The groin is a complex anatomic region that has traditionally been ignored by radiologists because most lesions can be diagnosed from clinical data and physical examination. Nevertheless, ultrasound examinations of the groin are increasingly being requested to confirm injury or to resolve diagnostic uncertainty. On the other hand, some conditions involving the groin are found only in paediatric patients. This article describes the key imaging findings in paediatric groin injuries, placing special emphasis on the ultrasound appearance.
ConclusionsKnowledge about conditions that can affect the groin in paediatric patients and the key imaging findings associated with them helps improve the diagnostic performance of ultrasound.
La región inguinal es un área anatómica compleja que ha sido tradicionalmente olvidada por los radiólogos dado que la mayoría de las lesiones pueden diagnosticarse mediante datos clínicos y con la exploración física. No obstante, cada vez es más frecuente la solicitud de ecografías, bien para confirmar la existencia de patología o para resolver casos dudosos. Por otra parte, la patología inguinal incluye entidades únicas de la edad infantil. El objetivo de este trabajo es describir los hallazgos radiológicos clave de las lesiones inguinales pediátricas, poniendo especial énfasis en los datos ecográficos.
ConclusionesEl conocimiento de la patología inguinal pediátrica y sus claves en imagen ayudan a mejorar el rendimiento diagnóstico de la ecografía.
The inguinal canal is a very important anatomical area for paediatric surgeons, since it is the site of most of their procedures.1
Although physical examination is the primary diagnostic tool,2 imaging techniques play an increasingly important role, especially ultrasound and magnetic resonance imaging (MRI).
The objectives of this study are to show the most common paediatric inguinal diseases and to describe their characteristic findings (Table 1).
Paediatric inguinal pathology: primary ultrasound findings.
| Hernias | In general indirect: the abdominal contents penetrate the inguinal canal through the deep inguinal ring. In boys this may lead to testicular ischaemia. In girls these may contain the ovary and become complicated with ovarian torsion. |
| Hydroceles | Collections of fluid which may or may not have a communication into the peritoneum. In girls they are called abnormalities of the canal of Nuck. Abdominoscrotal hydroceles and Type 3 canal of Nuck abnormalities have an hourglass morphology. |
| Cryptorchidism | The inguinal canal is the most common location of cryptorchid testes. Their appearance on ultrasound is variable. If they show a heterogeneous structure, then they should be analysed with colour Doppler imaging due to potential malignancy. |
| Lymphadenopathy | Inguinal lymphadenitis manifests as enlarged lymph nodes with inflammation in the adjacent fat. Lymphadenopathy may present anechogenic areas in relation to necrosis or abscessification. |
| Tumours | Benign, such as lipoma. Notable in children under 3 years of age are fibrous hamartoma and lipoblastoma, both with some sort of fat component. |
| Malignant, in general sarcomas. They do not show specific findings. | |
| Vascular lesions | Pseudoaneurysms: anechogenic lesions with turbulent flow (yin-yang image on colour Doppler imaging).Vascular malformations: hypoechogenic/anechogenic lesions with phleboliths in venous malformations and possible flow in their interior. |
The inguinal canal is a diagonal passage parallel to the inguinal ligament (part of the aponeurosis of the external oblique muscle) which crosses the abdominal wall and ends in the scrotum in boys and the labia majora in girls. Its length increases with age.3
It has four walls formed by the abdominal musculature (anterior, posterior, superior and inferior) and two rings, one deep and one superficial (Fig. 1). The location of the deep ring is lateral to the origin of the inferior epigastric vessels. The superficial ring is found at the height of the pubic tubercle,4 a bony prominence adjacent to the pubic symphysis.
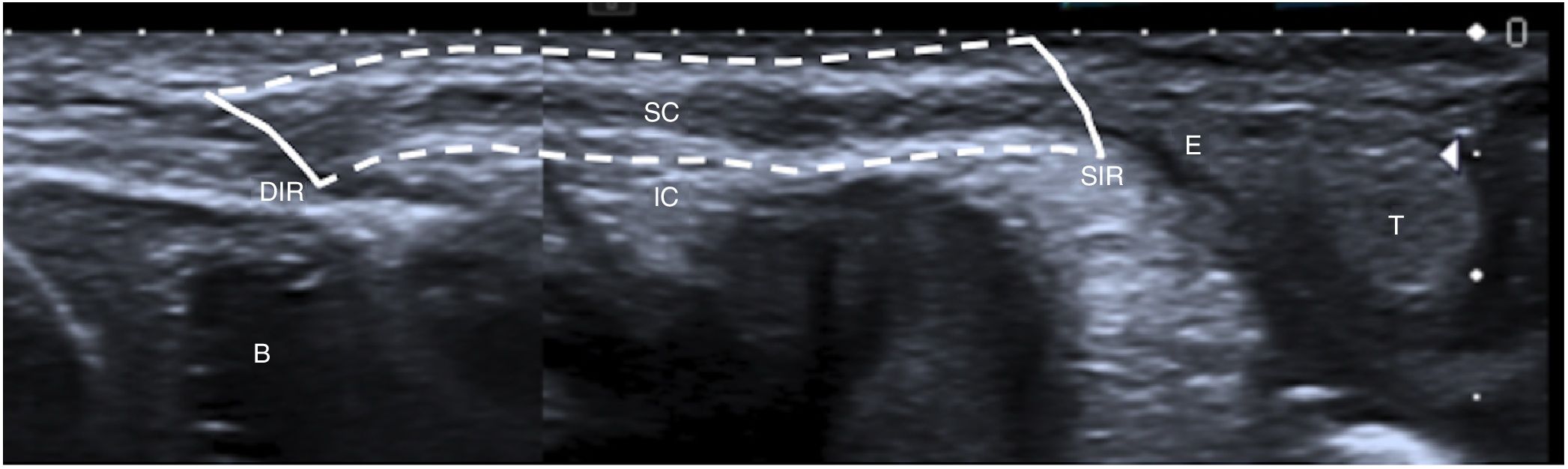
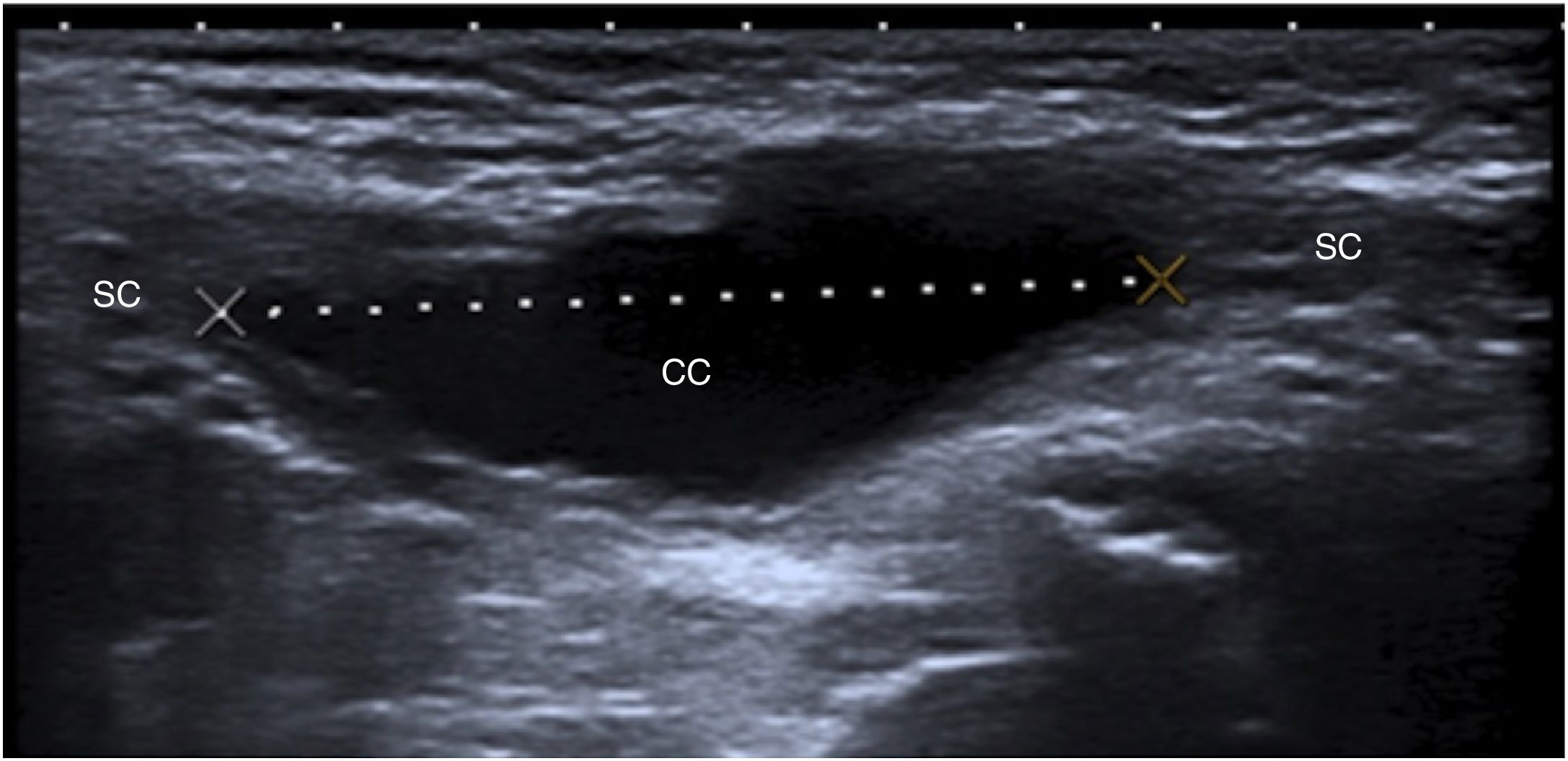
