The West Nile virus (WNV) is an arbovirus than can infect human beings and cause severe neuroinvasive disease. Taking the outbreak that occurred in Spain in 2020 as a reference, this article reviews the clinical and imaging findings for neuroinvasive disease due to WNV. We collected demographic, clinical, laboratory, and imaging (CT and MRI) variables for 30 patients with WNV infection diagnosed at our center. The main clinical findings were fever, headache, and altered levels of consciousness. Neuroimaging studies, especially MRI, are very useful in the diagnosis and follow-up of these patients. The most common imaging findings were foci of increased signal intensity in the thalamus and brainstem in T2-weighted sequences; we illustrate these findings in cases from our hospital.
El virus del Nilo Occidental es un arbovirus que puede infectar al ser humano y causar una enfermedad neuroinvasiva grave. Tomando como referencia el brote que tuvo lugar en España en 2020, se ha realizado una revisión clínica y de neuroimagen de dicha patología. Para ello, se han recogido datos demográficos, clínicos, analíticos y pruebas de imagen (tomografía computarizada y resonancia magnética) de 30 pacientes diagnosticados de infección por virus del Nilo Occidental en nuestro centro. Las principales manifestaciones clínicas fueron fiebre, cefalea y alteración del nivel de conciencia. Los estudios de neuroimagen, especialmente la resonancia, son de gran importancia para el diagnóstico y seguimiento. Los hallazgos más frecuentes fueron focos de aumento de señal en T2 en el tálamo y tronco del encéfalo, que ilustramos en esta revisión con casos de nuestro centro hospitalario.
West Nile virus (WNV) is a re-emerging pathogen. Its natural reservoir is birds and it is transmitted through the bite of a mosquito of the Culex genus which lives in damp areas and is more active during the summer period.1,2 Although the virus is maintained in nature thanks to the bird-mosquito transmission cycle, it can sometimes incidentally infect humans.
Since the first cases of infection in humans in Africa in 1937, WNV has gradually spread to countries around the world and, over this time, the number of outbreaks has increased and they have become more virulent. There has been a marked increase in the incidence of WNV outbreaks in the last 10 years, particularly in southern Europe.3
The vast majority of WNV infections are asymptomatic, but those who develop symptoms (20%–40%) will very often have a flu-like illness (West Nile fever). In a small percentage of cases (<1%), particularly in older or immunosuppressed people, WNV affects the central nervous system (CNS), causing neuroinvasive disease which manifests as meningoencephalitis and/or acute flaccid paralysis.4 Diagnosis is usually through detection of specific antibodies in serum or cerebrospinal fluid (CSF).5 Imaging tests are a very useful tool for the diagnosis, monitoring and prognosis of neurological involvement. CNS infection by WNV produces characteristic magnetic resonance imaging (MRI) findings that radiologists must be able to recognise. In 2020, 75 cases of WNV infection were documented in Spain (40 confirmed and 35 probable), with a total of seven deaths,4 this being the largest outbreak to date at our hospital, the reference centre in the most affected region, and in the country as a whole. The purpose of this article is to present our experience during the recent epidemic outbreak and to review the clinical and neuroimaging characteristics of WNV neuroinvasive disease.
Material and methodsWe collected data from a total of 30 patients diagnosed with WNV infection at our centre from 1 to 31 August 2020. These patients’ diagnoses were confirmed by the detection of specific antibodies in CSF and/or serum, or detection of viral RNA. Probable cases without microbiological confirmation were excluded.
Demographic data (age, gender, and place of residence) were documented, as well as the main clinical manifestations of the infection, the need for admission, and the results of the CSF analyses.
In terms of imaging tests, computed tomography (CT) and MRI were performed based on clinical criteria. All patients had head CT without intravenous contrast initially, and two had follow-up CT. Brain MRI was performed in 18 of the patients, and three of them underwent additional follow-up brain MRI due to worsening of their clinical condition. One of the patients had cervical and dorsal spinal cord MRI as they had symptoms suggestive of spinal cord involvement. A total of 21 studies were carried out on MRI equipment (Ingenia, Philips Medical Systems, Best, the Netherlands), 19 of them on 1.5 T scanners and two studies on 3 T scanners. The standard brain MRI protocol included T1-weighted 3D sequences (ultrafast gradient echo T1), T2-weighted sequences (fast spin echo T2), gradient echo T2*-weighted, 3D T2-weighted FLAIR (Fluid-Attenuated Inversion Recovery), and diffusion weighted imaging (DWI) with apparent diffusion coefficient (ADC) map. 3D T1-weighted sequences were obtained after administration of gadolinium in 16 of the scans. The spinal cord MRI protocol included the acquisition of sagittal T1-weighted (ultrafast spin echo T1), T2-weighted (ultrafast spin echo T2) and short time inversion recovery (STIR) sequences, and axial T2-weighted (ultrafast spin echo T2) sequences.
ResultsWe included a total of 30 patients diagnosed with WNV infection at our centre, 15 male and 15 female (age range: 11–87 years, mean age: 57.8, SD: 22.5 years). Most of the patients came from towns located on the banks of the river Guadalquivir, specifically Coria del Río (16), La Puebla del Río (10) and Seville capital (4).
Signs and symptomsThe most common symptom was fever (96.7%), followed by headache (46.7%) and gastrointestinal symptoms (46.7%). All cases had one or more neurological symptom, the most common being headache (46.7%), decreased level of consciousness (43.3%) and instability and altered gait (36.7%). The rest of the symptoms are shown in Table 1.
