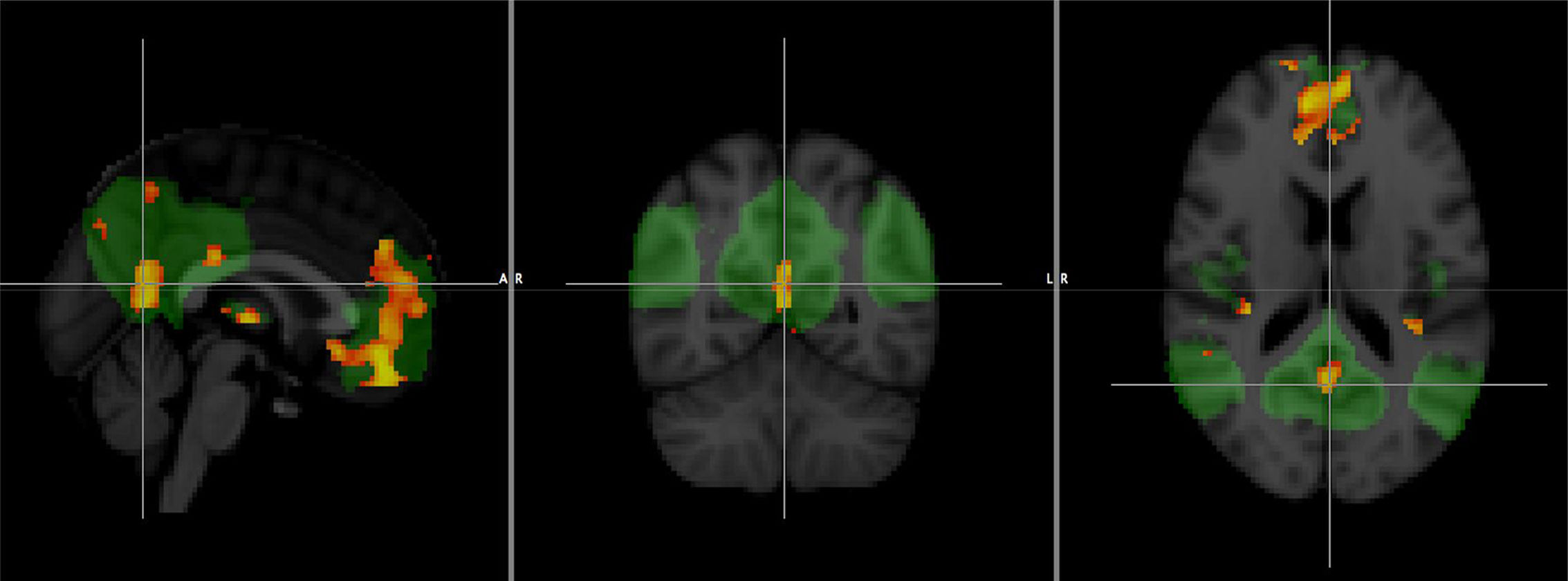
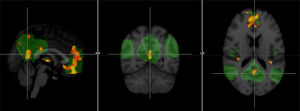
Other techniques, such as functional MRI and diffusion tensor imaging (DTI), are offering promising results, although their lower accessibility, as well as the greater technical difficulty and especially the lack of standardisation, mean that their use is currently restricted to the field of research. Resting-state functional MRI allows the study of connectivity in brain networks, and it has been shown that changes in the default network can be observed even in the preclinical phase (Fig. 7), so in the future it could be very useful in screening studies.22
Comparison of a group of patients with Alzheimer's disease and a healthy group with functional connectivity value in the default network obtained by resting-state functional magnetic resonance imaging. The areas in orange correspond to significant decrease (p<0.001) in the group with Alzheimer's disease.
FTD includes a series of degenerative processes characterised by a predominant involvement of the anterior brain regions.23 In our setting, it represents 10% of cases of dementia, and it is more common in forms of presenile initiation (under 65 years of age). About 50% of cases present with a brain accumulation of tau protein. The remaining cases present deposits of other proteins, such as TDP-43. It is estimated that 40% of cases of FTD have an autosomal dominant genetic origin. The main genes involved are MAPT, GRN, C9orf72, FUS, CHMP2B, TARDBP and VCP.
