Aortitis is a pathological term designating inflammation of the aortic wall, regardless of its cause. The clinical presentation of aortitis is nonspecific and variable. Symptoms include abdominal pain, fever, and weight loss; acute phase reactants may also be elevated. Aortitis can be caused by a wide spectrum of entities, including from infectious processes to autoimmune diseases (Takayasu arteritis and giant cell arteritis are among the most common of these causing aortitis), and the prognosis and treatment of these entities vary widely. Various imaging techniques can be used to evaluate the lumen and wall of the aorta (such as multidetector computed tomography, magnetic resonance imaging, angiography, or PET-CT). This review focuses on the most common diseases that cause aortitis and on the clinical and radiological findings that are most useful for diagnosing and treating this condition appropriately.
Aortitis es un término patológico que designa la inflamación de la pared aórtica, independientemente de su causa. Su presentación clínica es inespecífica y variable, con síntomas como dolor abdominal, fiebre y pérdida de peso. También pueden estar elevados los reactantes de fase aguda. Un amplio espectro de entidades puede ocasionar aortitis, desde procesos infecciosos hasta enfermedades autoinmunes (de las que las más frecuentes son la arteritis de Takayasu y la arteritis de células gigantes), cuyo pronóstico y tratamiento son muy variables. Son varias las técnicas de imagen que permiten evaluar tanto la luz como la pared vascular (como la tomografía computarizada multidetector, la resonancia magnética, la angiografía o la PET-TC). Esta revisión se centra en las enfermedades más frecuentes que provocan aortitis y en los hallazgos clínicos y radiológicos más relevantes que ayudan a diagnosticar y tratar adecuadamente esta entidad.
Aortitis is a general term encompassing many infectious and non-infectious diseases that have in common the fact that they cause inflammation of aortic wall. Presentation of aortitis is very variable, with unspecific symptoms and signs such as abdominal or back pain, fever, vascular insufficiency, valvular aortic insufficiency or acute aortic syndrome.1 The disease causing aortitis may appear with more specific symptoms that make it possible for diagnosis to be reached.
The objective of this article is the physiopathological, epidemiological and radiological revision of some of the frequent causes of aortitis in our milieu.
Image techniquesThe physician does not usually suspect aortitis due to the unspecificity of its symptoms. It is here that the radiologist's competence is key to reach diagnosis and that is why image techniques are a great help. They allow us to study the distribution of aortic affection and the characteristics that may distinguish the infectious from the non-infectious causes and they may also be useful for monitoring the disease's evolution and activity.2 The interventionist techniques play an important role in the treatment of some of these diseases. The image modalities must evaluate both the aortic lumen and wall.
Multidetector computerized tomography (CT) (with intravenous iodized contrast) is usually, due to its availability, the first image test requested. It allows us to rule out an acute aortic affection and to assess stenosing lesions of the aorta and the large arteries, mural thickening (Fig. 1a), aneurysms and thrombosis, thanks to its excellent spatial resolution and the possibility of making multiplane reconstructions in 3D. It is more useful than other techniques to observe mural calcifications, which are frequent in aortitis of long evolution. Its disadvantage is ionizing radiation and iodized contrast, which may pose a problem for patients with allergy or kidney failure.
Takayasu's arteritis in a 42-year-old woman with abdominal pain. (a) Axial CT image showing concentric thickening of abdominal aortic wall with contrast mural uptake (arrow head). (b) MR sagittal image (T1 sequence with fat saturation in black blood, with and without contrast). Gadolinium uptake of aortic wall (arrow heads), indicating active inflammation.
Magnetic resonance (MR), which does not use ionizing radiations, is the test recommended for patients who need repeated or follow-up examinations. Like CT, it is useful in evaluating stenosis, mural thickening or aneurysms (Fig. 1b). In addition, it is very useful in identifying wall edema, which has been related with the disease's activity.1,2 Angiographic images (angio-MR), obtained with different sequences, with or without gadolinium, make it possible to assess stenosis and vascular occlusion areas. Contrariwise, MR is less available, it cannot be used in patients with certain types of pacemakers, and gadolinium is contraindicated when glomerular filtration is less than 30ml/min/m2.
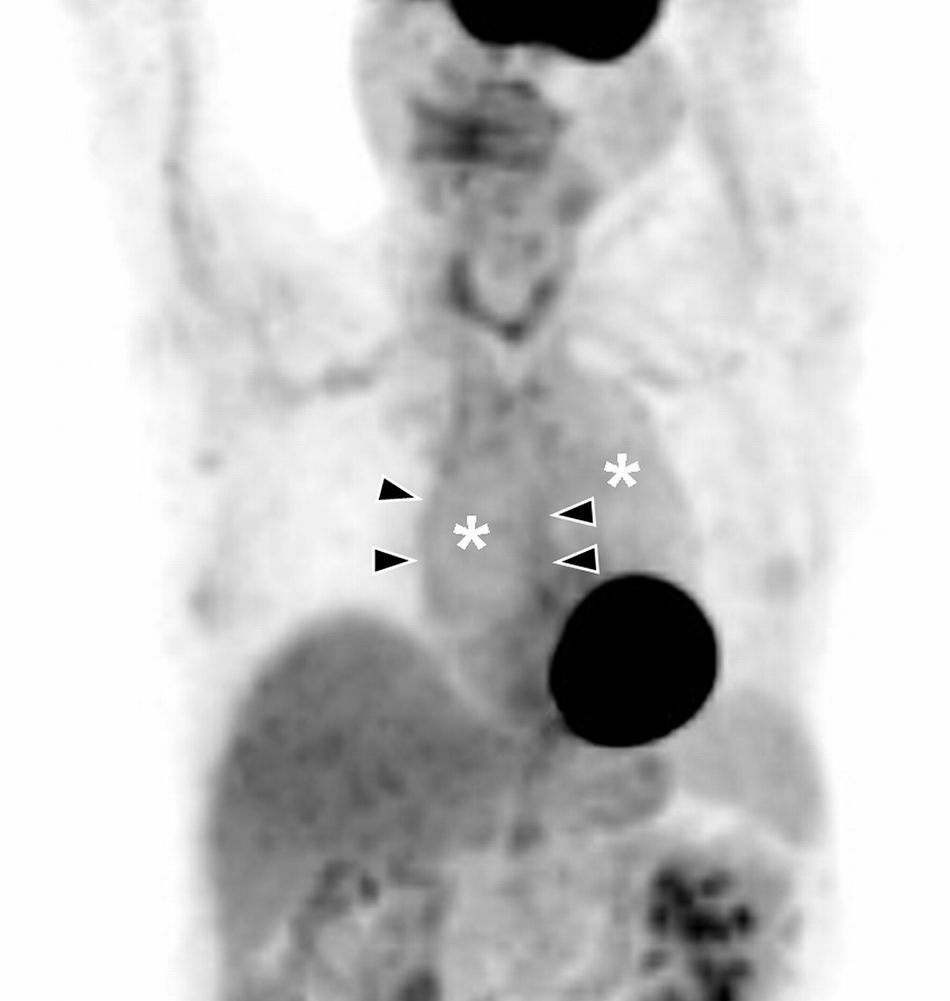
Positron emission tomography (PET) is being used more and more frequently to diagnose aortitis, because it detects increase of metabolism on aortic wall represented by the accumulation of fluorodeoxyglucose in acute phases (Fig. 2). It is also useful to follow the disease up and control response to treatment.3 Its greatest defect is its low spatial resolution; therefore it is usually combined with CT to gain anatomical precision, with which 77–92% sensitivity and 89–100% specificity are attained.3
Aortitis: Thoracic PET coronal image. Diffuse hyperactivity in ascending and descending aorta (asterisks) with more focal images delimiting aortic wall in the ascending segment (arrow heads). Image by courtesy of Doctor Albert Flotats Giralt. Nuclear Medicine Service. Hospital de la Santa Creu i Sant Pau in Barcelona.
Angiography, traditionally considered the reference pattern to study aortic lumen, has been replaced by CT and MR both to diagnose and follow up evolution. The radiation dose is high and it does not contribute information about the vascular wall. Its use is reserved for interventionist therapeutic procedures.