Clinical characteristics of West Nile virus neuroinvasive disease.
| Signs and symptoms | No. of patients | Percentage (%) |
|---|---|---|
| Prodromal symptoms | 21 | 70.0 |
| Fever | 6 | 28.6 |
| Flu-like syndrome | 5 | 23.8 |
| Headache | 3 | 14.3 |
| Gastrointestinal symptoms | 7 | 33.3 |
| Fever (>38 °C) | 29 | 96.7 |
| Neurological symptoms | 29 | 100 |
| Headache | 14 | 46.7 |
| Altered level of consciousness | 13 | 43.3 |
| Imbalance, altered gait | 11 | 36.7 |
| Altered speech | 9 | 30.0 |
| Neck stiffness or pain | 8 | 26.7 |
| Focal or generalised weakness | 8 | 26.7 |
| Confusion | 7 | 23.3 |
| Coma | 5 | 16.7 |
| Vertigo | 4 | 13.3 |
| Paraplegia or quadriplegia | 4 | 13.3 |
| Tremor | 3 | 10.0 |
| Diplopia | 3 | 10.0 |
| Nystagmus | 3 | 10.0 |
| Myoclonus | 2 | 6.7 |
| Seizures | 2 | 6.7 |
| Numbness | 1 | 3.3 |
| Hallucinations | 1 | 3.3 |
| Fatigue | 7 | 23.3 |
| Maculopapular exanthema | 4 | 13.3 |
| Gastrointestinal symptoms | 14 | 46.7 |
| Otalgia | 2 | 6.7 |
| Myalgia | 1 | 3.3 |
| Shock | 1 | 3.3 |
| Death | 4 | 13.3 |
Twenty-six patients required admission, eight of whom were seriously ill with progressive alteration in level of consciousness (6), septic shock (1), flaccid paralysis (3) or severe brainstem involvement (2). Four of these patients died (mean age: 77.3; SD: 6.1 years). Three of those who died had one or more cardiovascular risk factors (hypertension, diabetes, hyperlipidaemia and obesity), one also having had a history of ischaemic heart disease with revascularisation. The fourth person who died had a previous medical history of liver cirrhosis due to hepatitis B virus which had required liver transplantation.
Laboratory resultsTwenty-seven of the patients had lumbar puncture with samples taken of cerebrospinal fluid. The majority showed biochemical abnormalities consisting of high CSF protein (>45 mg/dl) (85.2%) and pleocytosis (92.6%), mainly lymphocytic (80.0%) and to a lesser extent involving polymorphonuclear cells (20.0%), with normal glucose (50−80 mg/dl) (85.2%).
NeuroimagingOf all CT scans performed, only two showed abnormalities. In the first case, symmetrical bilateral hypodense areas were identified in the mesial region of the temporal lobes (Fig. 1). In the second case, bilateral hypodense areas were detected in the area of the basal ganglia and thalamus, showing greater involvement of the left side (Fig. 2).
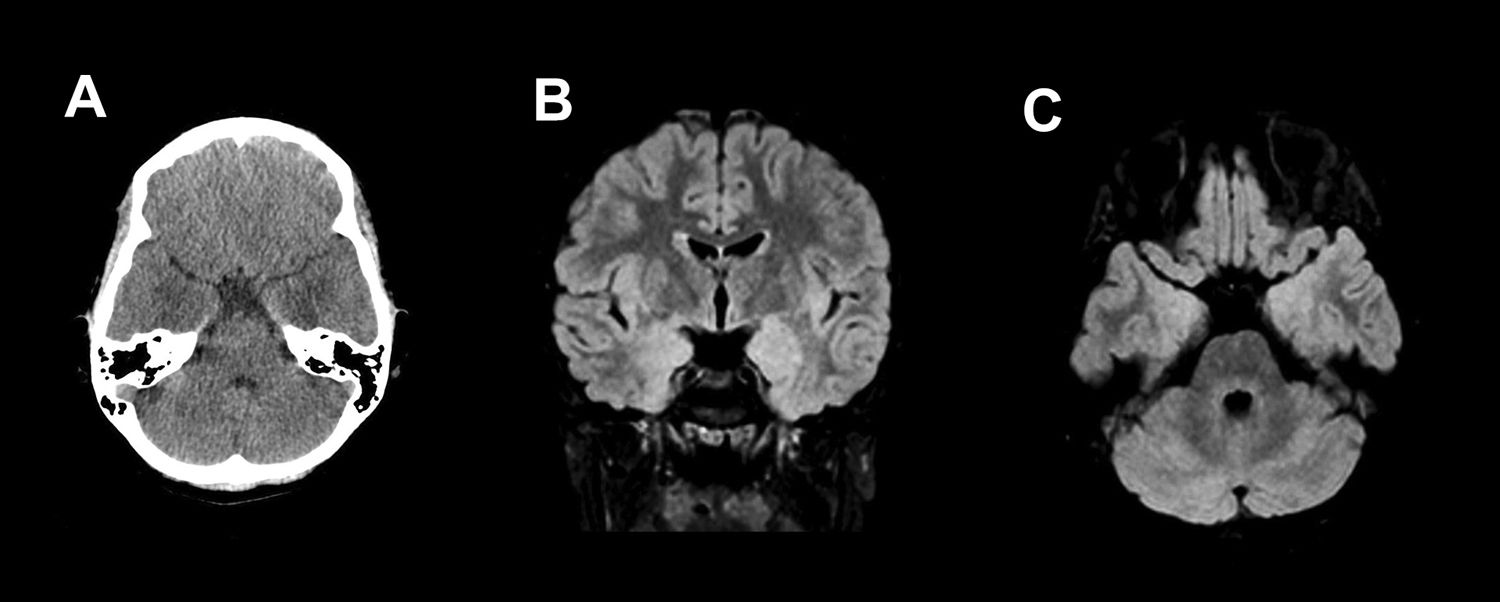
Eleven-year-old male with 2-day history of symptoms of fever, headache, behavioural changes, vertigo, vomiting and altered gait. Computed tomography (A) of the brain showed a bilateral poorly defined hypodense area in the temporal lobes adjacent to the amygdala. These findings were confirmed on an MRI performed three days later, where the oedema appears as hyperintense areas on T2 and FLAIR (B and C). No contrast enhancement was observed.