A 1-month-old boy with a normal inguinal canal. An ultrasound image parallel to the spermatic cord (SC) is shown. The inguinal canal (IC) is represented with dotted lines, and the deep inguinal ring (DIR) and the superficial inguinal rings (SIR) are represented with solid lines. The testicle (T), the epididymis (E) and the bladder (B) are also marked.
In boys it contains the spermatic cord (formed by the vas deferens; the artery of vas deferens, the testicular artery and the cremasteric artery; the pampiniform plexus; the genital branch of the genitofemoral nerve; lymphatic vessels; and sympathetic fibres), and in girls it contains the round ligament of the uterus. In both sexes there is also fat, connective tissue, lymphatic vessels, the ilioinguinal nerve and the vaginal process.3,4
EmbryologyThe development of the inguinal canal has not been well described,1 but it is known that the most important structures are the gubernaculum and the vaginal process.
The gubernaculum is a fibromuscular ligament, formed in the 7th week of gestation, that in boys guides the descent of the testicles from the abdomen to the scrotum.1,3–5 In girls its medial third inserts into the uterus and it prevents the ovaries from penetrating the inguinal canals.3
The vaginal process is a peritoneal recess, formed in the 2nd and 3rd months of gestation, which herniates through the defect in the anterior abdominal wall created by the gubernaculum.3,4 This recess is obliterated in a craniocaudal direction during a few weeks before and after birth. In boys, the inferior part constitutes the testicular tunica vaginalis and remains permeable with a small amount of fluid, but in girls, it is completely obliterated.3,5 The permeable vaginal process in girls is called the canal of Nuck.3,4
Evaluation by imagingThe main imaging techniques used for evaluating the inguinal canal are ultrasound and MRI.3
Ultrasound should be performed with high-frequency linear probes (at least 10MHz), since lesions tend to be superficial. In obese children and children in whom a deeper evaluation is needed, lower-frequency probes (3–5MHz) are used. It is advisable to start with images that are longitudinal, parallel in relation to the spermatic cord (Fig. 1), and subsequently transversal. It is also useful to perform the examination with the child standing or performing Valsalva manoeuvres to identify hernias and communicating hydroceles.
Doppler imaging provides important information on the viability of herniated intestinal loops, as well as undescended testicles (cryptorchidism), but it should be optimised since these are diseases particular to small children with low flow in the gonads. Therefore, it is important to increase the Doppler gain, reduce the scale of velocities (to 1–2kHz) and use low wall filter values (<100MHz).
MRI is the second most important technique. It is especially useful in the evaluation of tumour masses due to its anatomical resolution and capacity for tissue characterisation. Apart from morphological sequences, sequences enhanced in diffusion should be included, taking into consideration that normal testes and ovaries show restricted diffusion.
PathologyCongenital inguinal herniasThese affect 1%–5% of newborns and 9%–11% of prematurely born infants.6 They are almost always indirect,7,8 lateral in relation to the inferior epigastric vessels, and may be classified as reducible, incarcerated or strangulated. In reducible cases, the herniated intestine returns easily to the abdomen, whereas in incarcerated cases, it is found to be thickened and oedematous and it becomes trapped in the inguinal canal. Cases that also feature ischaemia of the herniated loops are termed strangulated cases; these represent a surgical emergency due to a risk of intestinal necrosis.
They are more common in boys (the ratio of boys to girls is 3–5:1); it is also more common for them to be unilateral and right-sided, since the right vaginal process closes after the left.1,4,6
In general, they present as masses that appear with Valsalva manoeuvres.
Ultrasound plays an important role in confirming uncertain cases, assessing the contents of the hernia (Figs. 2 and 3) and providing evidence of strangulation of the herniated loops such as the absence of peristalsis therein. In addition, with colour and pulsed Doppler imaging, the vascularisation of the contents of the hernia and of the ipsilateral testis can be evaluated4 (Fig. 2).