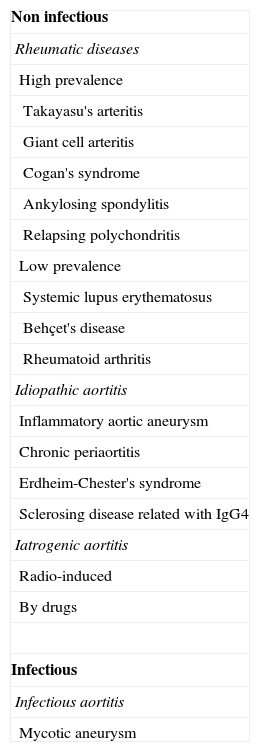
ClassificationFor this revision, the diseases that co-occur with aortitis have been grouped into infectious and non-infectious (Table 1). The aortic affectation pattern and the radiological findings help us differentiate between these two causes.2
Classification of aortitis.
| Non infectious |
| Rheumatic diseases |
| High prevalence |
| Takayasu's arteritis |
| Giant cell arteritis |
| Cogan's syndrome |
| Ankylosing spondylitis |
| Relapsing polychondritis |
| Low prevalence |
| Systemic lupus erythematosus |
| Behçet's disease |
| Rheumatoid arthritis |
| Idiopathic aortitis |
| Inflammatory aortic aneurysm |
| Chronic periaortitis |
| Erdheim-Chester's syndrome |
| Sclerosing disease related with IgG4 |
| Iatrogenic aortitis |
| Radio-induced |
| By drugs |
| Infectious |
| Infectious aortitis |
| Mycotic aneurysm |
Most aortitis are not infectious. Among them the rheumatic diseases that may affect the aorta are included. The commonest causes are large vessel vasculitis: Takayasu's arteritis and giant cell arteritis.2 Within this group idiopathic aortitis are also found: inflammatory aortic aneurysms, chronic periaortitis and other causes of retroperitoneal fibrosis. Finally, we must not forget aortic iatrogenic pathologies, whether due to drugs or secondary to radiotherapy.
Rheumatic diseasesTakayasu's arteritisTakayasu's arteritis is an idiopathic vasculitis that may affect the thoracoabdominal aorta and its branches, as well as the pulmonary arteries.4,5
It more frequently affects young patients, mostly women (75–90%)1 and typically those of Asian origin.
It is a large vessel arteritis, histologically characterized by granulomatous inflammation of arterial wall, with marked infiltration and proliferation of the intima in the initial stages, and fibrosis of the medium and adventitia in the tardive stages. The result is stenosis, occlusion and formation of aneurysms.1,2,5
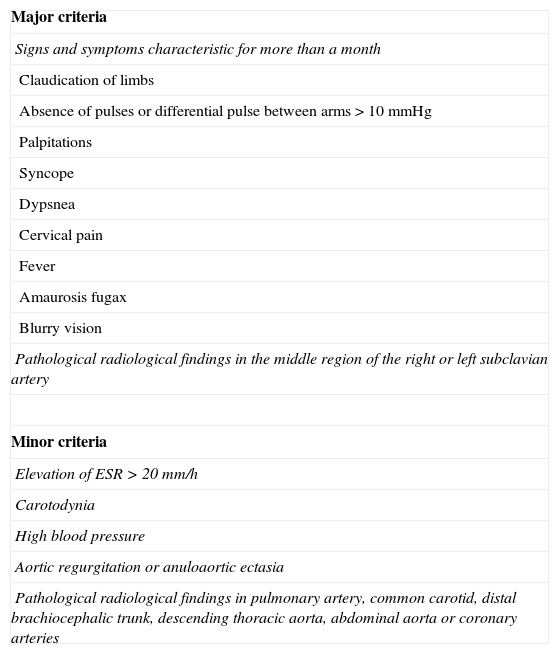
Clinically 2 stages of the disease are distinguished. In the acute stage the symptoms are unspecific: fever, malaise, night sweat, arthralgia, anorexia and weight loss, with increase of PCR and VSG.6 In the chronic stage (without pulse) there appear symptoms derived from vascular occlusion depending on the affected arteries: claudication of limbs, cerebral ischemia, syncope, high blood pressure and acute heart disease (up to 40% of the patients).1 Due to the unspecificity of the symptoms and the serologic tests, the disease is usually suspected in the radiology service. Definite diagnosis is based on clinical-radiological criteria (Table 2). Association of two major criteria, one major criterion and two minor ones or 4 minor ones are considered diagnostic of Takayasu's arteritis.7
Takayasu's arteritis. Diagnostic criteria according to Sharma et al. (1996).
| Major criteria |
| Signs and symptoms characteristic for more than a month |
| Claudication of limbs |
| Absence of pulses or differential pulse between arms>10mmHg |
| Palpitations |
| Syncope |
| Dypsnea |
| Cervical pain |
| Fever |
| Amaurosis fugax |
| Blurry vision |
| Pathological radiological findings in the middle region of the right or left subclavian artery |
| Minor criteria |
| Elevation of ESR>20mm/h |
| Carotodynia |
| High blood pressure |
| Aortic regurgitation or anuloaortic ectasia |
| Pathological radiological findings in pulmonary artery, common carotid, distal brachiocephalic trunk, descending thoracic aorta, abdominal aorta or coronary arteries |
CT with intravenous contrast, in the initial stages of the disease, shows concentric wall thickening, which has been described as «double ring»2 (edematous and hypodense intima in the inside with the medium and the adventitia inflamed around, hypercaptant); in advanced or tardive stages stenosis, thrombosis and occlusions may be observed (Fig. 3a). Other findings associated are extensive mural calcifications, aneurysms and ulcers (Fig. 4a–c).
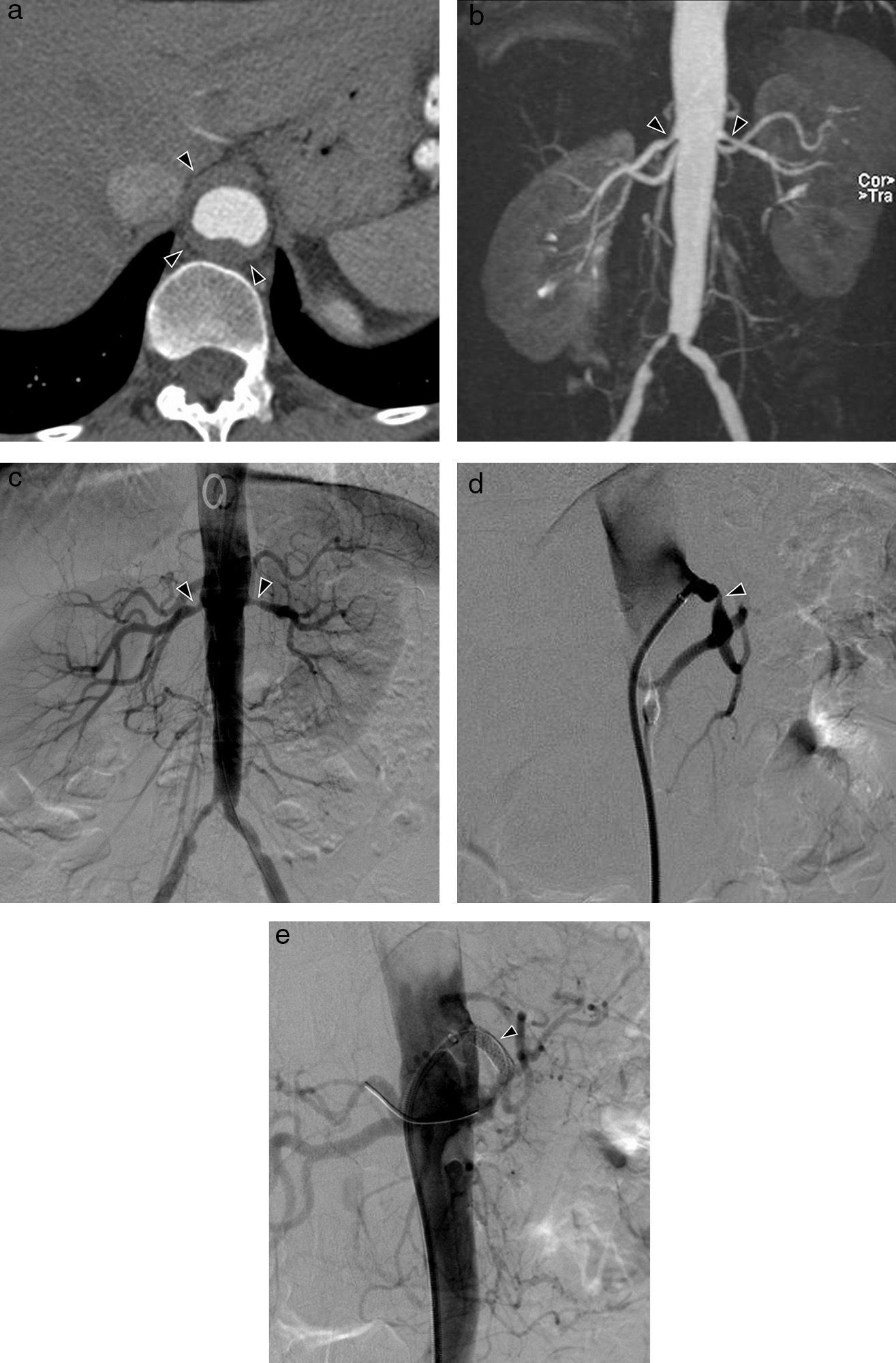
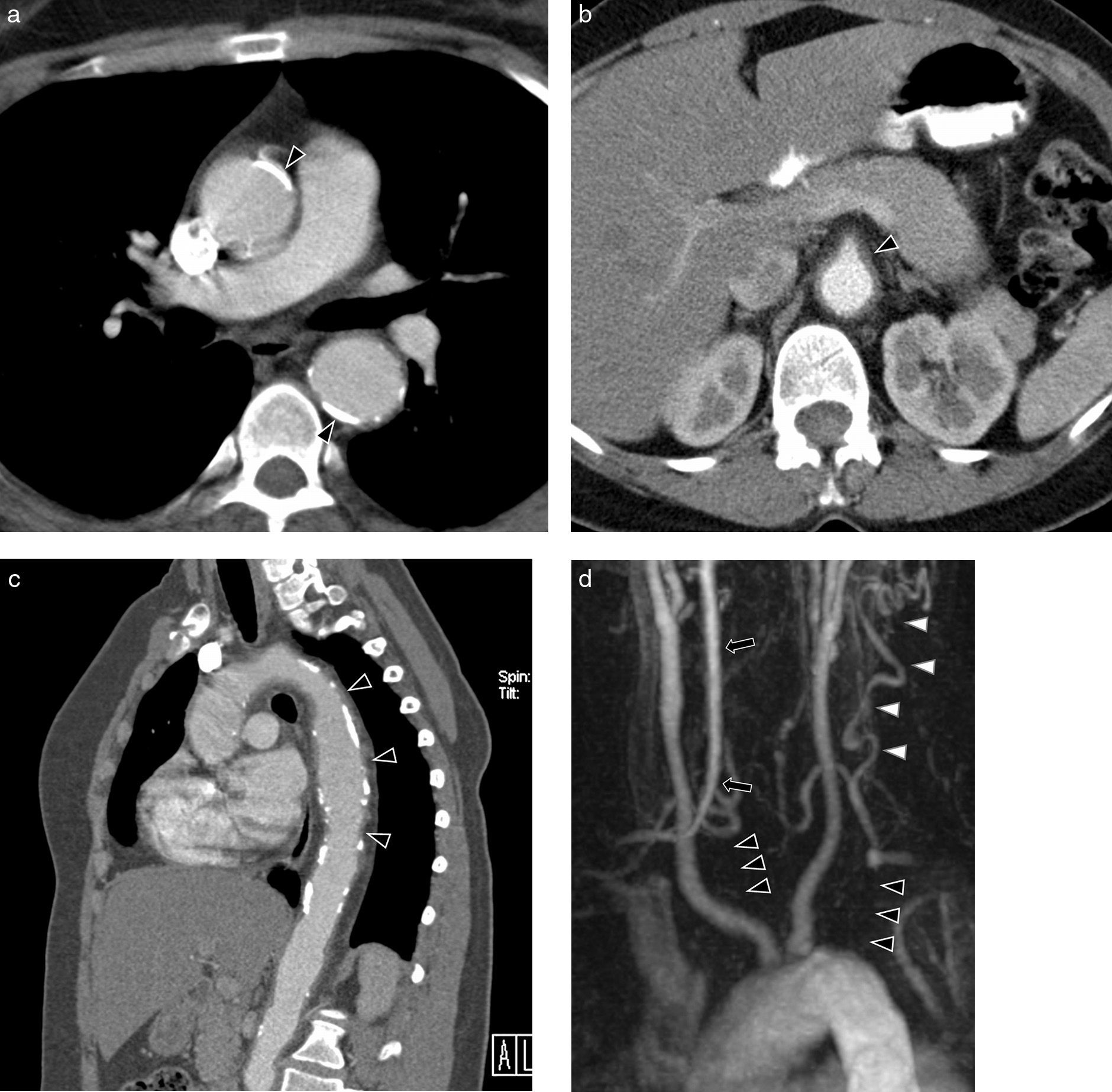
Takayasu's Arteritis of a 41-year-old woman with intestinal angor and right leg claudication. (a) CT study with contrast. Irregular-looking thoracoabdominal aorta with wall thickening (arrow heads). (b) MIP image of angio-MR showing stenosis in the ostium of both renal arteries (arrow heads) and in the primitive iliac arteries. (c) Angiographic correlation of the MR image findings. (d) Selective arteriography of the celiac trunk showing critical stenosis (arrow head) of 90% in the proximal portion thereof without distal affectation. (e) Successful implantation of Herculink low-profile expansible balloon endoprosthesis (arrow head) (of 7×18mm).
Takayasu's arteritis of a 34-year-old woman. (a) Axial CT image with contrast showing dilation and significant calcification of the ascending and descending thoracic aorta (arrow heads). (b) Axial CT cut with contrast. Abdominal aorta and upper mesenteric ostial artery wall thickening (arrow head). (c) MPR sagittal reconstruction. Parietal irregularity (arrow heads) and extensive calcification of the aorta. (d) Coronal MIP reconstruction of angio-MR of supraaortic trunks. Complete occlusion of both subclavian arteries in their prevertebral portions (black arrow heads) with posterior recannulation on the left side by cervical arteries (white arrow heads) and on the right side by the vertebral artery (arrow).