The clinical presentation usually corresponds to one of these three syndromes:
- •
Frontal dementia or behavioural variant is associated with atrophy of frontal predominance and presents with a variable combination of apathy, disinhibition, lack of introspection and executive dysfunction.
- •
In progressive non-fluent aphasia, atrophy is focussed in left perisylvian areas and is characterised by a predominantly expressive language disorder. These cases are usually associated with tau deposits.
- •
Semantic dementia is characterised by a predominant atrophy of the left temporal lobe and is accompanied by a progressive loss of knowledge of the meanings of words. In the initial phases, patients usually use language that is fluent, even verbose, but with little content and abundant circumlocutions. The underlying pathology usually consists of deposits of TDP-43.
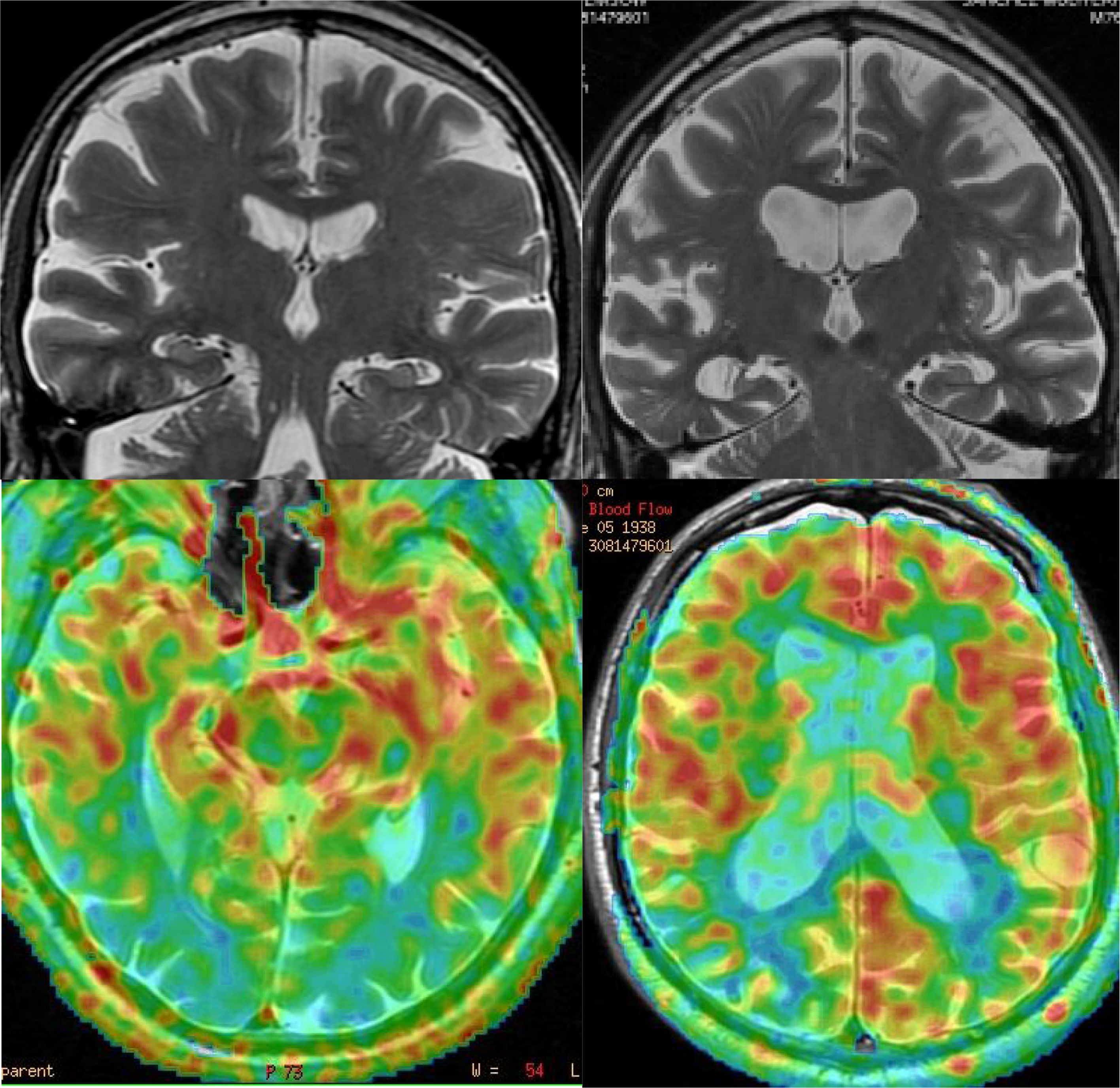
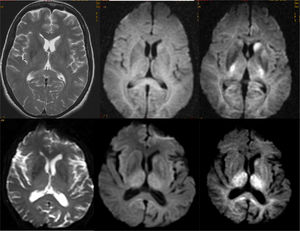
The image findings also vary depending on the clinical syndrome,24 although the most common pattern is frontotemporal atrophy, which is usually asymmetric. In addition, white matter signal abnormalities can be seen in FTD (Fig. 8). Atrophy patterns usually correspond to clinical subtypes (Fig. 9) and show correlation with the pathological subtype, although there may be individual variations.25 Functional studies show hypofunction, related to neurodegeneration, which usually coincides with areas of greater loss of volume, with the advantage that the sensitivity of functional studies is greater in the early stages26–28 (Fig. 10). In some cases, patients with FTD may present a functional pattern similar to AD, although there is almost always a frontal hypofunction, which is exceptional in AD up to the advanced stages (Fig. 11). ASL perfusion studies correlate with FDG-PET studies, although there is less experience than in AD.21 Amyloid markers are negative in these patients (unless there is comorbidity with AD),29 which can be very useful for the differential diagnosis in doubtful cases, especially in the temporary involvement forms.