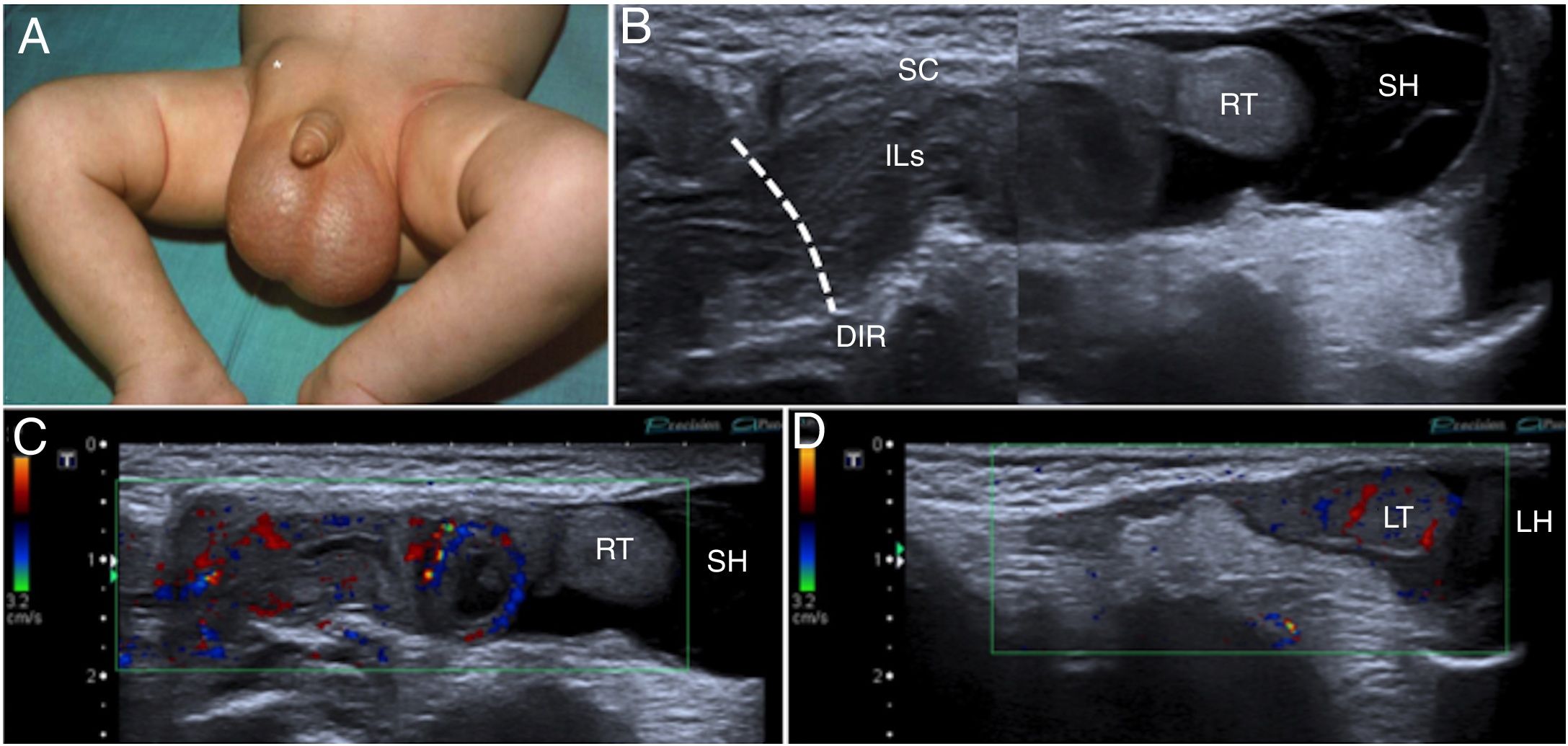
A 3-month-old boy with a right inguinal hernia. (A) The photograph shows said hernia (*) and an increase in scrotal volume. (B) Ultrasound of right inguinal hernia in B mode, slice parallel to the spermatic cord (SC), confirming the presence of an inguinal hernia with passage of intestinal loops (ILs) through the deep inguinal ring (DIR) and a septated hydrocele (SH). (C and D) The colour Doppler imaging performed on the same plane as the previous image identifies the presence of flow in the intestinal loops (ILs), suggesting their viability, but the absence of flow in the right testis (RT) compared to the left testis (LT), probably in relation to ischaemia. Note the left scrotal hydrocele (LH).
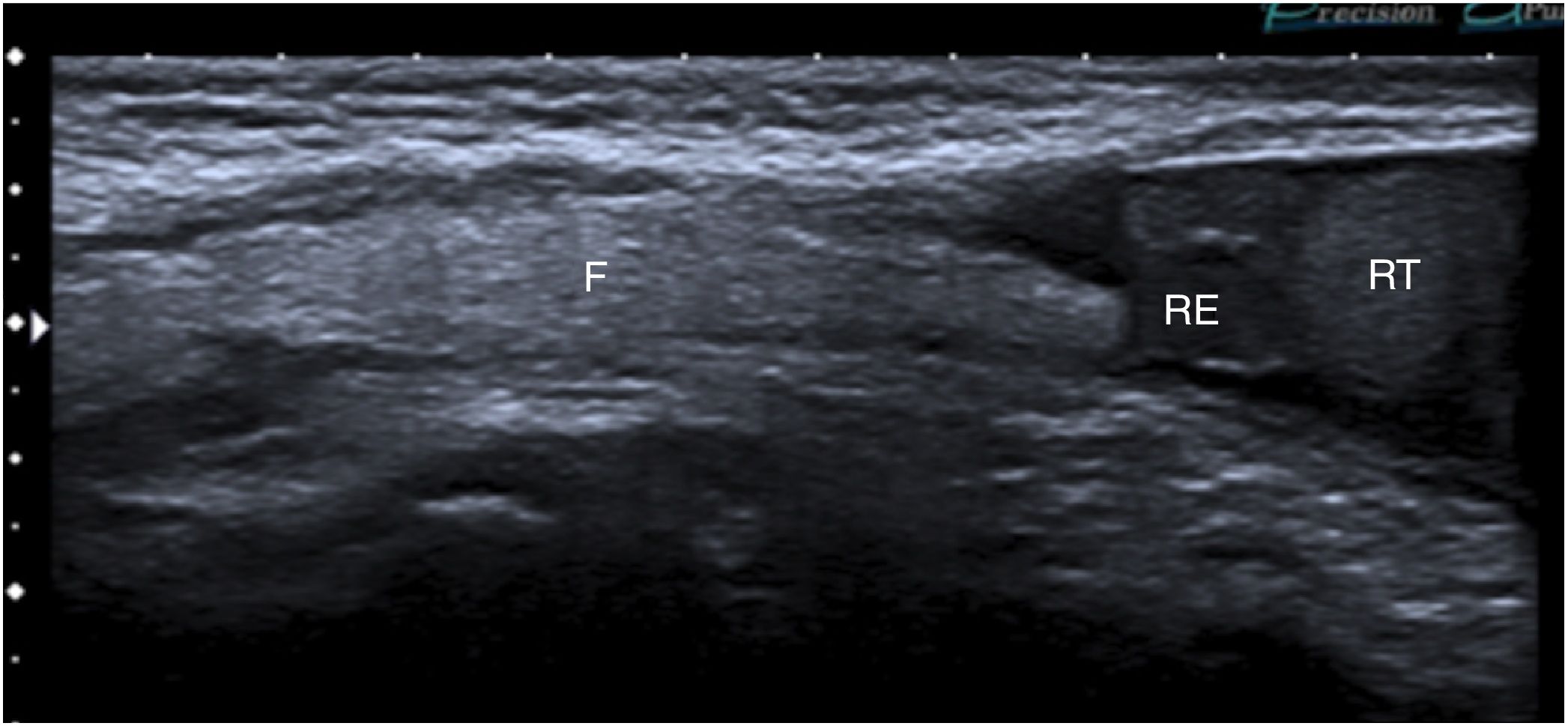
A 3-year-old boy with a right inguinal hernia with fat content. Ultrasound in B mode, longitudinal slice parallel to the inguinal canal, showing an image that occupies the inguinal canal and approaches the right epididymis and right testicle (RE and RT, respectively) in relation to abdominal fat.