MR is very useful to detect precocious changes in the aortic wall and it is the technique of choice to follow up the disease's evolution, especially in young patients for whom it avoids radiation.4,5 Enhanced sequences on T1 are used with fat saturation and contrast to show the tardive enhancement of the wall, which has been related with the disease's activity (Fig. 1b),7–10 although Isobe, in a recent study of 150 patients, disagrees with the clinical meaning of this finding that is observed in patients both with and without an active disease.11 A hyperintensity of the sequences enhanced on T2 is indicative of wall edema. Like CT, MR allows to identify wall thickening, stenosis (Figs. 3b and 4d), thrombi and aneurysms. In addition, it may prove thickening of the aortic valves and pericardial affectation.4
PET-CT contributes information about the disease's activity.12 It has been proved that when metabolic activity of the affected vessels drops, the inflammation markers normalize and the symptoms improve,3 therefore PET-CT is also useful to monitor therapeutic response.
Treatment of the acute phase is based on glucocorticoids and immunosuppressors. Treatment of vascular stenosis is the surgical bypass or endovascular intervention in critical stenosis (Fig. 3c–e).6
Giant cell arteritisGiant cell arteritis or temporal arteritis is a disease affecting almost exclusively subjects who are older than 50 years, with predilection for the female sex (3:1)13 and a tendency to family aggregation.6 It is a granulomatous vasculitis of medium and large caliber very frequently affecting the external carotid branches, especially the upper temporal artery and also the vertebral and coronary arteries, and the aorta (in up to 15% of the cases).2
Its anatomopathological characteristics are panarteritis with granulomatous infiltrate, multinucleated giant cells, elastic lamina fragmentation, thinning of medium lamina and occlusion of vascular lumen.13 Vascular affectation is typically segmentary, alternating pathological areas with respected areas.
Most giant cell arteritis symptoms are unspecific: fever, malaise, fatigue, anorexia, weight loss, sweating and arthralgia. Headache is more typical with pathological signs in the physical examination of the temporal artery, which is nodular and it is thickened, and in advanced stages, the pulse is lost. In the chronic stage there appear symptoms by occlusion of the affected vessels: claudication of the chewing muscles and the tongue, ischemic optic neuropathy, myocardial infarction and ictus.6 In the aorta it produces abdominal aneurysms, acute dissection, anuloaortic ectasia and acute aortic valvular failure. Thoracic aorta aneurysms are a tardive complication of this disease.2 It is frequently accompanied by rheumatic polymyalgia (up to 60% of giant cell arteritis) with stiffness and pain in the neck, shoulders, back and hips. Up to 10–20% of the patients with rheumatic polymyalgia will develop giant cell arteritis along their lives.13
At present the reference standard to diagnose it is biopsy of the temporal artery in patients with high clinical suspicion.13 A long enough segment of the artery (>20mm) must be obtained so as to prevent segmentary affectation from leading to a false negative.14
Like in Takayasu's arteritis, CT and MR will prove wall thickening, with stenosis areas, occlusion or aneurysms of the affected vessels. CT allows us to observe changes on the wall such as calcifications and thrombosis (Fig. 5). MR detects in addition wall edema in the vessels where the disease is active.2 PET-CT is very useful to detect extracranial affectation, with 85 and 95% sensitivity and specificity respectively, but not so for the intracranial one due to its spatial resolution. More than 80% of the patients with recent diagnosis of giant cell arteritis show aortitis in the PET-CT examinations.2,13
Treatment for giant cell arteritis consists in glucocorticoids, which improve the symptoms quickly and normalize analytical alterations. In case this treatment is not effective, alternative treatment must be resorted to.13
Cogan's syndromeIt is a very rare systemic inflammatory disease, of unknown etiology,15 with only about 250 cases described in the literature.16 It affects equally men and women of different races, above all in the third decade of life.15
The usual signs are ophthalmic and audiovestibular. Ocular affection includes interstitial keratitis (in the typical form), and conjunctivitis, uveitis and scleritis (in the atypical form). La audiovestibular affectation is similar to Ménière's syndrome, with sudden onset of dizziness, vertigo, instability, nausea, vomiting and irreversible hearing loss.17 Moreover, it may be accompanied by other unspecific signs: fever, arthritis and arthralgia, and weight loss. Aortitis has been described in approximately 10% of the cases and it may cause aortic valvular failure, coronary affection and thoracic and abdominal aneurysms.15 Biopsies of the aortic wall show lymphocytic inflammation, destruction of the elastic lamina, fibrosis and aneurysms.2
Radiological findings are the same as in other aortitis: aortic wall thickening, stenosis and aneurysms (Fig. 6). PET-CT has also been used at times to diagnose and monitor the disease and the therapeutic response.18
Cogan's syndrome of a 45-year-old man with episcleritis and neurosensory deafness. (a) Axial CT image with contrast showing mural thickening of the abdominal aorta (arrow head). (b) Sagittal MIP reconstruction. Stenosis of the celiac trunk exit and the upper and lower mesenteric arteries (arrow heads).