LBD represents around 20% of all cases of dementia and is characterised by the presence of cortical and subcortical Lewy bodies constituted by the protein α-synuclein.30 Most of the cases are sporadic. The clinical diagnosis requires the presence of a dementia, which is usually predominantly dysexecutive and visuospatial, and a variable combination of spontaneous parkinsonism, visual hallucinations and severe fluctuations. These patients have a special sensitivity to classical neuroleptics (e.g. haloperidol).
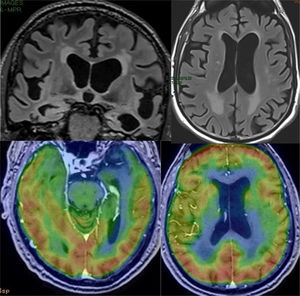
The findings in structural MRI are not very expressive. In early stages, MRI is usually normal and later atrophy may occur, which tends to have a diffuse distribution, so the findings are not specific.31 The existence of atrophy has been attributed to comorbidity with AD, since studies with subjects with LBD, but without AD, have not shown significant loss of volume.31 However, in a patient with normal dementia and hippocampus, this possibility should be considered, since it is a relatively common cause of dementia: the third most common, after AD and vascular dementia. Functional studies are very useful in these cases, since LBD characteristically presents occipital hypofunction (Fig. 11), in addition to other common areas with AD, while in AD functional involvement of the occipital lobes is very rare, except in the phenotype known as posterior cortical degeneration (Fig. 12). The presence of occipital hypometabolism in PET in LBD has a sensitivity and specificity of 90%.32 PET with amyloid markers is not useful in the differential diagnosis with AD, since LBD very frequently presents positive studies with these markers.33 In these cases, dopamine markers (e.g. ioflupane) are indicated, which show reduced uptake in striata, which is not observed in AD.34
Examples of the usefulness of ASL perfusion. In the left row, patient with Lewy Body Dementia, with minimal diffuse atrophy, with marked bilateral occipital hypoperfusion. In the right row, patient with Alzheimer's disease, which presents diffuse atrophy, without clear temporal predominance, which nonetheless shows a typical pattern of parietal hypoperfusion.
