To assess the importance of false-negative and false-positive findings in computed tomography (CT) and 18F-FDG positron emission tomography (PET) in mediastinal lymph node staging in patients undergoing surgery for non-small cell lung cancer (NSCLC).
Material and methodsThis retrospective study included 113 consecutive patients and 120 resected NSCLCs; 22 patients received neoadjuvant treatment. We compared the findings on preoperative 18F-FDG PET-CT studies with the postoperative pathology findings. Lymph node size and primary tumor size were measured with CT, and lymph nodes and primary tumors were evaluated qualitatively and semiquantitatively (using standardized uptake values (SUVmax)) with PET.
ResultsMetastatic lymph nodes were found in 26 (21.7%) of the 120 tumors and in 41 (7.7%) of the 528 lymph node stations analyzed. 18F-FDG PET-CT yielded 53.8% sensitivity, 76.6% specificity, 38.9% positive predictive value, 85.7% negative predictive value, and 71.7% diagnostic accuracy. The false-negative rate was 14.2%. Multivariable analysis found that the factors associated with false-negative findings were a moderate degree of differentiation in the primary tumor (p=0.005) and an SUVmax of the primary tumor >4 (p=0.027). The false-positive rate was 61.1%, and the multivariable analysis found that lymph node size >1cm was associated with false-positive findings (p<0.001).
ConclusionsIn mediastinal lymph node staging in patients with NSCLC, 18F-FDG PET-CT improves the specificity and negative predictive value and helps clinicians to select the patients that will benefit from surgery. Given the high rate of false positives, histological confirmation of positive cases is recommendable.
Valorar las implicaciones de los falsos negativos (FN) y de los falsos positivos (FP) de la tomografía computarizada (TC) y la tomografía por emisión de positrones (PET) con fluorodesoxiglucosa (18F-FDG) en nuestro medio en la estadificación ganglionar mediastínica de pacientes operados de carcinoma de pulmón de células no pequeñas (CPCNP).
Material y métodosEstudio retrospectivo de 113 pacientes consecutivos con 120 CPCNP operados; 22 pacientes recibieron tratamiento neoadyuvante. Se compararon los resultados obtenidos en la 18F-FDG PET-TC prequirúrgica con los patológicos. Se analizaron el tamaño ganglionar y del tumor primario en la TC, y su valoración cualitativa y semicuantitativa (SUVmáx) en la PET.
ResultadosSe encontraron ganglios metastásicos en el 21,7% de los 120 tumores y en el 7,7% de las 528 estaciones ganglionares analizadas. La 18F-FDG PET-TC en el estudio por tumor mostró una sensibilidad del 53,8%, una especificidad del 76,6%, un valor predictivo positivo del 38,9%, un valor predictivo negativo del 85,7% y una precisión diagnóstica del 71,7%. La tasa de FN fue del 14,2%. El análisis multivariable mostró que un grado de diferenciación moderado del tumor primario (p = 0,005) y una SUVmáx del tumor primario >4 (p = 0,027) eran los factores asociados con los FN. La tasa de FP fue del 61,1% y el tamaño ganglionar >1cm era el factor asociado con los FP (p < 0,001).
ConclusionesLa 18F-FDG PET-TC en la estadificación ganglionar mediastínica de pacientes con CPCNP mejora la especificidad y el valor predictivo negativo, y ayuda al clínico a seleccionar los pacientes que se beneficiarán de la cirugía. Dada la elevada tasa de FP, es recomendable que, antes de excluir a pacientes de la cirugía, se confirmen histológicamente los casos positivos.
Lung cancer is the first cause of cancer-related death in the world; in the year 2012 it caused 1.6 million deaths.1 Mean survival at five years in Europe is 12–14 per cent. 80–87 per cent are non-small cell carcinomas (NSCC) that basically include the histological subtypes of adenocarcinoma, epidermoid or squamous-cell carcinoma, and large-cell carcinoma. The remaining 15–20 per cent are small-cell carcinomas.2
It is essential to perform an accurate, detailed staging of the disease or order to choose the most suitable treatment and be able to make a prognosis. In patients with NSCC who do not have distant metastasis, the most important factor influencing the therapeutic decision is mediastinal lymph node damage, since it allows us to distinguish among patients with resectable tumors at initial stages of the disease (stages I and II), stage IIIA patients with potentially resectable tumors and the rest of patients with unresectable stage-III tumors.3
Computed tomography (CT) is the most widely used imaging modality when staging NSCC. It provides us with excellent anatomical and morphological information of the primary tumor and the infiltration of neighboring structures, but it has limited sensitivity (51–70 per cent)4,5 in lymph node staging when ganglion damage is not bulky, since the only criterion used is ganglion size.