Oral corticosteroids are administered in order to control symptoms. For treatment of ocular affectation topical corticoid drops are used. In the refractory cases, use of immunosuppressors such as methotrexate or cyclophosphamide may be considered.17
Ankylosing spondylitisIt is an inflammatory disease of unknown cause which has manifestations in the axial skeleton, peripheral joints and extra-articular structures. It more often than not affects men (2–3:1) in the second or third decades of life. It has an important association with the histocompatibility antigen HLA-B27, which is expressed in up to 90% of the patients.19
Usual clinical presentation is articular pain (the sacroiliac joint is typically affected) of inflammatory characteristics and morning stiffness that lasts for several hours and it improves with physical activity. The most frequent extra-articular manifestations are anterior uveitis (40%) and inflammatory diseases of the intestines (60%).19
Cardiovascular affectation has been described in up to 80% of the ankylosing spondylitis patients,20 typically aortic root dilatation, valvular regurgitation and conduction alterations. Aortic valvular disease is related with the duration of the disease.21 Anatomopathological characteristics are cellular inflammation in the aortic root with fibroblastic repair that produces thickening of the adventitia, focal destruction of the elastic lamina and intimal proliferation. The process expands to the aortic valvular ring where it retracts the valves.20
Treatment for aortic valvular affection is changing the valves and for conduction alterations, implanting a pacemaker.19
Relapsing polychondritisIt is a connective tissue disease that may occur in a progressive manner or, more typically, with cartilaginous inflammatory outbreaks. Both the elastic cartilage of the nose and ears and the hyaline cartilage of the joints are affected, as well as the axial skeleton fibrocartilage, the tracheobronchial tree cartilage and other structures with a high proteoglycans content (eyes, heart, blood vessels and kidneys). The median age for the onset of the disease is 47 years, with certain prevalence among females (1–3:1).22 It is often associated to other autoimmune diseases.
Clinical presentation will depend on the affected organs. Diagnosis is based on meeting the following 7 criteria: (1) symmetric inflammation of the ear cartilage; (2) inflammation of nasal cartilage with deformity in the bridge; (3) inflammation of the respiratory tract cartilage; (4) non-erosive seronegative arthritis; (5) ocular tissue inflammation; (6) Inner ear and labyrinth affectation; and (7) anatomopathological confirmation.23
Cardiovascular affection appears in about 25% of the patients, and it includes aortic and mitral regurgitation and aortic aneurysms, aortic dissection, myocarditis, pericarditis and conduction alterations. Anatomopathological characteristics are lymphocyte infiltrate around the vasa vasorum and the medium, with fragmentation or complete loss of the elastic lamina and hyaline substitution.24
The first line of treatment for relapsing polychondritis is corticoids. Immunosuppressors are used in refractory cases, in which they may decrease the frequency, duration and seriousness of the episodes.24
Systemic lupus erythematosusIt is an autoimmune disease measured by autoantibodies and immune complexes, occurring more frequently in women in fertile age.25
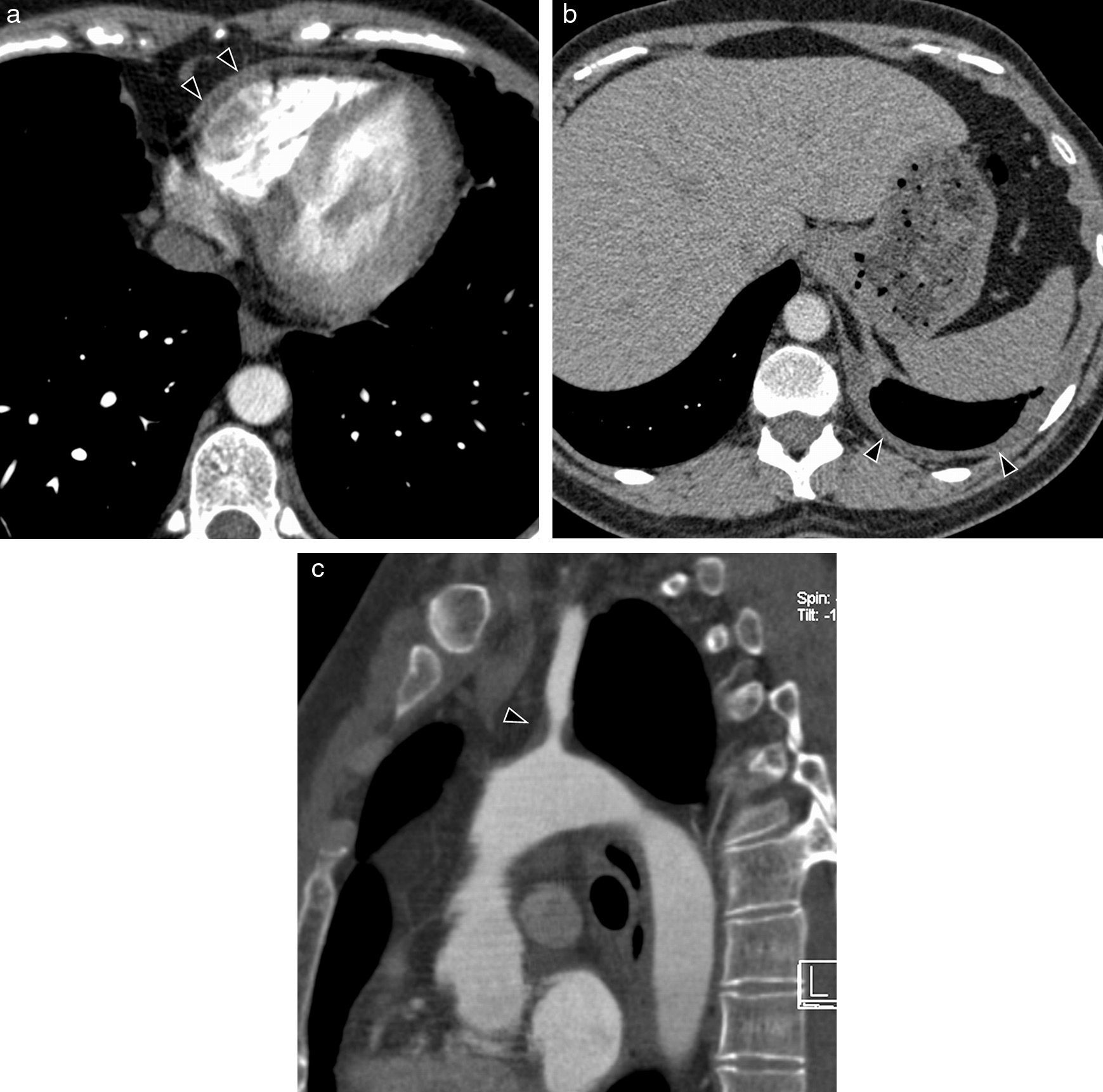
Clinical signs will depend on the organs affected, although pleuropulmonary affectation is the most frequent (50–60%). In the cardiovascular system the most common one is pericarditis with pericardial effusion (Fig. 7).
Systemic lupus erythematosus in a 36-year-old woman. Axial CT images with contrast showing affectation of serosas: (a) pericardial thickening (arrow heads) and (b) effusion and left pleural thickening (arrow heads). (c) CT Sagittal MPR reconstruction with contrast showing mural thickening and left subclavian artery stenosis (arrow head).