Cerebral vascular lesions cause 20% of all dementias and are often associated with Alzheimer's-type degenerative lesions. The characteristic clinical picture consists of an acute focal deficit that precedes or accompanies cognitive deterioration.35 However, there are cases that evolve progressively, simulating a degenerative disease.
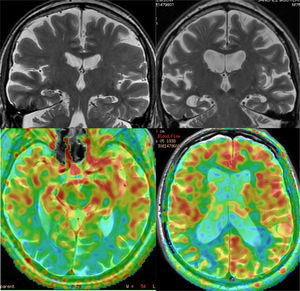
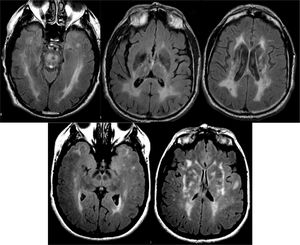
Ischaemic lesions may correspond to lesions of large vessels, typical of territorial infarcts, or lesions of small vessels, which cause lacunar infarcts and subcortical ischaemic leukoencephalopathy. The pattern of involvement typically includes the periventricular regions, the basal ganglia area, and the central region of the pons (Fig. 13). The degree of cognitive impairment is usually related to the extent of the lesions, which is usually assessed by qualitative scales. One of the most used is the Fazekas scale, which establishes four grades: (0) no lesions, (1) small non-confluent lesions, (2) partially confluent lesions and (3) diffuse periventricular involvement. Cases of dementia associated with small lesions (strategic infarcts) have been reported, such as lacunar infarcts in the territory of the paramedian thalamic arteries, infarcts of the lower knee of the internal capsule, infarcts of the head of the caudate nucleus and bilateral infarcts of pale nuclei. The main diagnostic problem is the frequent coexistence of vascular dementia with AD. In these cases, functional studies may be useful. In cases of vascular dementia, the typical pattern of AD is not observed, but it is common to observe a heterogeneous pattern, depending on the ischaemic areas, which usually include the basal ganglia; this is not observed in AD.36 When the typical pattern of AD is observed, comorbidity should be suspected. Hereditary forms (CADASIL and the recessive CARASIL form) are rare and usually present early. The pattern of involvement is similar to subcortical ischaemic leukoencephalopathy, with the particularity that in hereditary forms the white matter of the temporal poles and the external capsule can frequently be affected, which do not usually present in the first (Fig. 13).
Cerebral haemorrhages are classified, depending on their location, into intraparenchymal, subarachnoid, subdural and epidural haemorrhages. Deep intraparenchymal haemorrhages usually have a hypertensive origin, while non-traumatic superficial haemorrhages, especially in the parietal-occipital regions, usually depend on amyloid angiopathy. Amyloid angiopathy deserves special mention because of its clinical–pathological peculiarities.37 Most cases appear sporadically in elderly patients, and in this group the underlying lesions consist of deposits of peptide Aβ at the level of the leptomeningeal and cortical arteries. Hereditary forms are rare and usually present at younger ages. Spontaneous subarachnoid haemorrhages are usually secondary to the rupture of cerebral aneurysms. Subdural and epidural haemorrhages have a traumatic origin in most cases.