Ultrasound is also useful in hernias of the canal of Nuck in girls, as they may contain the ovary (Fig. 4), incarcerate and cause torsion of the ovary.9
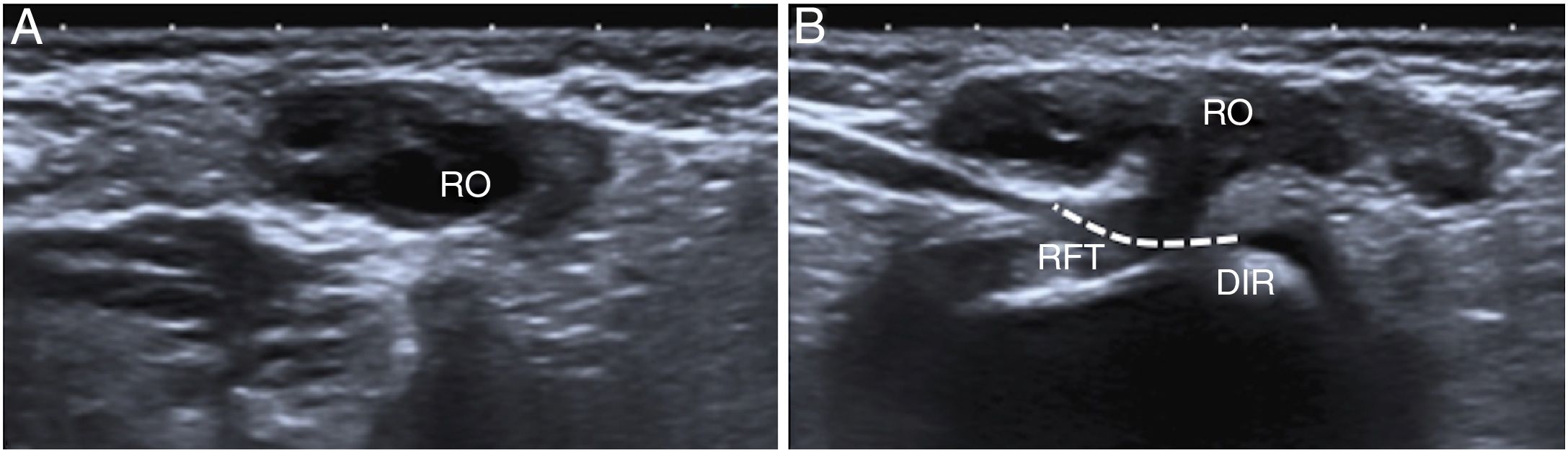
A 3-month-old girl with a right Nuck hernia with the ovary inside. (A) Ultrasound in B mode on the axial plane showed the right ovary (RO) in the inguinal canal. (B) The passage of the right Fallopian tube (RFT) through the deep inguinal ring (DIR) is identified on the sagittal plane.
These are abnormal collections in the vaginal process. They are classified as communicating or non-communicating depending on whether or not have a communication, or opening, into the peritoneal cavity. Communicating hydroceles are more common and are usually associated with indirect inguinal hernias.3
In neonates, the majority are congenital.10 They affect 4.7% of newborns11,12 and in general they resolve spontaneously before the age of 2.10–12 In older boys and adolescents they may be idiopathic or secondary to orchiepididymitis, trauma, tumours, testicular torsion, etc.10
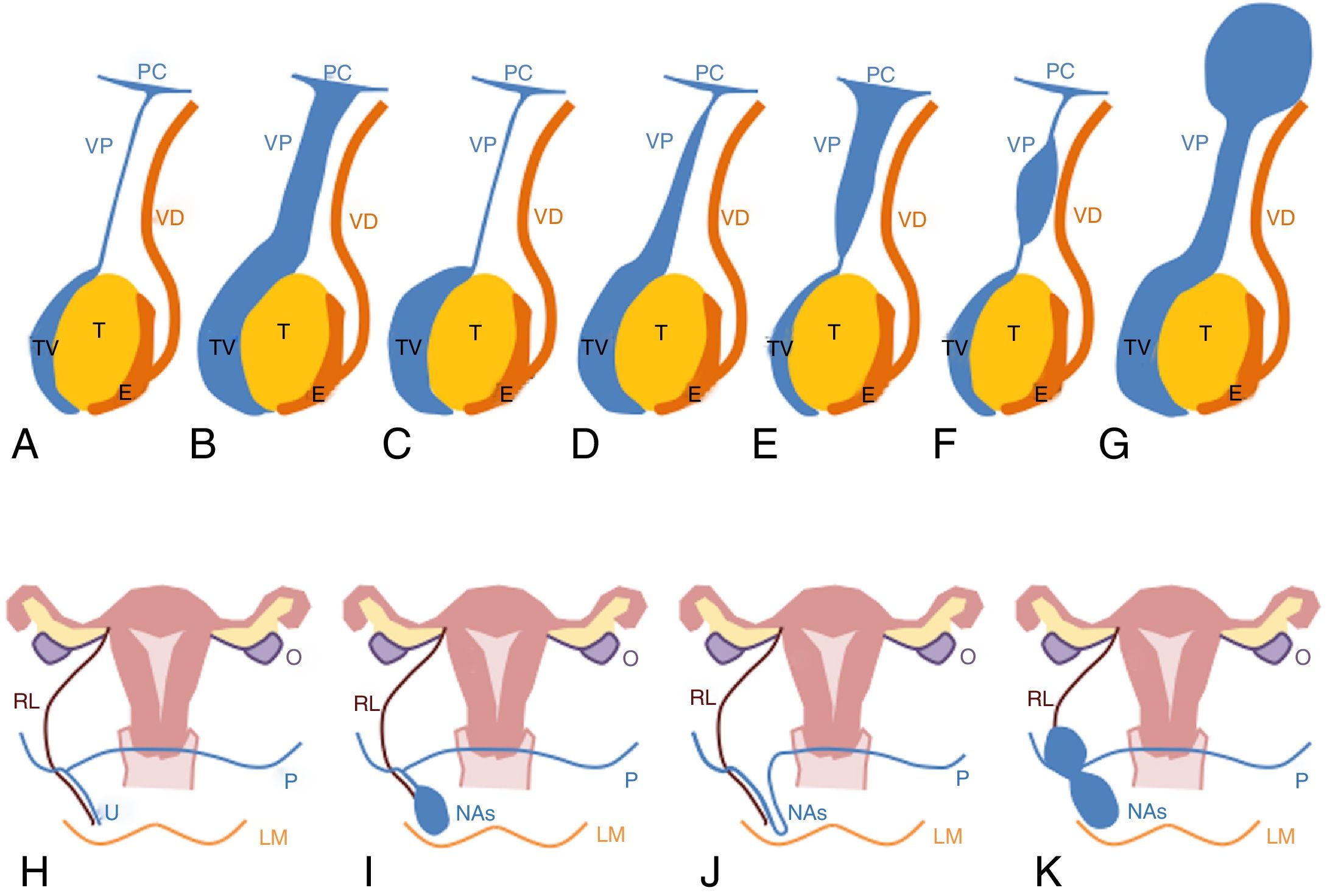
In boys, a distinction is made between several types of congenital hydroceles,4,13 which can be diagnosed with ultrasound (Fig. 5A–G). In a communicating hydrocele, the entire length of the vaginal process is permeable. When it is found to be entirely obliterated, it is called a scrotal or adult hydrocele.13
Diagram of hydrocele types. (A) Representation of a normal boy with the vaginal process obliterated (T: testis, E: epididymis, VD: vas deferens, VP: vaginal process, TV: tunica vaginalis, PC: peritoneal cavity). (B) Communicating hydrocele. (C) Scrotal or adult hydrocele. (D) Inguinoscrotal hydrocele. (E) Funicular hydrocele. (F) Cord cyst. (G) Abdominoscrotal hydrocele. (H) Representation of a normal girl with the vaginal process obliterated (U: uterus, O: ovary, RL: round ligament, LM: labia majora, P: peritoneum, VP: vaginal process). (I–K) Canal of Nuck abnormalities (NAs) Types I, II and III, respectively.
An inguinoscrotal hydrocele affects the testicular tunica vaginalis and part of the vaginal process (Figs. 5D and 6B). When the collection is located in the vaginal process and does not extend towards the scrotum, it is a spermatic cord hydrocele. If it has a communication into the peritoneum, then it is called a funicular hydrocele; if it does not, then it is a cord cyst (Fig. 7).