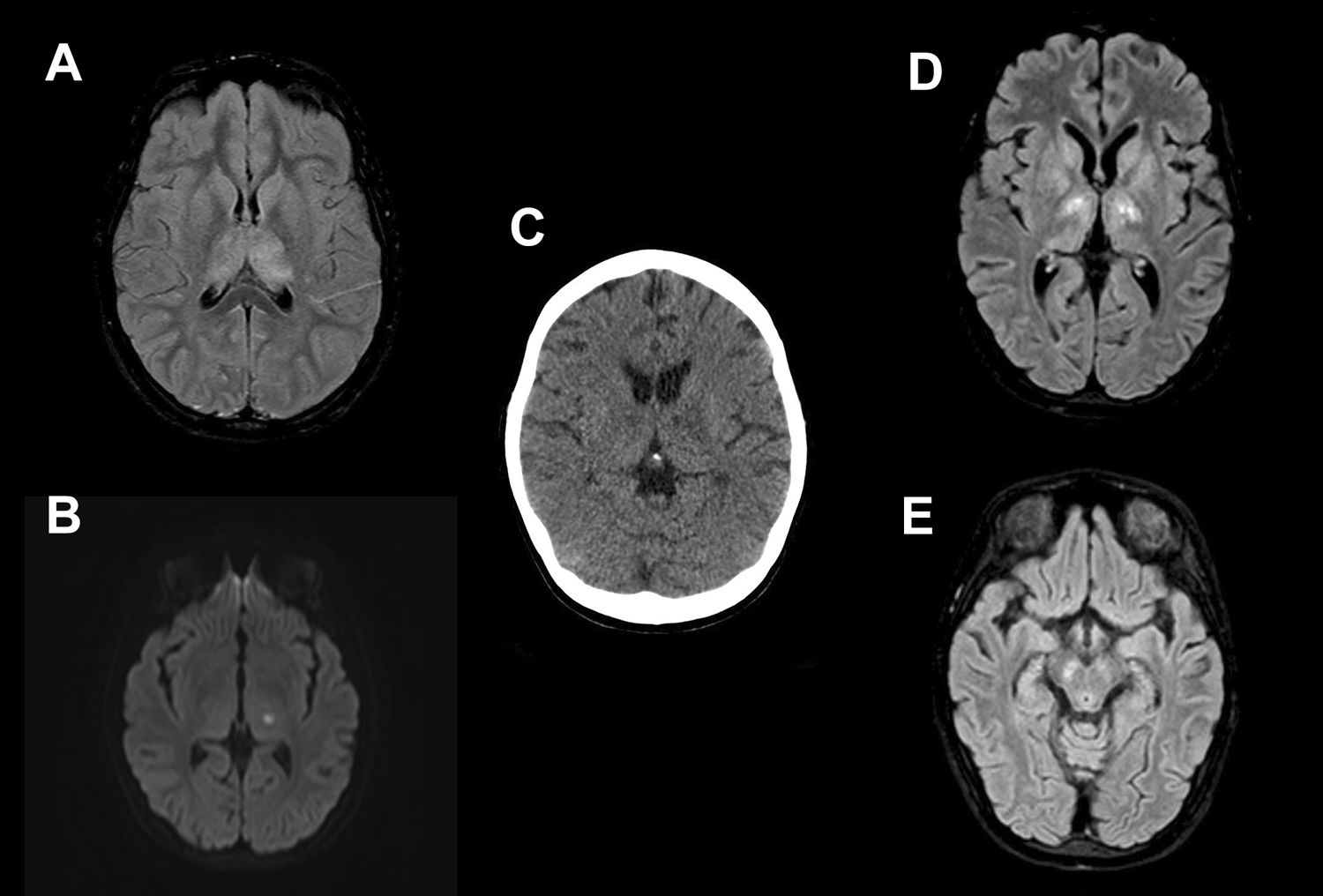
Fourteen-year-old female with fever, nausea, vomiting and vertigo. Petechial rash and meningeal signs on physical examination. She required admission to the ICU and mechanical ventilation due to the severity of her symptoms. Brain magnetic resonance imaging shows a diffuse increase in the T2/FLAIR signal intensity in the thalamus (A) and a focus of restricted diffusion (B) in relation to an acute lacunar infarct in the left thalamus. Brain computed tomography scan performed 20 days later shows bilateral hypodensity in the thalamus and basal ganglia. Four days later, a follow-up MRI showed oedema on T2/FLAIR (D) in the thalamus, globus pallidus, and caudate nuclei bilaterally. Involvement of the periaqueductal region and the mesencephalic substantia nigra (E) was also identified.