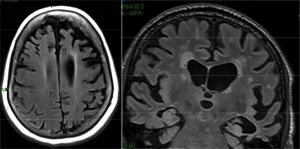
Adult hydrocephalusThe characteristic clinical picture of this entity consists of Hakim's triad: dementia, abnormal gait and incontinence.38 The cognitive impairment is of the frontal-subcortical type and usually appears after the alteration of the gait. Cases associated with haemorrhages or previous inflammatory processes usually respond well to ventricular drainage. Primary cases often have a partial and transient response. It is suspected that a significant percentage of these primary forms have underlying vascular or degenerative pathology. Frequently, prominence of the lateral ventricles and the third ventricle is observed, with signal void in the aqueduct of Sylvius in the T2 sequences due to the increase in CSF flow. Studies of CSF dynamics may facilitate the selection of patients for surgery, but all of them have false positives and negatives and their clinical utility is debated. More recently, a characteristic pattern has been described in MRI, with disproportionate enlargement of the subarachnoid space in Sylvian regions, associated with effacement of the grooves in the convexity (disproportionately enlarged subarachnoid space hydrocephalus [DESH]), that translates to an alteration in the drainage of the CSF into the venous circulation mediated by aquaporins39,40 (Fig. 14).
Creutzfeldt–Jakob diseaseCreutzfeldt–Jakob disease is the most common prion disease.41 Its incidence is one case per million inhabitants and year. Most cases develop sporadically, but may also have a hereditary, in relation to mutations in the PRNP gene,42 iatrogenic (e.g. dura mater implants, non-recombinant growth hormone) or infectious origin (e.g. new variant). The characteristic clinical picture consists of a rapidly progressive myoclonic dementia. In some patients, it