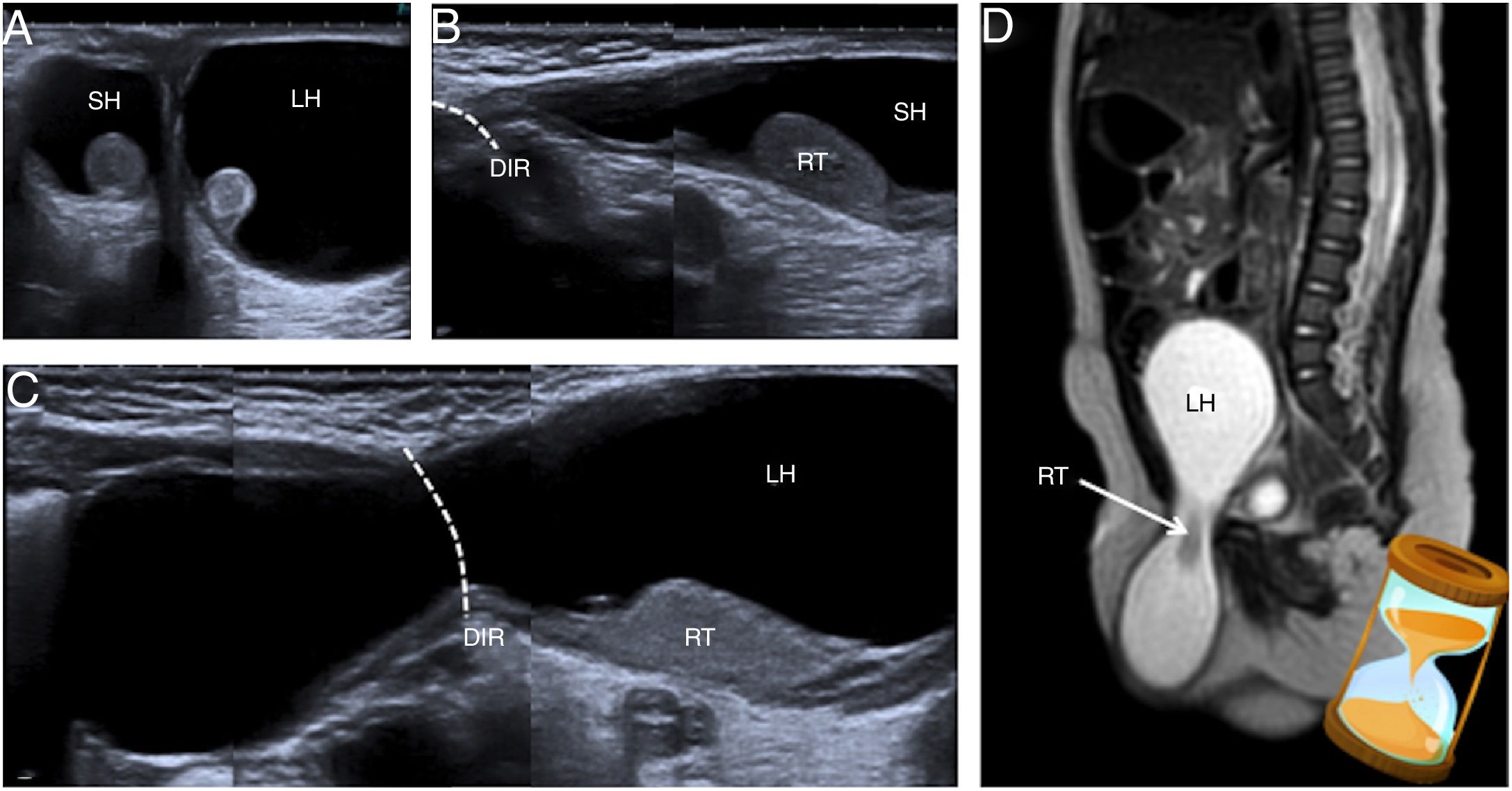
A 5-month-old boy with a right inguinoscrotal and left abdominoscrotal hydrocele. (A) Ultrasound on the transversal plane shows the hydrocele adjacent to the testes (right hydrocele [RH] and left hydrocele [LH]). (B and C) The images prepared on the plane of the inguinal canals shows that the right hydrocele (RH) extends to the deep inguinal ring (DIR) and the left hydrocele (LH) continues with a proximal abdominal cystic cavity in relation to said ring (DIR). (D) Similar findings are identified on magnetic resonance imaging: on the T2-enhanced imaging of the sagittal plane presented, the large left abdominoscrotal hydrocele (LH) with its typical hourglass morphology is delimited.
If the hydrocele shows an inguinoscrotal component and another abdominal component such that it has an hourglass morphology, it is called an abdominoscrotal hydrocele, which is the least common type (Fig. 6). When the abdominal component is large in size, it may cause complications due to a mass effect, such as obstructive uropathy or oedema of the ipsilateral lower limb.14
Congenital hydroceles are rare in girls and are known as abnormalities of the canal of Nuck. A distinction is made between three types4,15,16 (Fig. 5H–K). Type 1 is the most typical and consists of a collection in the canal of Nuck with no communication into the peritoneum. In Type 2, the canal of Nuck is completely permeable. Type 3, like abdominoscrotal hydroceles, is the least common and exhibits an hourglass morphology.
CryptorchidismA testis is considered cryptorchid, or undescended, if is it not found in the scrotum at the age of 4 months, since after that age spontaneous descent is rare. In fact, it is recommended that surgical descent of cryptorchid testes be performed as early as possible before 2 years of age, since it improves their growth and function.17
Cryptorchidism is the most common genital abnormality17–19 and may be caused by a failure in the descent of the testis from the abdomen or, in rare cases, by the location of the testis outside of this trajectory, in which it is called an ectopic testis.3
This condition affects 1%–3% of full-term newborns and 30% of prematurely born infants,19 and it tends to be unilateral.3 In 70% of patients the testis is identified in the inguinal canal, in 20% of cases it is adjacent to the superficial inguinal ring, and in 10% of patients it is intra-abdominal.20
The importance of its early diagnosis lies in its association with the development of testicular tumours and in its risk of infertility, torsion and trauma.17
The use of imaging techniques is debated. Some studies have not shown ultrasound to be of any benefit in locating undescended testes, given that sensitivity and specificity are low, 45% and 78%, respectively.19,21 However, other authors such as Vijayaraghavan have advocated for the use of this technique to select the surgical approach.22
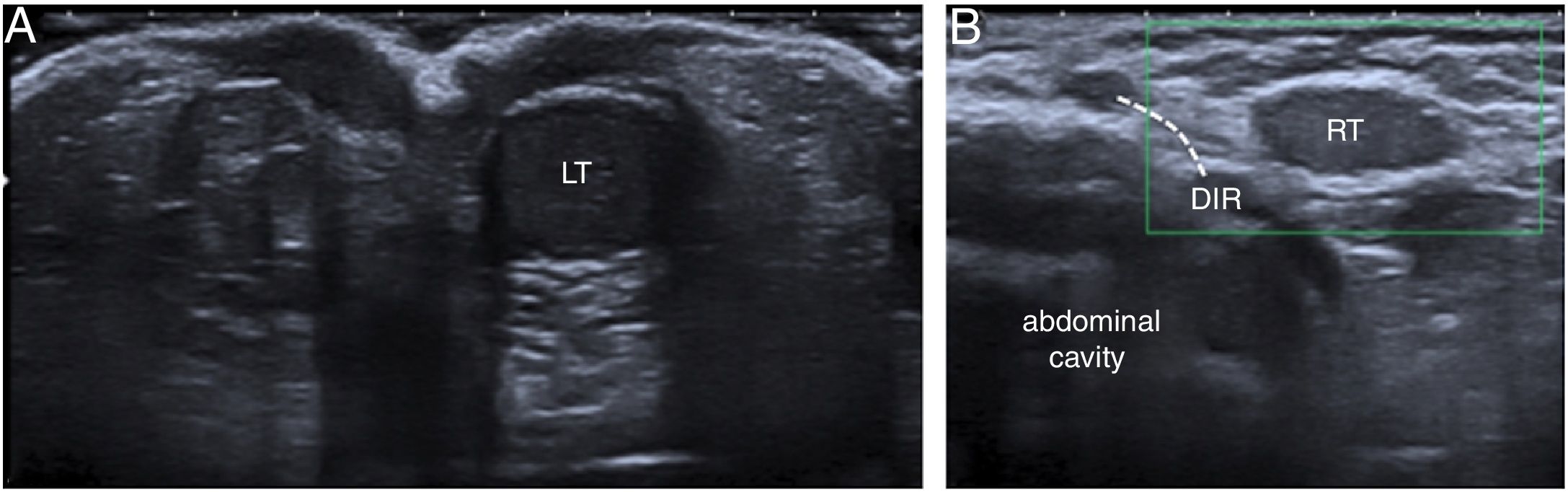
The appearance on ultrasound of undescended testes is variable, since they may be homogeneous, hypoechogenic (Fig. 8) or hyperechogenic and they may have calcifications or be heterogeneous. In the latter case, Doppler imaging is important to rule out an underlying neoplasm.3
An 8-month-old boy with right cryptorchidism. (A) Ultrasound on the axial plane at the height of the scrotum showing only the left testis (LT). (B) Sagittal image at the height of the right deep inguinal ring (DIR) showing the testis on that side (RT) in the inguinal canal. Colour Doppler imaging did not manage to identify flow in that testis, probably due to low physiological flow given the absence of symptoms suggestive of complication.