The disease's diagnosis is clinical-analytical when 4 or more of the following criteria are met: (1) malar erythema; (2) discoid erythema; (3) photosensitivity; (4) oral ulcer; (5) non-erosive arthritis; (6) serositis (pleuritis and/or pericarditis); (7) kidney disorder; (8) neurologic disorder; (9) hematologic disorders; (10) immunologic disorders; and (11) antinuclear antibodies.5
Aortitis is unusual, but cases associated with aortic dissection, aneurysms and thrombi have been described. The aneurysms related with lupus occur in younger patients, they are characterized by destruction of middle lamina, and they have been related with long periods of corticotherapy and vascular mural lesion typical of vasculitis.2
Behçet's diseaseIt is a chronic autoimmune disease characterized by mucocutaneous, ocular, vascular and central nervous system affection, that affects young patients (between 18 and 40 years) of Mediterranean, Middle Eastern and Far Eastern origin, with similar prevalence between sexes (although occurrence in men is more serious).26
Patients suffering from Behçet's disease present oral and genital cankers, cutaneous lesions as erythema nodosum and pseudofolliculitis. Ocular affectation is frequent and serious, with loss of vision. Neurologic signs are varied, including headache, meningitis/meningoencephalitis, comicial crises, neurologic focality and signs of intracranial hypertension.27
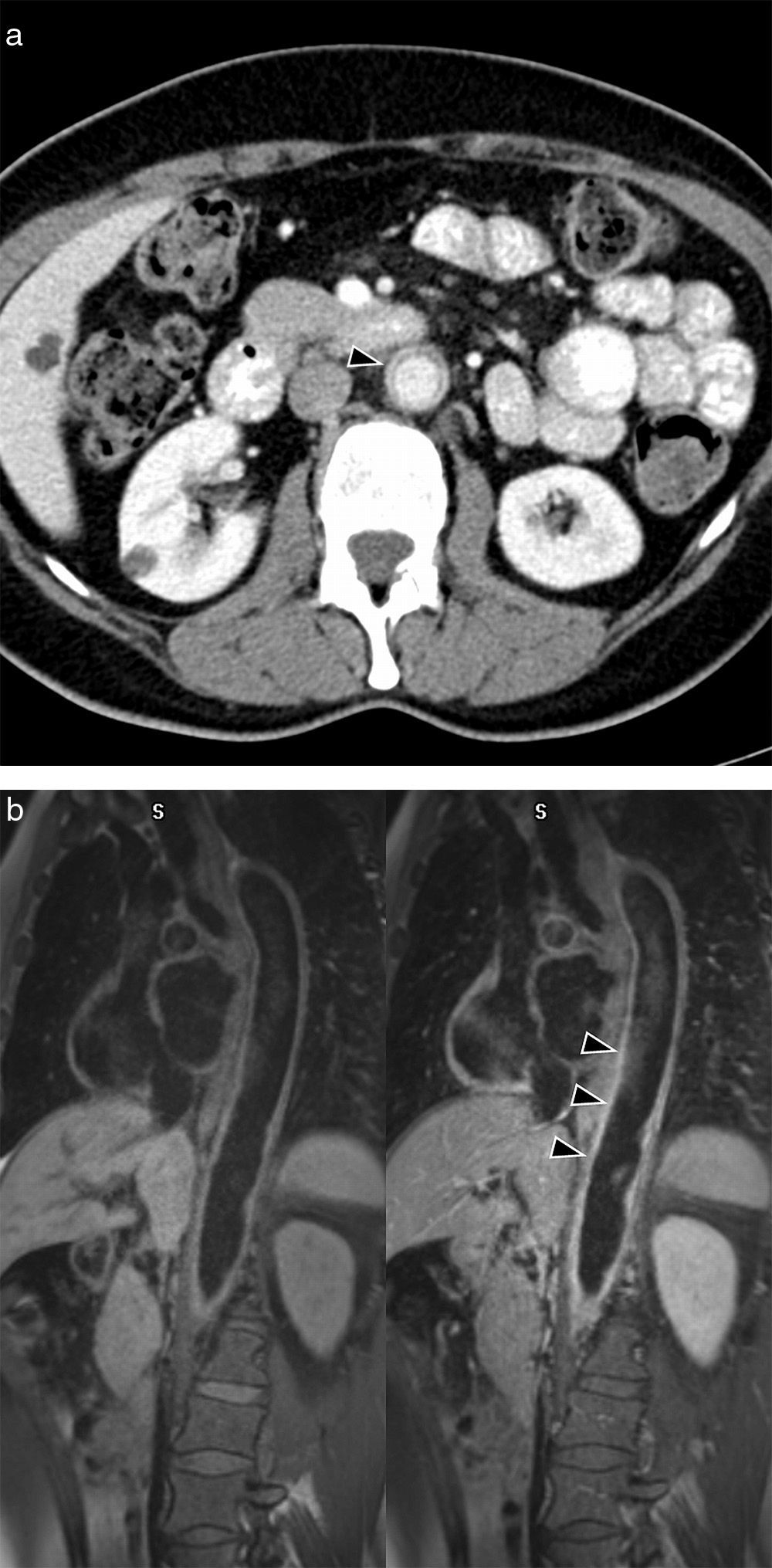
In the vessels, it occurs as a vasculitis that affects both arteries and veins of all sizes. The most common presentation is thromboflebitis and deep venous thrombosis, which occurs in up to 30% of the patients. In the lung, there appear aneurysms of pulmonary arteries that may become complicated with massive hemoptysis. Affectation of the aorta and its branches occurs in less than 5% of the patients, and it occurs as aortitis, aneurysms or arterial thrombosis (Fig. 8).27
Behçet's disease in a 44-year-old woman with genital ulcers. (a) Axial CT image with contrast where proximal abdominal aorta aneurysm is observed, just at the exit of the celiac trunk (arrow head). (b) Sagittal MPR reconstruction showing occlusion distal to the celiac trunk aneurysm (arrow head). Notice the large subcutaneous thoracoabdominal emphysema, pneumoperitoneum and retropneumoperitoneum secondary to barotrauma by intubation.
Diagnosis is clinical when, in the presence of recurrent oral ulcers, two of the following criteria are met: (1) recurrent genital ulcers; (2) ocular lesions; (3) cutaneous lesions of the erythema nodosum or pseudofolliculitis type; and (4) sign of patergia.26
Both corticosteroid and immunosuppressor drugs have demonstrated their effectiveness to treat Behçet's disease.27 Aortic aneurysms are treated by placing endoprostheses or surgically.28
Idiopathic aortitisInflammatory aortic aneurysmThey are distinguished from the atherosclerotic ones by perianeurysmatic fibrosis and thickening of vascular wall.2 They represent 3–10% of all abdominal aorta aneurysms (very rare in the ascending aorta and the aortic arc), with a median age at the onset of the disease of 62 years and a clear predilection for the male sex (6–30:1).29
The disease's etiology is not clear and a multifactorial origin has been suggested, one where genetic factors are involved (certain HLA allele, T-cell function alterations, excessive proteolytic activity), endothelial (arterial hypertension, atherosclerosis) and environmental risk factors (tobacco, viral infections).29
Typical clinical presentation is the abdominal pulsatile mass triad with weight loss and elevation of the reactant in the acute stage, especially VSG. Other manifestations depend on obstructive uropathy and intestinal occlusion secondary to trapping of retroperitoneal structures by periaortic fibrosis.30
CT with intravenous contrast is highly sensitive and specific (84 and 100% respectively).2 Mural thickening is characteristic (typically the anterior wall) of aortic aneurysm and a density mass of soft parts around.