begins with ataxia or focal manifestations (e.g. subsequent Heidenhain variant). Typical cases have a survival of less than one year. Routine blood and CSF tests are usually normal. Typical cases usually show an increase in 14–3–3 protein levels in CSF. Recently, an analytical method called RT-QuIC has been described. When applied to the CSF or olfactory epithelium, it reaches sensitivity and specificity figures for the diagnosis of sporadic Creutzfeldt–Jakob disease of close to 100%.43
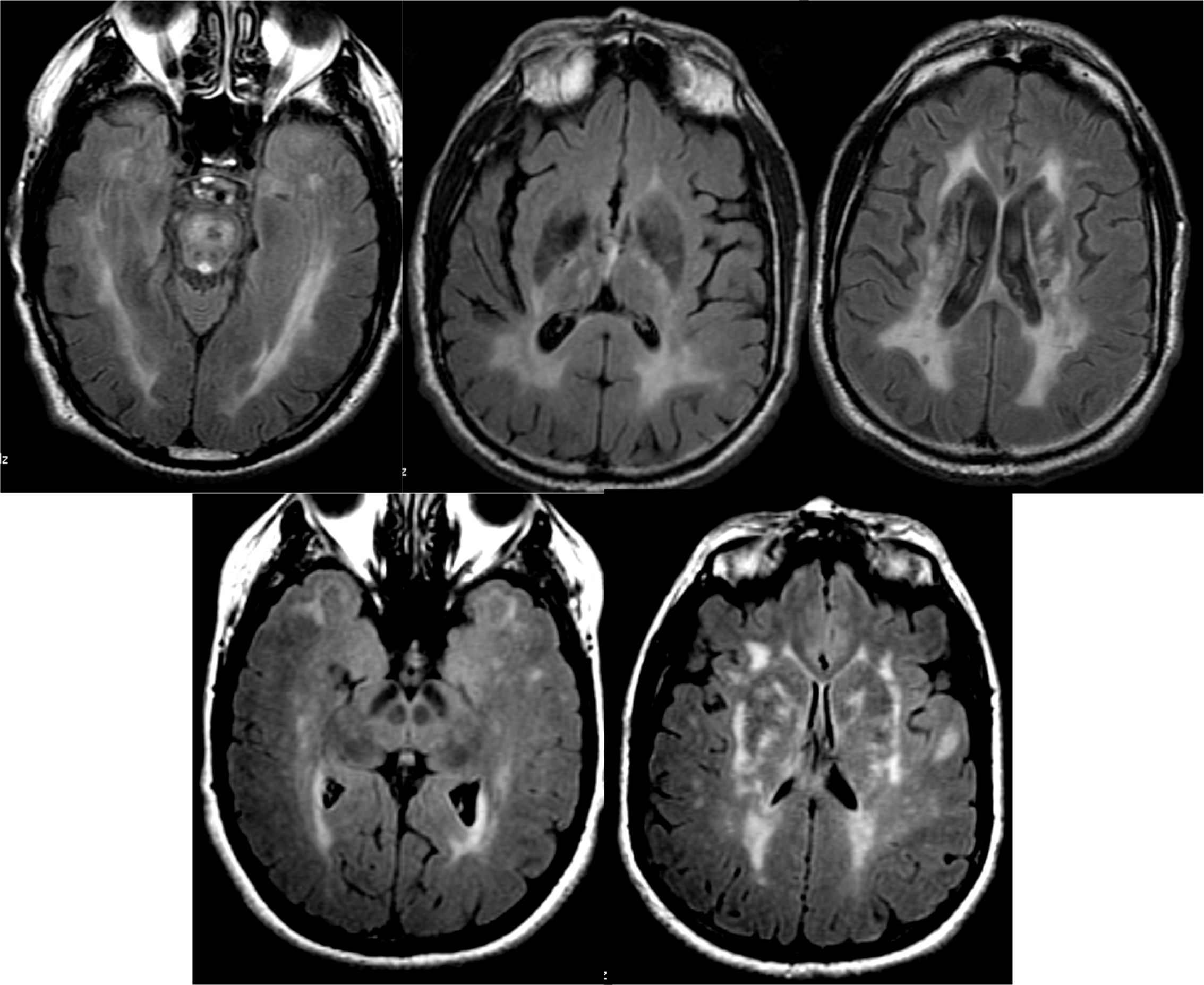
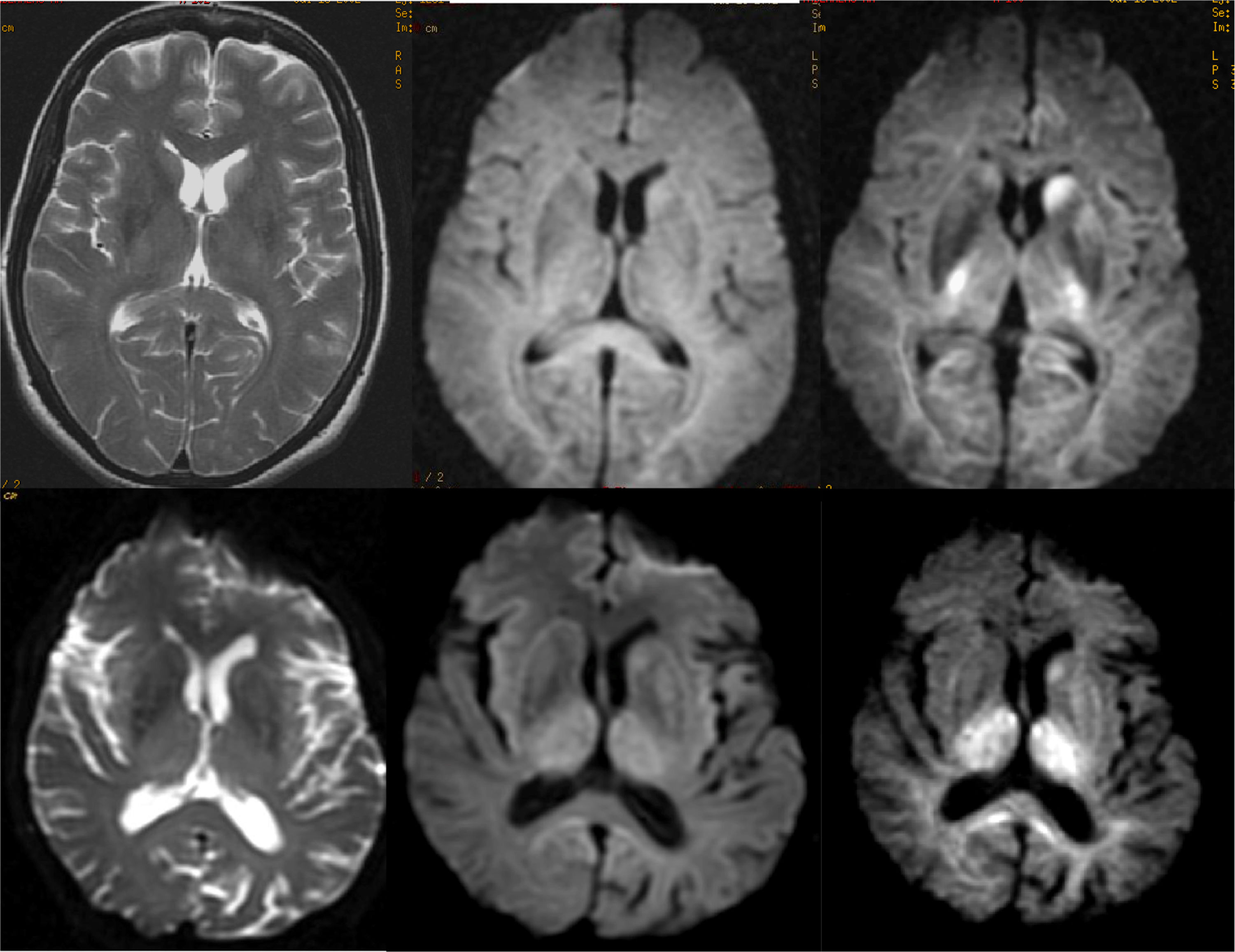
The characteristic image pattern in the sporadic form shows an increase in the T2/FLAIR signal in the striata, which may be associated with a variable degree of cortical signal alteration (in some forms it may be the predominant alteration).44 The diffusion sequences are especially indicated, since they present early restriction of diffusion in these locations, even in early phases in which significant alterations in conventional sequences are not yet observed. In some cases, it is necessary to use diffusion sequences with higher b values (2000–3000) to reveal these alterations (Fig. 15).45
Selection of imaging studiesThe brain imaging test of choice in most patients with cognitive impairment is MRI.46 In cases where it is not available or contraindicated, a brain CT scan will be performed instead. The recommended protocol includes a T1 sequence in 3D acquisition for assessment of atrophy patterns, a FLAIR sequence (also preferably in 3D) for assessment of white matter lesions and a T2* sequence susceptibility-weighted imaging (SWI) for assessment of microhaemorrhages and/or superficial siderosis. In addition, it is recommended to include a diffusion sequence, especially if the possibility of prion disease is suspected (Table 2). In selected cases, it may be necessary to perform studies with contrast or MR-angiography, but they are not routinely indicated.