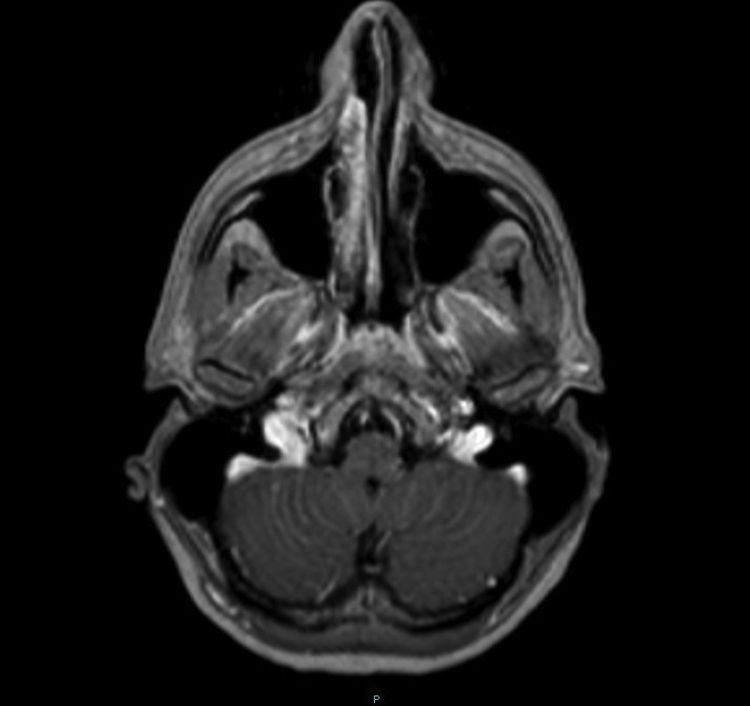
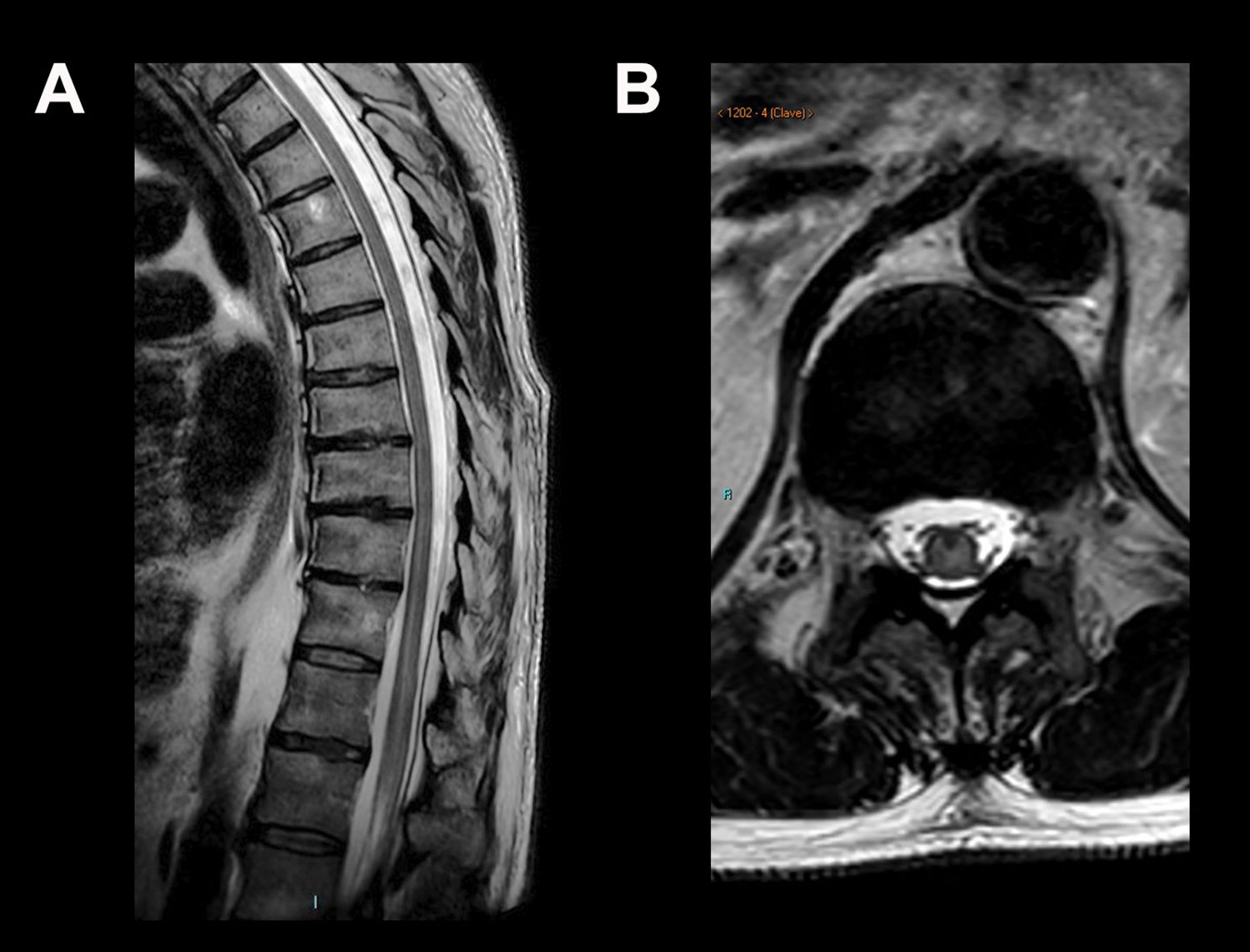
In 10 patients the MRI images showed abnormalities. In eight (44.4%) of the brain MRI scans, one or more findings were identified compatible with oedema due to meningoencephalitis, consisting of an increase in signal intensity in T2-weighted/FLAIR sequences located in the brainstem (38.9%), mainly bilaterally, affecting the mesencephalic substantia nigra; thalamus (33.3%); basal ganglia (5.6%); cerebellum (5.6%); hemispheric white matter (5.6%) (Figs. 2 and 3); and temporal lobes (5.6%) (Fig. 1). One of the scans showed infratentorial leptomeningeal enhancement after contrast administration (Fig. 4) and another showed a focus of restricted diffusion in the left thalamus. Spinal MRI showed areas of increased central intramedullary signal on T2-weighted/STIR sequences from T8 to the conus medullaris in relation to myelitis (Fig. 5).