MRI, including morphological sequences and diffusion weighted imaging, has high sensitivity and specificity (89.5% and 87.5%, respectively),19 but studies supporting its widespread use have not yet been conducted.
Prepubertal boys with properly descended testes often present retractile or hypermobile testes: these acquire a suprascrotal position with the cremasteric reflex, but unlike cryptorchid testes they can be descended manually.23
LymphadenopathyThe groin features physiological nodes that drain lymph from the lower limbs, pelvis and genitals.
As in other locations, they appear on ultrasound as hypoechoic oval structures with a hyperechoic central hilum and flow on Doppler imaging.
They can become enlarged in tumour diseases such as leukaemia and lymphoma or be reactive to infections of the perineum, genitals and ipsilateral lower limb.24,25 If in addition inflammation is present in the adjacent skin, the diagnosis will be inguinal lymphadenitis, whose aetiology tends to be infectious.24
Ultrasound in lymphadenitis shows enlarged lymph nodes, sometimes with anechogenic areas in relation to necrosis.25 There may also be hyperechogenicity and increased flow from adjacent subcutaneous cellular tissue (Fig. 9).
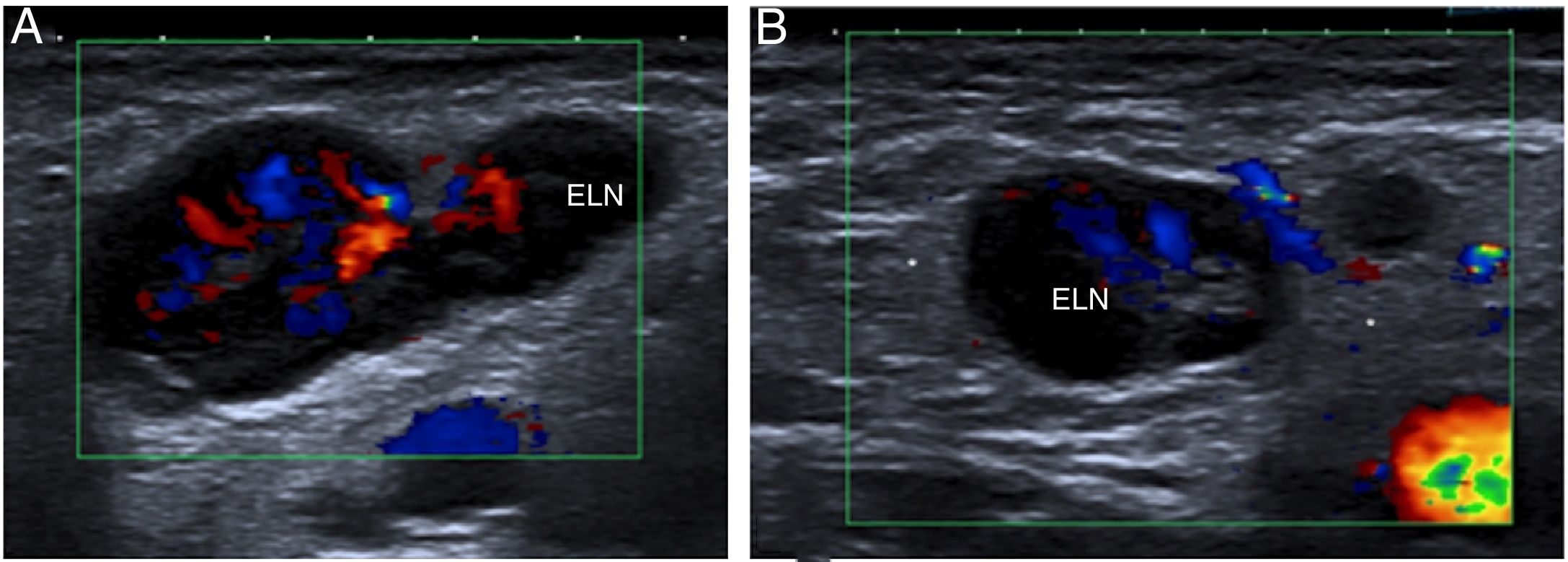
A 12-year-old boy with right inguinal lymphadenitis confirmed with ultrasound. The images show one enlarged lymph node (ELN) on the longitudinal and transversal planes (A and B, respectively) with a preserved structure, but a prominent size and marked flow on colour Doppler imaging. They also show areas of slightly increased echogenicity of adjacent fat (*) in relation to inflammatory changes.
Inguinal tumours are rare and comprise multiple lineages due to the large quantity of structures that converge in that area.3,24
The most common benign tumour in the general population is lipoma, but in children other entities should be considered such as fibrous hamartoma of infancy in those under 1 year of age (Fig. 10), lipoblastoma in those under 3 years of age and plexiform neurofibroma in patients with Type 1 neurofibromatosis.