Magnetic resonance protocol in a patient with dementia.
| 1. 3D-T1 isotropic sequence with voxel of 1.2–1.4mm (5–7min) |
| 2. FLAIR sequence (axial 2D 4–5mm or sagittal isotropic 3D) (3–5min) |
| 3. T2* axial sequence 3–5mm (1min) |
| 4. Axial diffusion (b 1000) (1min) |
| 5. Optional: coronal T2 3mm |
| 6. Optional: ASL perfusion |
The indication of functional studies should be assessed individually, preferably in dementia units.47 They are usually very useful for differential diagnosis in doubtful cases, especially in early stages and atypical forms. It is considered that a normal functional study in a patient with dementia makes the possibility of a neurodegenerative disease very unlikely, but its use in the screening of patients is not indicated. The new amyloid markers are indicated in atypical cases of AD (especially in the differential diagnosis with FTD) and have a very high negative predictive value for AD, although at very elderly ages false positives are common: it is possible to observe positive studies in patients without dementia, although it is believed that these are cases that in all likelihood will subsequently develop the disease. Considering that amyloid deposition occurs long before the development of dementia, many of these elderly patients will not develop dementia. In any case, unconventional studies are usually never indicated in the initial evaluation of a patient with dementia, but are indicated within protocols developed in dementia units, with a multidisciplinary approach.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Álvarez-Linera Prado J, Jiménez-Huete A. Neuroimagen en demencia. Correlación clínico-radiológica. Radiología. 2019;61:66–81.