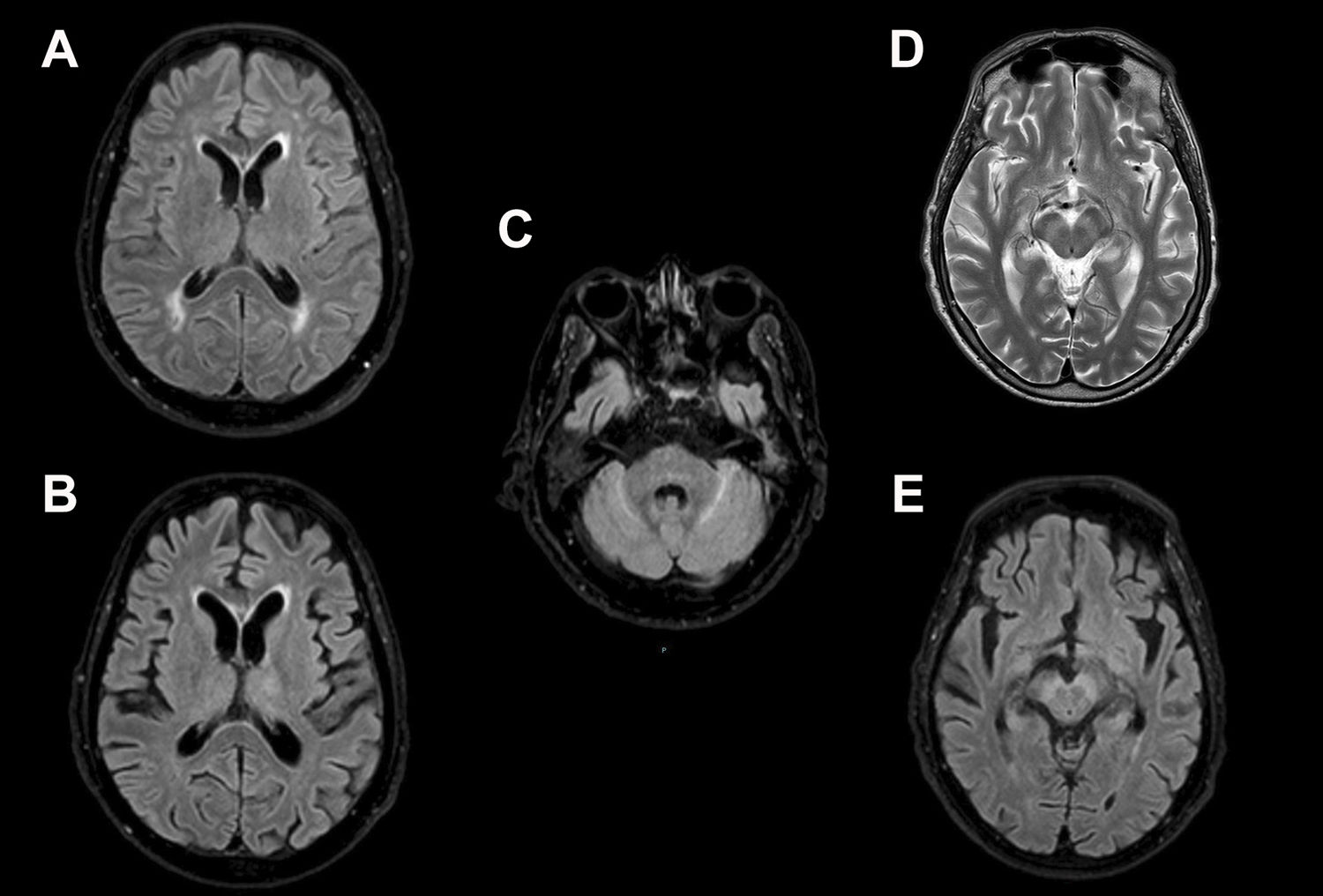
Twenty-year-old male, hospitalised for fever and progressive alteration of level of consciousness. Initial MRI (A) showed areas of increased signal intensity on T2/FLAIR in the periventricular and subcortical white matter which were attributed to chronic ischaemic microangiopathy. In a follow-up magnetic resonance imaging performed 13 days later (B and C), these findings had disappeared, but there were new foci of oedema in the left thalmus and cerebellum. Involvement of the mesencephalic substantia nigra was also seen on T2-weighted (D) and T2/FLAIR (E) sequences. This patient’s clinical condition was extremely serious and unfortunately he died.
Fifty-seven-year-old male with acute flaccid paralysis of the left lower limb. Sagittal T2-weighted (A) and axial T2-weighted magnetic resonance images are shown at the level of T12-L1 (B), where an increase in central spinal cord signal can be seen extending from T8 to the conus medullaris.
Details of the findings in the different imaging tests are provided in Tables 2 and 3.
Radiological findings on CT and MRI images in 30 patients with West Nile virus neuroinvasive disease.
| No. of cases | Percentage (%) | |
|---|---|---|
| Brain CT | 30 (+2 follow-up) | 100 |
| No changes | 28 | 93.3 |
| Basal ganglia hypodensity | 1 | 3.3 |
| Bilateral temporal lobe hypodensity | 1 | 3.3 |
| MRI | 18 (+3 follow-up) | 60.0 |
| T1-weighted contrast-enhanced | 16 | 88.9 |
| No changes | 8 | 44.4 |
| Changes | 10 | 55.6 |
| Brain stem | 7 | 38.9 |
| Thalamus | 6 | 33.3 |
| Basal ganglia | 1 | 5.6 |
| Cerebellum | 1 | 5.6 |
| White matter | 1 | 5.6 |
| Temporal lobes | 1 | 5.6 |
| Leptomeningeal enhancement | 1 | 5.6 |
| Myelitis | 1 | 5.6 |
| Ischaemic complications | 2 | 11.1 |
MRI: magnetic resonance imaging; CT: computed tomography.
Description of the findings in the CT and MRI studies of the 10 patients who showed abnormalities in the imaging tests.