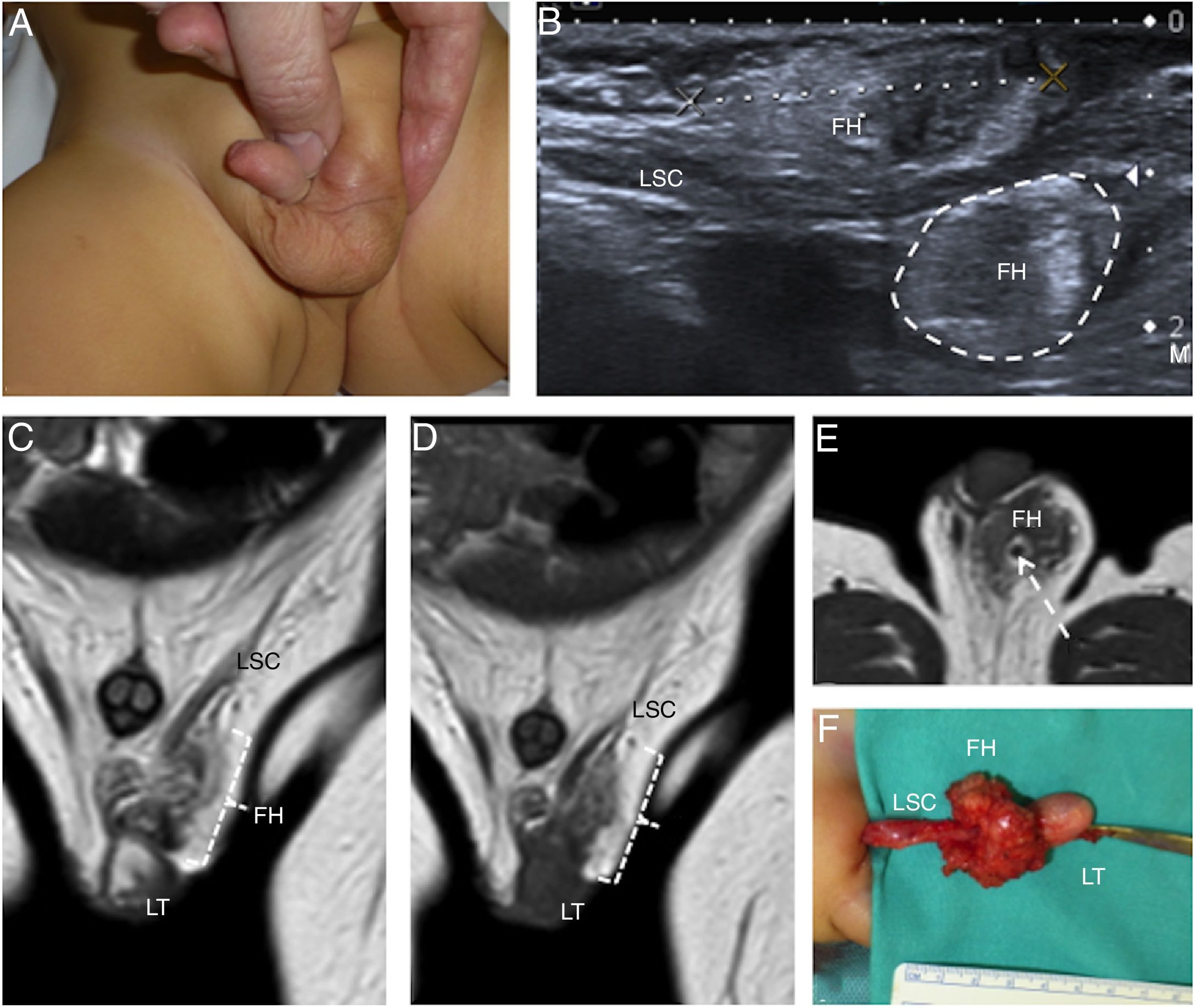
A 1-year-old boy with an inguinal fibrous hamartoma. (A) The photograph signals left inguinal swelling. (B) Ultrasound image on the plane of the inguinal canal showing a poorly defined mass (FH) around the left spermatic cord (LSC) with a superficial component marked with callipers and a deep component marked with a dotted line. Note the echostructure of the slightly heterogeneous lesion. (C and D) T2- and T1-enhanced, respectively, coronal magnetic resonance imaging (MRI) images confirming the presence of a mass (FH) adjacent to the left testis (LT) and to the spermatic cord (LSC), hypointense in both sequences and with some elongated areas isointense with fat. (E) The T1-enhanced axial MRI image best identifies the relationship of the tumour (FH) to the spermatic cord (LCS). (E) Surgical photograph showing the testis (LT), spermatic cord (LSC) and mass (FH).
Malignant tumours tend to be sarcomas, since most spermatic cord structures derive from mesodermic tissues,25 especially rhabdomyosarcomas in children under 3 years of age.
Imaging techniques help to locate the lesion, but they do not enable a definitive diagnosis to be made.3,5 However, they may demonstrate important findings, since benign tumours are usually homogeneous and well-defined, and malignant tumours are usually heterogeneous with areas of necrosis.
In addition, MRI determines the presence of intratumoural fat; this information is important since lipomas are typically isointense with fat and only present a thin fibrous capsule and a thin septum. Fibrous hamartoma of infancy and lipoblastoma also present fat, but with other components. The former shows hypointense fibrous tissue in T1- and T2-enhanced sequences, and the latter shows immature fat and myxoid stroma which may be so abundant that the fat is not detected.26,27
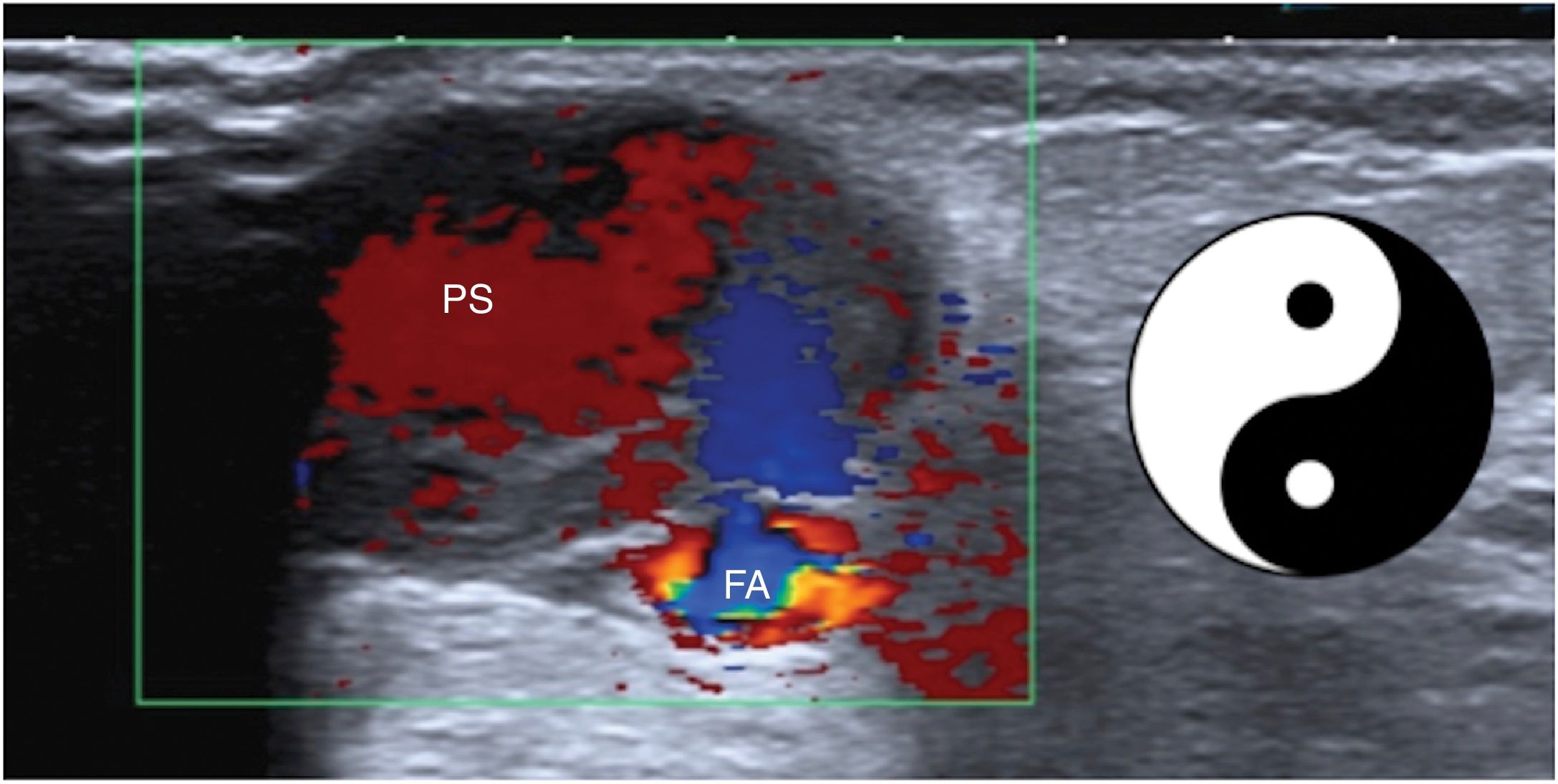
Vascular lesionsMost notable are complications of catheterisations of the femoral artery such as haematomas and pseudoaneurysms (Fig. 11).28 These present on ultrasound as cystic lesions with a communication into the arterial lumen. One blood component that enters the lesion and another that exits the lesion are identified in their interior, making them appear on colour Doppler imaging as a “yin-yang” sign.29
There may also be arteriovenous, lymphatic or venous paratesticular vascular malformations (Fig. 12) or mixed paratesticular vascular malformations.30
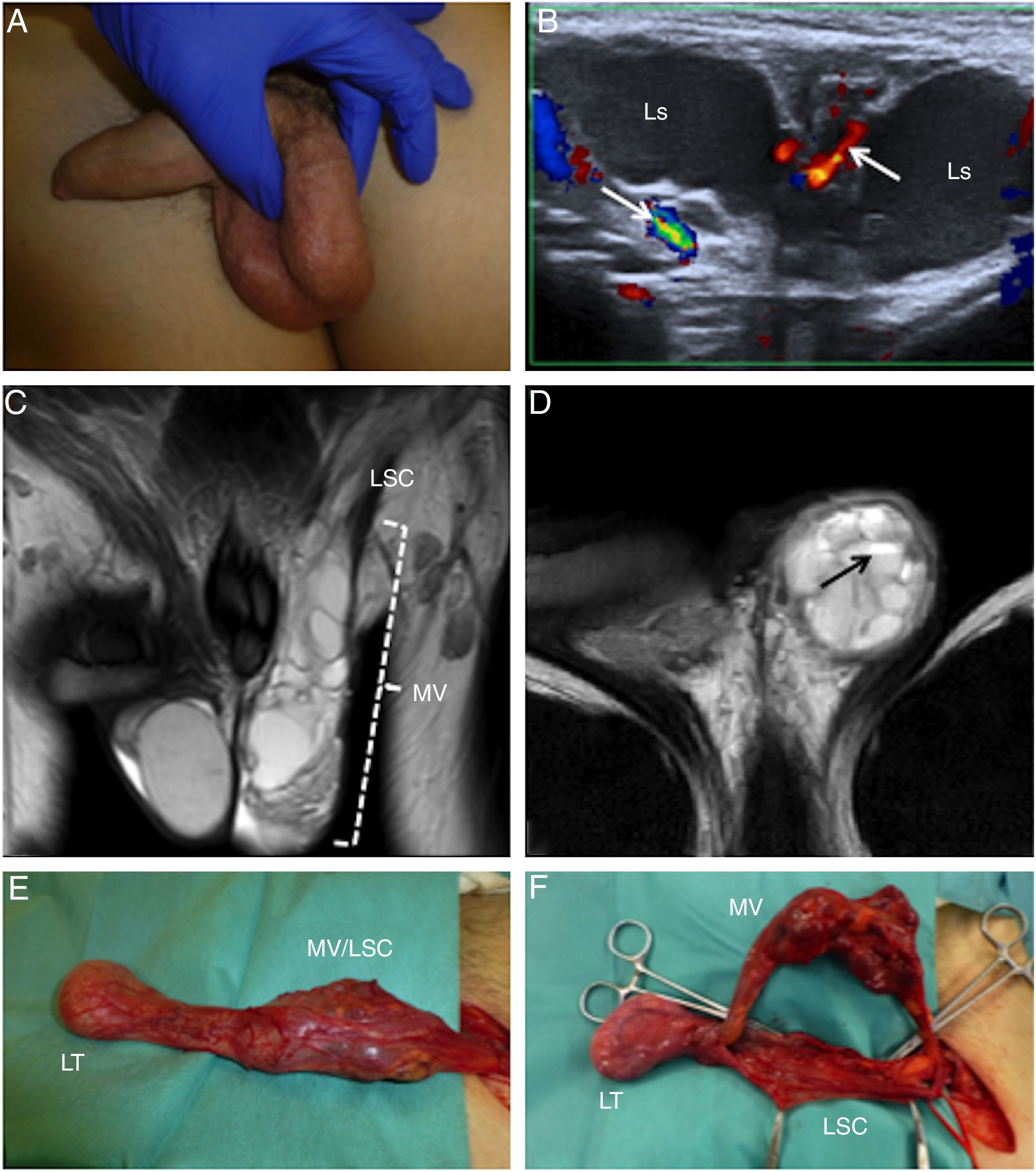
A 14-year-old boy with a left inguinoscrotal venous malformation. (A) The photograph signals the left parascrotal mass. (B) Doppler imaging shows a multiloculated cystic mass with vessels (arrows) between the locules (Ls). (C) T2-enhanced coronal magnetic resonance imaging (MRI) confirming the presence of an inguinoscrotal cystic mass (MV) in close relation to the left spermatic cord (LCS). (D) T2-enhanced axial MRI showing fluid–fluid levels (arrow). (E) Surgical photograph showing the testis (LT), spermatic cord (LSC) and mass (MV).