Treatment consists in repairing the aneurysm surgically, because its natural tendency is growth and rupture.29
Chronic periaortitisAlso called retroperitoneal fibrosis, Ormond's disease or sclerosing retroperitoneal granuloma, it is a systemic disease consisting in a retroperitoneal proliferation of fibrous tissue extending to the adjacent viscera, including the vena cava and the ureters.2 On the great majority of the occasions (>70%) it is idiopathic, and on the rest, it is related with inflammatory diseases or with an underlying neoplasia (lymphomas, retroperitoneal sarcomas, carcinoid tumors or metastasis). It is associated to immunologic diseases such as primary biliary cirrhosis, fibrosing mediastinitis, rheumatoid arthritis, systemic lupus erythematosus or sclerosing mesenteritis.31
It has an incidence of approximately 1/200,000 inhabitants, with a greater affectation in the population 40–60 years old, and it is more frequent in the male sex (2–3:1).31
In the initial stages the symptoms of the disease are unspecific, and as the degree of the fibrosis progresses, there appear symptoms derived from compression of neighboring structures. The most common presentation is intense lower lumbar and abdominal pain, as well as pain in the flanks. If the kidneys and the ureters are affected, it may cause obstructive uropathy.31
Anatomopathological characteristics are fibroblastic proliferation with inflammatory cell infiltrate consisting in lymphocytes, macrophages and vascular endothelium cells.31
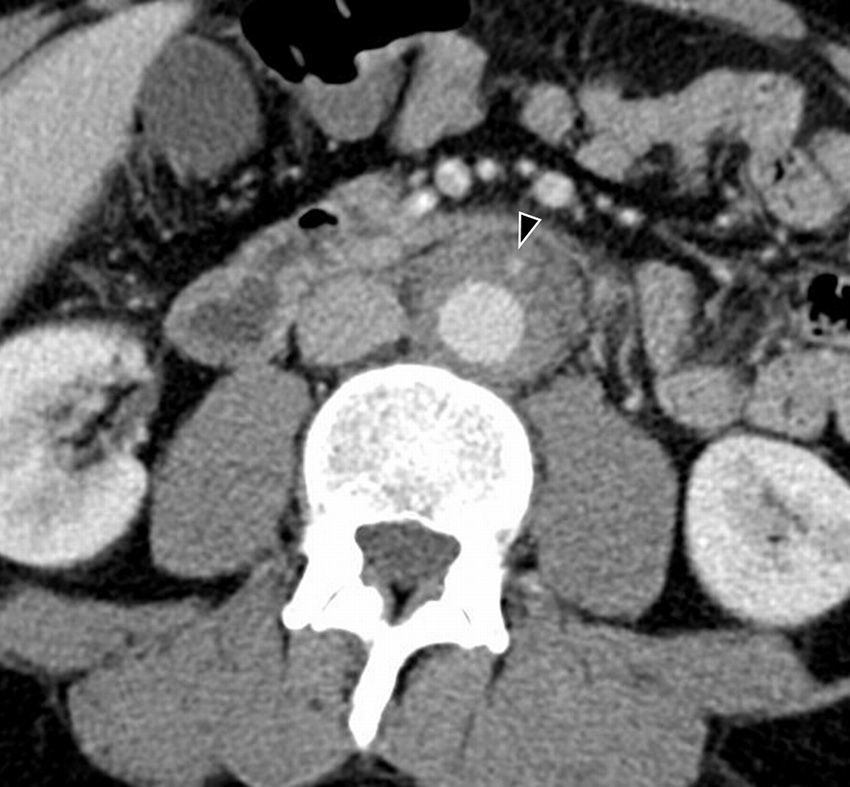
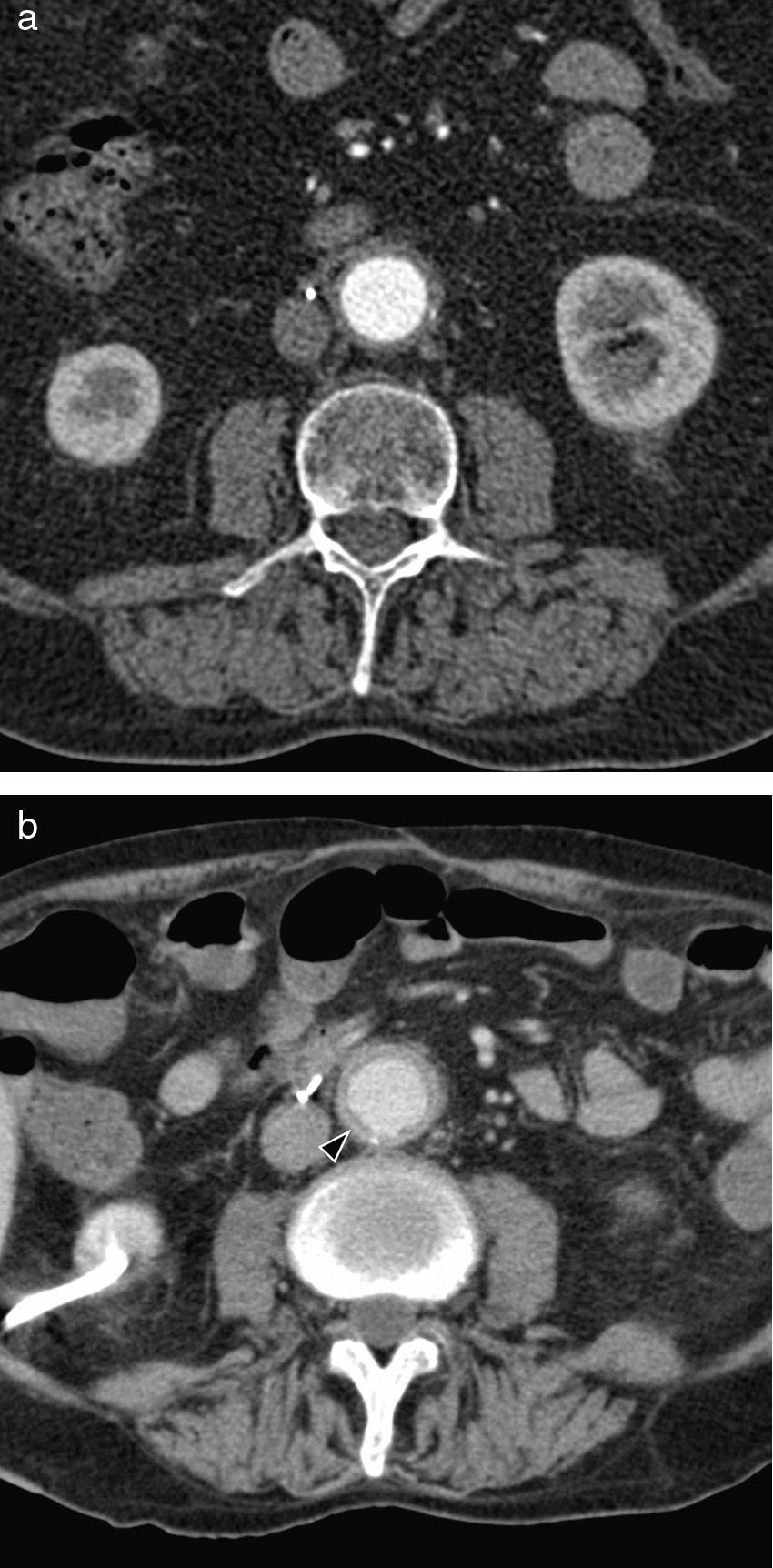
CT shows a retroperitoneal soft tissue mass, isodense with respect of the adjacent muscle (Fig. 9). Initial fibrosis tends to begin near the aortic bifurcation and the iliac arteries, and it may extend forward to the duodenum, pancreas and the spleen, and craniocaudally from the mediastinum to the sacrum. The degree of enhancement after injecting contrast correlates with the activity of the fibrotic process: it is marked in the acute stages, of up to 20–60UH; whereas in the advanced or chronic disease is it practically non-existent.31 In the MR the fibrotic tissue has low signal intensity in the adjusted images on T1. Signal intensity on T2 will vary depending on the degree of active inflammation (and therefore, of edema). At present image tests are insufficient to differentiate between an idiopathic retroperitoneal fibrosis and an underlying neoplastic process; therefore diagnosis will require anatomopathological confirmation.