| CT | MRI | |
|---|---|---|
| Case 1 | No findings | Symmetrical T2/FLAIR hyperintensity in both thalami and in the central region of the midbrain and the pons |
| Case 2 | No findings | Confluent hyperintense foci on FLAIR from the periaqueductal region of the pons to the central portion of the midbrain |
| Case 3 | No findings | Initial MRI: bilateral and relatively symmetrical hyperintense foci on T2/FLAIR, located in the frontoparietal subcortical white matter and in the periatrial regions |
| Follow-up MRI: • Significant decrease in white matter lesions visualised on initial MRI • Asymmetrical FLAIR hyperintensity in both thalami in the cerebral peduncles of the midbrain, central pontine region and cerebellar folia | ||
| Case 4 | Initial CT: no findings | Initial MRI: • Asymmetrical T2/FLAIR hyperintensity in both thalami • Focus of restricted diffusion in the left thalamus |
| Follow-up CT: bilateral, symmetrical gangliocapsular and thalamic hypodensity | Follow-up MRI: • Heterogeneous increase in signal intensity in both gradients of T2 of the thalamus due to cavitation/necrosis • Bilateral hyperintensity of the basal ganglia, mesencephalic substantia nigra and periaqueductal region • Supra and infratentorial diffuse corticosubcortical volume loss | |
| Case 5 | No findings | FLAIR hyperintensity of the medial region of the thalamus (pulvinar) and periaqueductal white matter |
| Case 6 | No findings | Right marginal focal and bilateral thalamic hyperintensity of the midbrain-pons transition |
| Case 7 | No findings | Asymmetrical T2/FLAIR hyperintensity in both thalami and pons |
| Left frontal subacute ischaemic lesion with petechial haemorrhagic transformation | ||
| Case 8 | Symmetrical, bilateral mesial temporal hypodensity | Symmetrical hyperintensity in T2/FLAIR of the medial cortex of both temporal lobes |
| Case 9 | No findings | Bilateral diffuse enhancement of the infratentorial leptomeningeal surface |
WNV is an arbovirus belonging to the Flavivirus genus, whose natural animal reservoir and main host are birds. It is transmitted through the bite of an infected vector, generally a mosquito of the Culex genus, which lives in damp areas near stagnant waters or rivers and which exhibits greater replication and activity in warm temperatures; hence the summer period from July until the end of September is the highest risk for the transmission of the disease. Isolated cases have been reported of other rare forms of transmission (through transfusion of blood products or solid organ transplant or vertical).6 Mammals such as humans and horses are incidental final hosts, and the viral load in such hosts is insufficient to contribute to the biological cycle.1,2 WNV infection in humans rarely causes disease, but when there are certain individual predisposing factors (advanced age, immunosuppression) and environmental factors (warmer temperatures, increased vector density, greater urbanisation of rural areas) it can cause outbreaks which have an impact on human and animal health.4,7
First described in Uganda in 1937, over the years WNV has gradually spread throughout the world, with an increasingly marked rise in the number of outbreaks, mainly in Europe. From the 1990s a significant increase was noted in the number of outbreaks and in their virulence, especially in the Americas. Since the beginning of the new millennium, and particularly in the last 10 years, an upsurge in disease activity has been documented in areas where it had already been reported and it has started to affect other areas where it had never been found before.8 In 2018, probably as a result of the transmission season being earlier due to warm spring temperatures, the number of reported infections in Europe was significantly higher than the total number of cases in the previous four years (1605 cases in 2018 compared to 654 in the period 2014–2017).3 In recent years, countries in Southern Europe have been affected and, to a lesser extent, Central Europe also.3
In Spain, the first case of WNV neuroinvasive disease was identified in 2004 in Barcelona, but no cases of meningoencephalitis in humans were recorded again until 2010 in the province of Cádiz.9 The last outbreak in humans reported in Spanish territory was in 2016 with three confirmed cases.4 In 2020, a total of 75 cases were reported, the majority in Seville province,5,7 with this being the largest outbreak ever in this country.3 It should be pointed out that most of the subjects lived in two towns by the banks of the river Guadalquivir, in an area of marshes and rice fields, an ideal environment for the proliferation of the mosquito vector (Fig. 3).
Signs and symptomsAfter the bite of the mosquito vector, the WNV replicates at the site of inoculation, from where it goes to the blood and lymphatic circulation. Approximately 80% of patients will be asymptomatic. However, 20% will develop symptoms10 after an incubation period of approximately 3–6 days, consisting of an acute flu-like illness known as West Nile virus fever; symptoms include fever, fatigue, malaise, headache, muscle pain and weakness, sometimes accompanied by a skin rash, and typically resolve within a week. The appearance of a maculopapular rash on the trunk and the development of lymphadenopathy is common.6
Neuroinvasive disease only occurs in a small percentage of cases (<1%),10 the main risk factor being advanced age and, to a lesser extent, states of immunosuppression.11 The virus reaches the CNS, crosses the blood-brain barrier and directly infects neurons, particularly those of the deep grey matter, brainstem and spinal cord.8,12
Neuroinvasive involvement, sometimes preceded by a prodromal phase of nonspecific symptoms (arthromyalgia, gastrointestinal or respiratory discomfort), can present as aseptic meningitis, acute flaccid paralysis similar to poliomyelitis, or more frequently encephalitis. This may be focal or diffuse and is characterised by the initial onset of fever, headache, vomiting and abdominal pain, and subsequent decreased level of consciousness, which may be accompanied by Parkinsonian syndrome, myoclonus and tremor, symptoms related to the affinity of the virus for the basal ganglia. Seizures are less common than in herpetic encephalitis.6,13
The clinical picture presented by the patients in our series was consistent with that described in the literature, with initial symptoms of fever, headache and gastrointestinal discomfort, as well as subsequent disorientation and progressive decrease in the level of consciousness, which may reflect involvement of the thalamus. About a third of the patients had instability, vertigo, tremor or altered gait, which clinically point towards a localised disturbance in the cerebellum or brainstem. In general, these clinical findings correlate with the abnormalities seen on neuroimaging.
A quarter of the patients had neck stiffness, which usually means meningeal irritation suggestive of meningitis or meningoencephalitis.
In the spinal cord, invasion of the anterior horn neurons causes a rapidly progressive flaccid paralysis with loss of deep tendon reflexes, which may be preceded by pain in the limb6. Four of our patients showed symptoms suggestive of spinal cord involvement, but only in one of the cases was this corroborated by abnormal imaging test findings.