The groin may be the site of various diseases, and increasing numbers of imaging studies are required to evaluate them. Therefore, the radiologist should be familiar the differential diagnosis to consider and attempt to make maximum use of imaging techniques.
Authorship- 1.
Responsible for the integrity of the study: AAD.
- 2.
Study conception: AAD, MVTA, MMLP.
- 3.
Study design: AAD, MVTA, MMLP.
- 4.
Data acquisition: NA.
- 5.
Data analysis and interpretation: NA.
- 6.
Statistical processing: NA.
- 7.
Literature search: SBG and MGP.
- 8.
Drafting of the article: AAD and SBG.
- 9.
Critical review of the manuscript with intellectually significant contributions: MVTA, MMLP, SBG and MGP.
- 10.
Approval of the final version: AAD, MVTA, MMLP, SBG and MGP.
The authors declare that they have no conflicts of interest.
We would like to thank Dr Adolfo Bautista Casasnovaspor for his splendid surgical images and his efforts in reviewing the manuscript.
Please cite this article as: Arango-Díaz A, Trujillo-Ariza MV, Liñares-Paz MM, Baleato-González S, García-Palacios M. Lesiones inguinales pediátricas: hallazgos radiológicos. Radiología. 2020;62:188–197.











![A 5-month-old boy with a right inguinoscrotal and left abdominoscrotal hydrocele. (A) Ultrasound on the transversal plane shows the hydrocele adjacent to the testes (right hydrocele [RH] and left hydrocele [LH]). (B and C) The images prepared on the plane of the inguinal canals shows that the right hydrocele (RH) extends to the deep inguinal ring (DIR) and the left hydrocele (LH) continues with a proximal abdominal cystic cavity in relation to said ring (DIR). (D) Similar findings are identified on magnetic resonance imaging: on the T2-enhanced imaging of the sagittal plane presented, the large left abdominoscrotal hydrocele (LH) with its typical hourglass morphology is delimited. A 5-month-old boy with a right inguinoscrotal and left abdominoscrotal hydrocele. (A) Ultrasound on the transversal plane shows the hydrocele adjacent to the testes (right hydrocele [RH] and left hydrocele [LH]). (B and C) The images prepared on the plane of the inguinal canals shows that the right hydrocele (RH) extends to the deep inguinal ring (DIR) and the left hydrocele (LH) continues with a proximal abdominal cystic cavity in relation to said ring (DIR). (D) Similar findings are identified on magnetic resonance imaging: on the T2-enhanced imaging of the sagittal plane presented, the large left abdominoscrotal hydrocele (LH) with its typical hourglass morphology is delimited.](https://static.elsevier.es/multimedia/21735107/0000006200000003/v1_202005140641/S2173510720300276/v1_202005140641/en/main.assets/thumbnail/gr6.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)