Corticosteroids alone or associated to other immunosuppressor agents improve the symptoms, reduce the size of retroperitoneal mass and on occasion, solve the obstructive complications, although in some cases surgical intervention may be necessary.31
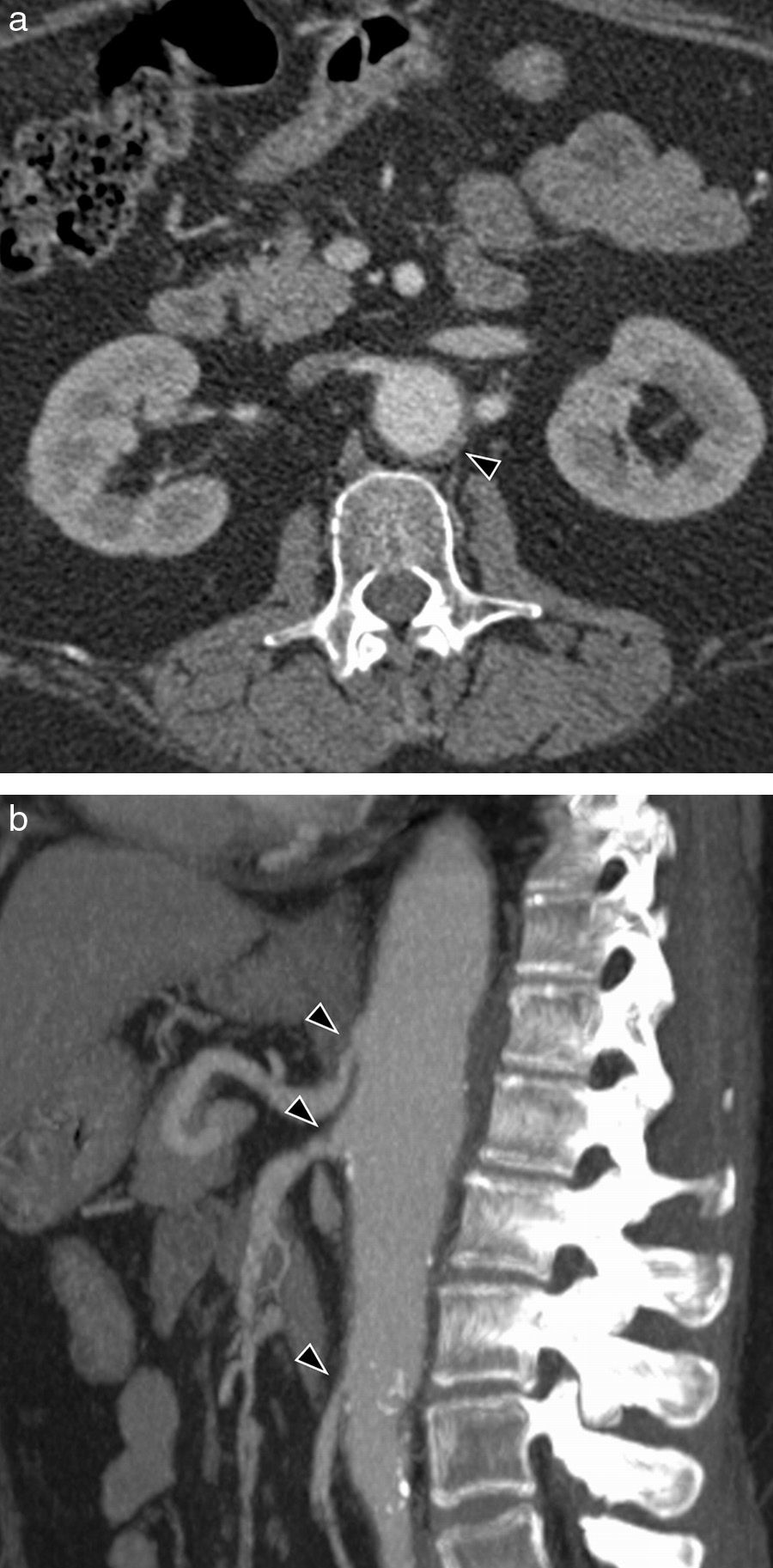
Erdheim-Chester's syndromeErdheim-Chester's syndrome is a rare systemic disease, which anatomopathologically corresponds to a histiocytosis of non-Langerhans cell. It may present various signs depending on the organs affected: osseous affection is practically constant (long bone metaphysodiaphysary and extraskeletal osteosclerosis (Fig. 10a)), occurs in almost half the patients, including retroperitoneal infiltration, cardiovascular affection, as well as that of the central nervous system, exophthalmos and interstitial pulmonary diseases. In the image tests, when the aorta is affected, periaortic tissue is infiltrated circumferentially from the ascending aorta to the iliac bifurcation (image of “coated aorta”) (Fig. 10b and c). This infiltrate is hypointense in the adjusted echo spin MR sequences on T1 and T2 (Fig. 10d).32,33
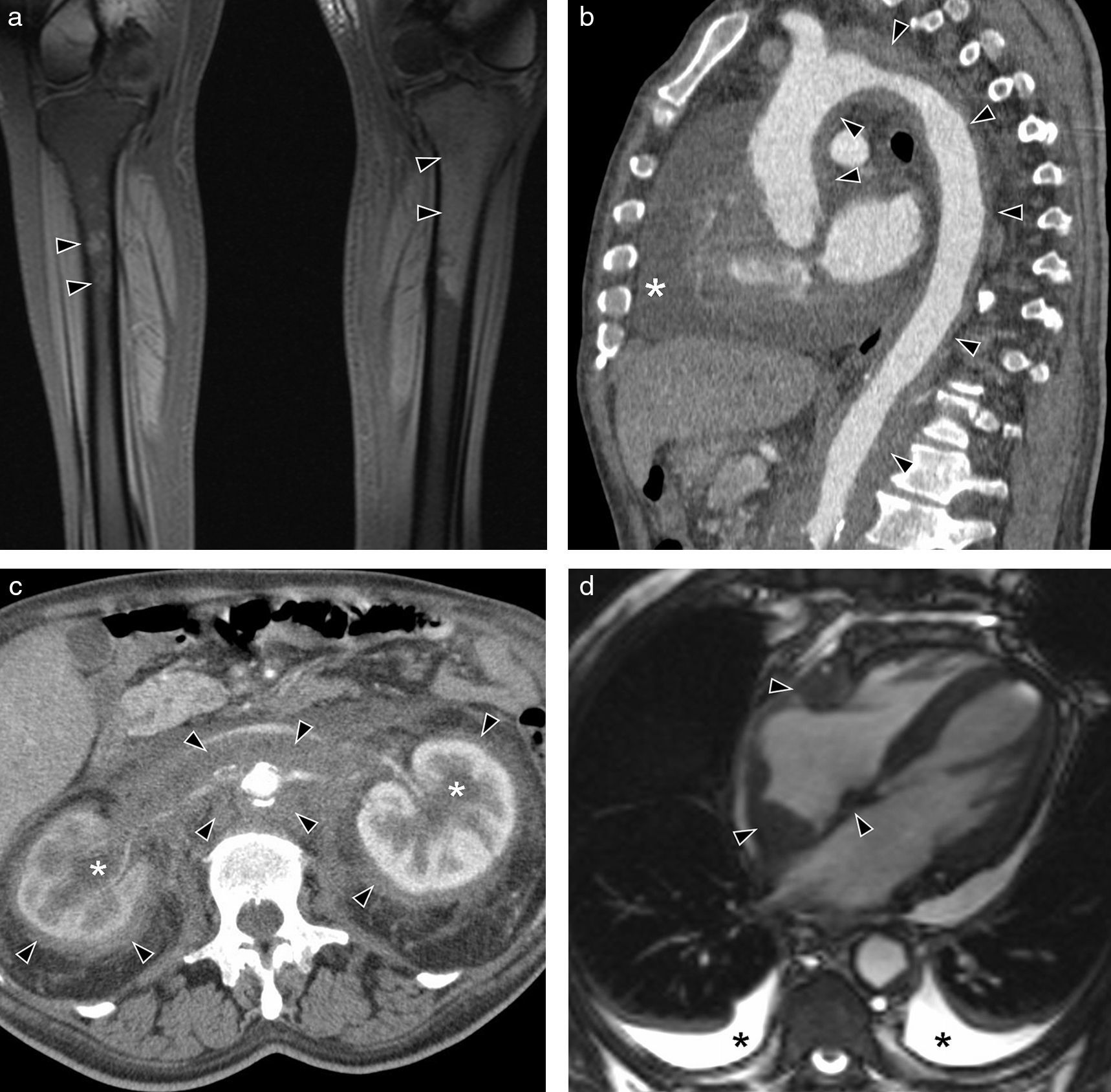
Erdheim-Chester's syndrome of a 66-year-old man with chronic thoracic pain and toxic manifestations. (a) MR coronal image of lower limbs (sequence T1 with fat saturation) showing a signal alteration in the left tibia upper third and patched affectation of right tibia upper diaphysis (arrow heads). (b) Sagittal MPR reconstruction of CT with contrast. Extensive periaortic thickening with vascular wall irregularity (arrow heads), from the aortic root to the abdominal aorta. Abundant pericardial effusion (asterisk). (c) Axial CT image with contrast showing hypodense tissue infiltrating periaortic and perirrenal regions (arrow heads), with compromise of renal hili and secondary bilateral hydronephrosis (asterisks). (d) MR axial image in 4 heart chambers (cine gradient sequence) showing hypointense tissue around the right auricle, interauricular septum and right coronary artery (arrow heads). Small bilateral pleural effusion (asterisks).