LaboratoryBlood, CSF and urine samples need to be taken to be certain about the diagnosis.10 The CSF is usually clear and in the biochemical analysis it shows normal glucose values, moderate elevation of proteins and pleocytosis of lymphocyte predominance, although occasionally up to 40% have a polymorphonuclear predominance in the initial phases of the disease.5 The presence of viral RNA or specific antigens in CSF has a specificity of 100%, but is relatively insensitive. For this reason, the detection of specific IgM in serum, CSF or both is the most useful diagnostic technique in WNV infection.5,10 IgM antibodies can be detected 3–8 days after the onset of the disease, and persist for up to 90 days.6 Most of our patients were diagnosed by detection of IgM in serum, CSF or both. In isolated cases only, viral RNA was detected in urine by PCR.
Imaging testsThe finding of abnormalities in imaging tests varies according to the technique used; MRI is the most sensitive imaging test to detect parenchymal alterations, mainly in T2-weighted and diffusion sequences. Despite its low sensitivity, CT is usually the first imaging technique used due to its speed and high availability, helping to rule out other aetiologies. The time since onset of the infection has to be taken into account, as imaging tests tend to be normal in the first hours.6,14
Consistent with the medical literature, no abnormalities were found in the brain CT images in most of our patients. Only two patients had abnormal images. In the first, the initial CT showed hypodense areas in the mesial regions of both temporal lobes. This finding is not typical of WNV infection, although it has been described in the literature as a rare finding.15 In the second patient, a follow-up CT scan revealed the development of bilateral gangliocapsular and thalamic hypodensities compared to the initial scan, which was normal. Perhaps the cerebral abnormalities take longer to evolve and the symptoms need to be more severe before the oedema becomes apparent in the CT images.
In contrast, MRI images are more sensitive for the detection of parenchymal abnormalities.15,16 Early signs of the disease, such as foci of restricted diffusion in the thalamus and basal ganglia bilaterally, with dissemination towards the white matter, have been reported in 50% of patients in DWI sequences.17–19 Diffusion abnormalities are the first to return to normal on follow-up MRI.17 It is thought that the restriction foci are the translation of a structural inflammatory infiltration, and not ischaemic foci, but there are no conclusive results in the literature.6 Most of our patients did not have abnormalities in DWI sequences. This discrepancy with respect to reports in the literature may be due to the fact that MRI scans were performed in subacute phases of the disease. Only in one case was a restriction focus identified in DWI in the initial MRI, in the left thalamus, corresponding to a focal infarction which, in the follow-up MRI, was already in the cavitation phase. The finding of this sign does not seem to be directly related to the invasion of the CNS by WNV and could be the consequence of a systemic prothrombotic state generated by the viral infection, as has already been described in other viruses.20
In more advanced stages of the infection, the most characteristic finding is the presence of areas of increased signal intensity on T2/FLAIR sequences bilaterally in the thalamus, basal ganglia (mainly the caudate and lenticular nuclei), brainstem and cerebellum, sometimes with some mass effect on adjacent structures.21,22 These abnormalities are usually bilateral and relatively symmetrical.6 In certain cases, cortical grey matter involvement or signs of bleeding can be seen on T2*-weighted sequences. The presence of contrast enhancement is variable.22 It should be noted that these abnormalities tend to become increasingly apparent as the disease progresses, so late studies will show abnormalities not visible during the acute phase.10 Approximately half our patients had these typical findings of viral encephalitis, and the most affected structures were the thalamus and the brainstem. Selective involvement of the substantia nigra of the midbrain was found in two cases, and this was related to the development of Parkinsonism symptoms.10,21 Unfortunately, both patients had a profoundly decreased level of consciousness, which made it impossible to correlate the findings clinically.
As a less common finding, there have been reports of signal hyperintensity in T2/FLAIR in the periventricular white matter, which can mimic microvascular involvement due to chronic ischaemia10. At least one of our patients had this finding in the initial MRI, the viral aetiology being corroborated after it had disappeared by the time of the follow-up MRI 13 days later. Other patients in our series had these periventricular hyperintensities but, due to their advanced age, they were attributed to chronic ischaemic small-vessel disease. As no follow-up MRI were performed, it was not possible to verify the stability or disappearance of these signs to confirm the diagnosis.
Lastly, in one of our patients, the only MRI abnormality was an increase in bilateral mesial temporal T2/FLAIR signal intensity. This finding has also been described in neuroinvasive WNV disease due to inflammatory changes, mimicking herpetic encephalitis, although the necrotising component of herpetic encephalitis is normally absent.6,10 Although involvement of the temporal lobe is usually more frequently due to herpes simplex virus (HSV), it is important to be aware of this possible abnormality in relation to WNV and, if there is an adequate epidemiological context, consider it in our differential diagnosis.
It should be noted that all the lesions were more evident in FLAIR than in T2-weighted sequences, mainly in cases in which the focus of oedema was small or dim. This may due to the fact that volumetric and thin-section FLAIR sequences are obtained at our centre, providing high-resolution images which are more sensitive to faint parenchymal abnormalities.
There are reports in the literature that in up to a third of cases a slight leptomeningeal or periventricular enhancement can be seen as a sign of meningeal irritation.21 Only one of our patients showed a slight pial enhancement in the posterior fossa, which may have been related to inflammation of the meninges, as clinically he had neck pain and neck stiffness. However, we cannot rule out that the increased uptake was associated with the lumbar puncture he had recently had, although the usual enhancement after this type of procedure is of the pachymeningeal type, leptomeningeal enhancement being very uncommon.23
In a small percentage of patients, WNV affects the spinal cord by invading the neurons of the anterior horns and causing an asymmetrical acute flaccid paralysis.24 Although imaging tests may be normal, on occasion we find signs of myelitis consisting of a patchy increase in the signal intensity in T2-weighted sequences in the area of the anterior horns, affecting more or less extensive segments of the spinal cord. Enhancement of the anterior horns with extension towards the anterior nerve roots can also be seen in patients with polyradiculitis.6,24 There have been reports of isolated cases of diffuse enhancement of the conus medullaris and the cauda equina, which would signify arachnoiditis.21,25 Although four of our patients had symptoms of spinal cord involvement, only one had an MRI of the neuraxis. In that scan, there were findings consistent with myelitis of intramedullary hyperintensity extending from the spinal level T8 to the conus medullaris. No sequences were acquired after gadolinium administration, so no spinal cord or meningeal enhancement could be confirmed.