This disease encompasses a range of processes that occur with fibrosis of several organs (predominantly glandular tissues), elevation of IgG and IgG4 serum and autoantibodies. Autoimmune pancreatitis is the most frequent manifestation within this disease. Aortic affection and that of its main branches are secondary to a lymphoplasmocytary infiltrate (with numerous plasmatic IgG-positive cells) with irregular fibrosis, located in the vessel's adventitia, which causes the characteristic artery wall thickening in the image studies.34
Iatrogenic aortitisRadio-induced aortitisVascular affectation by radiation often appears many years (more than 10) after exposure to high doses of radiotherapy. There appear thrombosis, pseudoaneurysms, ruptures, stenosis and accelerated mural calcification of the affected elastic arteries. By definition, it only affects the arteries included in the radiated area.2,35
Aortitis secondary to drugsDrug-induced vasculitis deserves a special section, since many therapeutic agents (such as propylthiouracil, minocyclin, retinoid derivatives or leukotriennes receptor antagonists)36 have been implicated in the development of an inflammatory vascular disease. The patients often come with varied symptoms, ranging from fever of unknown causes or myalgia, to an isolated cutaneous vasculitis or a generalized affectation of internal organs.37
Diagnosis is often made after increasing the dose or re-exposure to the drug. Vasculitis is typically resolved after discontinuing medication; if the symptoms are serious, treatment with corticosteroids, plasmapheresis, hemodialysis or cyclophosphamide may be resorted to.38
La gemcytabin is an analog of the nucleosides used to treat different cancers. Many cases of small and medium-caliber vasculitis as well as some of aortitis have been described as secondary to gemcytabin (Fig. 11).38
Infectious aortitisAlthough most aortitis cases have a non-infectious origin, the possibility of infection must always be considered because treatment is very different.1 In normal conditions, the aorta is very resistant to infection. Bacterial aortitis usually affects an aortic segment already injured with atherosclerosis plaques or an aneurysm.1 Dissemination is by closeness from adjacent structures, although it may also be hematogenous by septic emboli in sepsis or bacterial endocarditis.2,39
Mycotic aneurysms (infected aneurysms) are those secondary to an infectious aortitis in a vulnerable vessel that eventually weakens its wall creating a pseudoaneurysm.2
CT is the test of choice. With it, it is possible to see aortic wall thickening, periaortic fluid or soft tissue accumulation, rapidly progressive saccular aneurysm and, occasionally, air in the aortic wall (Fig. 12).39
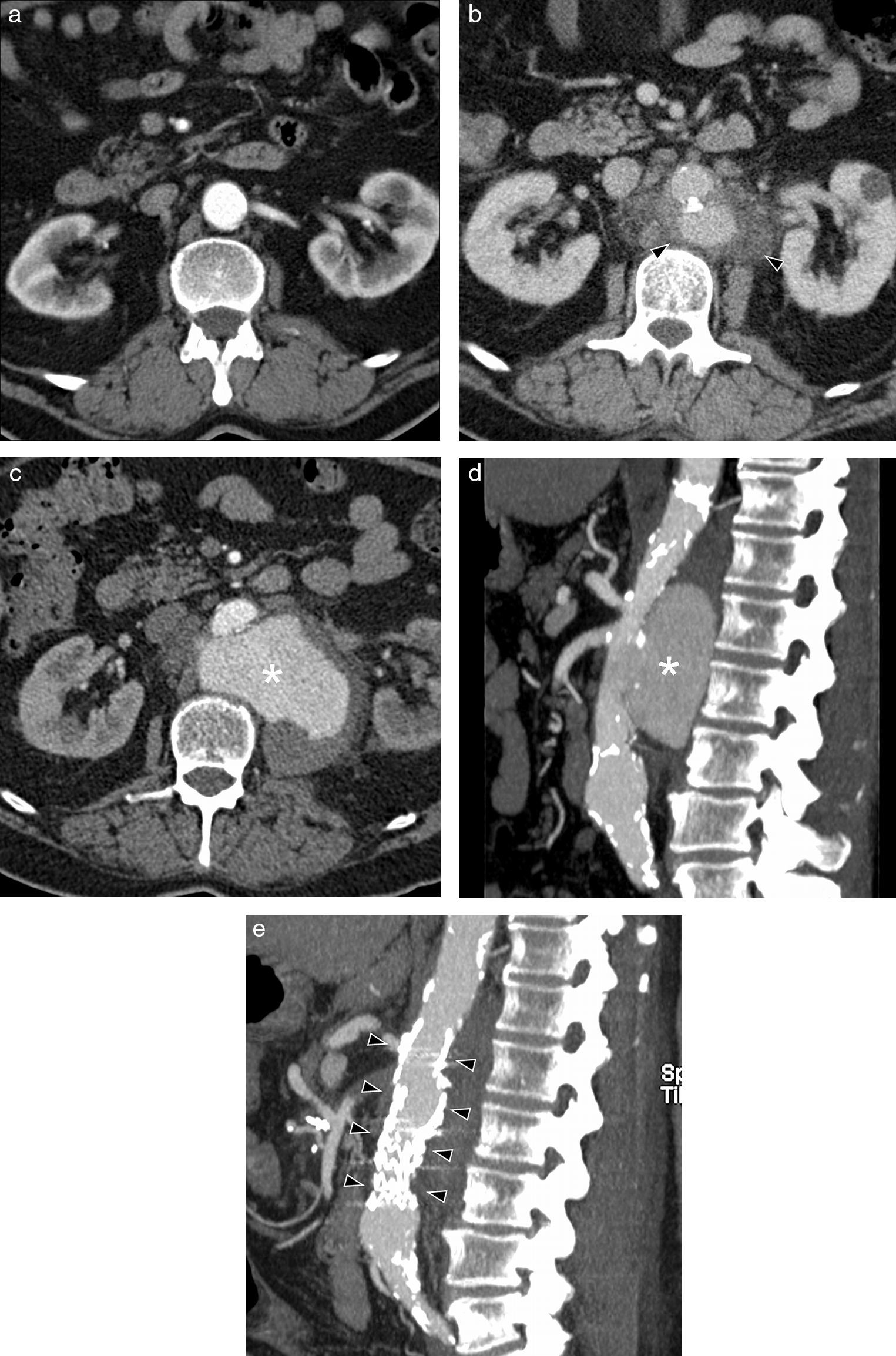
Mycotic aneurysm of a 70-year-old man with lumbar pain and low-grade fever. CT with intravenous contrast: (a) Axial image showing aorta with normal walls; (b) periaortic soft part mass (arrow heads), affecting the upper mesenteric artery ostium and that of the renal arteries (the same patient, a year after Image a). Axial CT image with contrast (c) and sagittal MIP reconstruction (d), showing size increase of aneurysm (asterisk), with very irregular walls (months after Image b). (e) Sagittal CT MIP image with contrast after treatment with aortic endoprosthesis Endurant 25mm×70mm (arrow heads), due to high risk of aneurysm rupture.
Salmonella infection is the most common cause of bacterial aortitis due to its tendency to infect damaged tissues, such as the arteriosclerotic vascular endothelium. It represents one-third of the cases. Many times there is no history of previous gastroenteritis and the blood cultures may be negative in up to 50% of the cases; therefore diagnosis is often retrospective after surgical intervention.40 Other frequent causes of infectious aortitis are Staphylococcus aureus, Escherichia coli and Streptococcus pneumoniae.41,42
Tuberculous aortitis is not frequent, although an increase of its prevalence is expected due to increase of co-infection with HIV and the appearance of multiresistant stocks.2 It normally occurs by direct extension from an adjacent focus of tuberculous infection, such as adenopathies, pericarditis, empyema, spondylitis o paravertebral abscesses. Caseous necrosis may affect the whole aortic wall thickness, causing perforation and massive bleeding, or a perivascular hematoma and pseudoaneurysms.43
Infection by Treponema pallidum may affect the cardiovascular system in its tertiary stage, usually as aortitis. It affects the ascending thoracic aorta in 60% of the cases and the aortic arch in 30%.2 The usual complications are aortic regurgitation, coronary ostial stenosis and the formation of aneurysms.44
ConclusionsUnspecific symptoms cause aortitis not to be suspected and the radiologist ends up establishing diagnosis. Etiological diagnosis must be performed taking into account other data such as the patient's age and sex, pathological antecedents and clinical findings, since many of them are part of systemic diseases.
It is essential for the radiologist to know the characteristics that arouse suspicion of an inflammatory aortic disease with image techniques: wall thickening, stenosis vascular occlusions, mural contrast uptake, mass of periaortic soft parts, intramural gas, aneurysms, calcifications and mural thrombi.
The radiologist must know the advantages and inconveniences of the different image techniques that contribute relevant information to treat and follow up the evolution of aortitis. Both the CT and the MR give us information about aortic wall and affectation of vascular lumen, and PET-CT and MR are useful to monitor inflammatory activity and response to treatment.
Ethical responsibilitiesProtection of people and animalsThe authors declare that no experiments were performed on humans or animals for this research.
Data confidentialityThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Authors- (1)
Person responsible for the study's integrity: JC, MA.
- (2)
Conception of the study: JC, MA.
- (3)
Design of the study: JC, MA.
- (4)
Data acquisition: JC, MA, EC, XG, EB.
- (5)
Data analysis and interpretation: JC, MA, EC, XG, EB.
- (6)
Statistic treatment: not applicable.
- (7)
Bibliographic search: JC, MA.
- (8)
Writing of the paper: JC, MA.
- (9)
Critical revision of the manuscript with intellectually relevant contributions: XG, EC, EB.
- (10)
Approval of final version: JC, MA, EC, XG, EB.
The authors declare that they do not have any conflict of interests.
Please cite this article as: Cabero Moyano J, Andreu Magarolas M, Castañer González E, Gallardo Cistaré X, Belmonte Castan E. Patología aórtica no urgente: diagnóstico clínico-radiológico de la aortitis. Radiología. 2013;55:469–482.