The imaging findings of WNV neuroinvasive disease are not pathognomonic and overlap with other CNS diseases.
Viruses usually affect the deep grey matter of the brain, sometimes at specific locations depending on the aetiology. Therefore, in the appropriate clinical context, certain viruses such as Japanese encephalitis virus, St. Louis virus, Epstein–Barr virus, influenza virus or dengue,22 which have a special predilection for the thalamus and the basal ganglia, should be included in the differential diagnosis.21 Other causes of abnormalities in the deep grey matter of the brain need to be taken into account, such as neoplasms, vascular abnormalities (arteriovenous fistulas, deep cerebral vein thrombosis, artery of Percheron infarction), acute disseminated encephalitis, acute necrotising encephalopathy, hypoxic-ischaemic encephalopathy, Wernicke’s encephalopathy and toluene poisoning.22 The imaging findings of these diseases tend to be superimposable, and the clinical-analytical context is important when considering the differential diagnosis.
In the case of temporal lobe involvement, the main differential diagnosis should be HSV encephalitis, which is far more common and tends to appear more aggressive.6
The involvement of the anterior horns of the medulla can be a consequence of other infections, mainly poliovirus and, less frequently, enterovirus, dengue virus, rabies, CMV, Rickettsia or Lyme disease. This finding can also be of non-infectious origin, as it occurs in acute disseminated encephalomyelitis, multiple sclerosis, spinal cord ischaemia and idiopathic transverse myelitis. The presence of arachnoid enhancement in the cauda equina should raise suspicions of cancer spread, sarcoidosis or Guillain-Barré syndrome.24
PrognosisAlthough WNV neuroinvasive disease is the least common form of presentation of this infection, it is associated with high morbidity and mortality rates. The fatality rate in Europe is around 11%–12% according to data collected by the European Centre for Disease Prevention and Control (ECDC) in the period 2016–2019,26 although this may be higher in older patients, with rates of up to 35% reported in older patients in the United States.27
The main risk factor for the development of a severe neuroinvasive condition is advanced age.27–29 Ageing is associated with a progressive decrease in immune activity, which may contribute to increased permeability of the blood–brain barrier and favour neuroinvasion by the virus.7 Other possible individual predisposing factors have been described, such as hypertension, cardiovascular disease, chronic kidney disease and diabetes.7,29 States of immunosuppression, as in transplant recipients or people with blood cancers, can also contribute to a more severe disease course than in immunocompetent patients.30 Lastly, some genetic susceptibility polymorphisms have been described, mostly related to the response pathways of the immune system.7
In our case series, 73.3% of the patients made a complete clinical recovery; seven of them reported persistent mild symptoms (asthenia and/or headache), but these resolved after a few weeks. Four patients (13.3%) had permanent sequelae: tetraparesis (two cases); paraparesis of the lower limbs (one case); and persistent asthenia (one case). Another four patients (13.3%) died.
The patients whose conditions were severe, requiring prolonged hospital stays or in intensive care units, had an older mean age (65). The mean age was highest for the patients who died (77.3; SD: 6.1), in contrast to the mean age of the patients who recovered (56; SD: 25.2). Only one of the patients who made poor progress was a 14-year-old girl who presented with flaccid tetraparesis.
In terms of the imaging findings, a large proportion of those whose conditions were very poor had a greater number of abnormalities in their MRI scans; three of the four patients who died (the fourth did not have an MRI due to his rapidly deteriorating clinical condition) and two of the four with permanent sequelae, compared to five of the 22 who recovered. The correlation between the imaging findings and the disease prognosis is not understood, but it has been suggested that patients with no abnormalities in their MRI images or with findings detected only in DWI diffusion sequences have a better prognosis than those with abnormalities in T2-weighted or FLAIR sequences.27
LimitationsIt is important to point out some of the limitations of this study. Although the cases in the recent outbreak in our region surpassed the numbers in previous outbreaks, the total number of patients included is relatively low and only some of them had MRI scans. In some cases, MRI could not be performed due to the extremely poor clinical condition of the patient. There was no established imaging protocol, so the initial and follow-up studies were performed according to the severity of symptoms and clinical criteria. Despite these limitations, we believe that we have been able to adequately describe the clinical and imaging findings of a cohort of patients from the largest WNV outbreak to date in Spain.
ConclusionsWNV infection is a re-emerging disease which occurs in areas near rivers or wetlands during the hot times of the year and is experiencing a resurgence in the number and virulence of outbreaks.
Although the virus rarely invades the CNS, it can produce severe meningoencephalitis or myelitis associated with high mortality rates and frequently with permanent sequelae. The main clinical manifestations in our cohort were fever, headache and altered level of consciousness. Although the diagnosis is clinical and requires the detection of specific IgM antibodies, neuroimaging, especially MRI, is very useful for the diagnosis and follow-up of these patients, with certain typical patterns of involvement that should alert the radiologist. In specific, the most common findings are signal alterations in the thalamus and in the brainstem in FLAIR and DWI sequences. Our case series reproduces these typical radiological findings, which are described in the medical literature on other epidemic outbreaks, in addition to some less common neurological abnormalities.
Authorship- 1.
Responsible for the integrity of the study:
- 2.
Study conception: CMR, MMM.
- 3.
Study design: CMR, MMM.
- 4.
Data collection: CMR, MMM.
- 5.
Data analysis and interpretation: CMR, MMM.
- 6.
Statistical processing: N/A.
- 7.
Literature search: N/A.
- 8.
Drafting of the article: CMR, MMM.
- 9.
Critical review of the manuscript with intellectually relevant contributions: PPG, PCD.
- 10.
Approval of the final version: CMR, MMM, PPG, PCD.
The authors declare that they have no conflicts of interest.