Positron emission tomography (PET) with fluorodeoxyglucose (18F-FDG) provides us with additional metabolic information, giving it a greater sensitivity and specificity than the CT for the detection and characterization of lymph node metastases. Several meta-analyses5–8 have reported a 79–90 per cent sensitivity and a 89–91 per cent specificity when it comes to differentiating patients with lymph node damage N0-1 from N2-3.
Since the year 2001, the multimodality technique 18F-FDG PET-CT has replaced the PET in the staging of NSCC. 18F-FDG PET-CT improves spatial resolution of PET and it is more accurate than CT or PET separately in staging NSCC. Also, the 18F-FDG PET-CT is useful in the selection of the most convenient invasive diagnostic procedure and the most suitable treatment, and to rule out distant metastatic damage.9–11 Although countless papers have demonstrated the role of 18F-FDG PET-CT in the detection of lymph node metastasis in NSCC12–17 and its cost-effectiveness relation has been validated,18 the incidence of hidden lymph node metastases that do not show 18F-FDG uptake in patients with NSCC is around 7–16 per cent19,20 and the rate of false positives (FP) due to inflammatory or granulomatous lesions continues to be a problem in PET- CT scans.21
The goal of this paper is to assess the validity in our center of 18F-FDG PET-CT lymph node staging of patients undergoing surgery for NSCC, whether they have received or not induction treatment prior to the surgery, and assess the characteristics of false negatives (FN) and the FP found.
Material and methodsThis work has been approved by our hospital ethics committee in compliance with the Declaration of Helsinki, 2013. All patients signed a prior written informed consent to be part of the 18F-FDG PET-CT scan.
A retrospective, descriptive study of case series was designed, with transversal evaluation regardless of the methods studied.
Selection of patients157 consecutive patients who had been diagnosed with NSCC and undergone surgery at our hospital thoracic surgery unit between May, 2011 and December 2012 were included retrospectively. Forty-four of these patients were excluded because their PET-CT scan was not performed in our hospital, because they were claustrophobic or because a mediastinal lymph node resection could not be performed at the moment of the surgery due to limited pulmonary function. Therefore, 113 patients with 120 NSCCs make up the basis of this work (five patients had two tumors and one patient had three primary tumors).
Of the 113 patients, 22 received neoadjuvant treatment, all of them with chemotherapy, and four of them with additional radiotherapy.
The results obtained in the pre-surgical 18F-FDG PET-CT were analyzed in all patients and the pathological findings were compared. In those patients who received neoadjuvant treatment who had been part of two different studies - before and after therapy, the post-therapy PET-CT outcome was considered, since it was the closest to surgery.
Definition of variablesFor each lymph node station, the 18F-FDG PET-CT studies analyzed the size on the CT (diameter of the lymph node short axis >1cm were considered pathological), and the qualitative or visual and semiquantitative assessment through the SUVmax of the lymph node on the PET. Any SUVmax >2.5 were considered positive. In each primary lung tumor, the histology, the degree of cellular differentiation, the location, the size and the SUVmax were assessed. Also all extrathoracic pathological uptakes were assessed and the possibility that it was a metastatic lesion or a second primary tumor was ruled out though puncture –aspiration/percutaneous biopsy, endoscopic ultrasound (EUS), ultrasound or control CT, or surgery. Also, the lymph node component (N) was analyzed in each tumor and the presurgical clinical staging conducted based on the 18F-FDG PET-CT findings, comparing such staging with the pathological lymph node staging obtained after the surgery. The TNM classification (7ª ed., 2009)22 was used. Hilar damage N1 could be determined when there were positive lymph nodes in the hilar region on the PET-CT; mediastinal damage N2 when homolateral lymph nodes were found with pathologic uptake, with or without hilar damage.
PET-CT scansThey were performed using one Siemens Biograph TruePoint machine (Siemens Medical Solutions, Erlanger, Germany) with a six-row detector CT. The procedure followed was the one recommended by the European Association of Nuclear Medicine.23
All patients fasted for at least 6h. It was verified that their blood glucose levels were within the reference limits before the administration of 18F-FDG. The 18F-FDG dose administered was normally 370MBq. After the injection of 18FDG, the patients remained at rest for around 60min in a room prepared to this end.
First, a chest CT study was obtained at inspiration with a slice thickness of 2.5mm, 60mA and 110 KV, with a tube rotation time of 0.6s and a 1.2 pitch. Then another body CT study was obtained from the base of the skull to the root of the thigh in a cranial-caudal direction using a free-breathing respiration signal. IV iodinated contrast was administered (130ml of Iohexol, Omnipaque®, 300mg/ml) in cases where patients had not undergone a previous contrast CT (one month before). Finally, the PET was performed on the same locations as the CT study, in free respiration. Acquisition time was 3min per bed (position of the bed); each bed equals 13.5cm of length in the cranial-caudal axis.
The 18F-FDG PET-CT scans were analyzed by a radiologist and a nuclear physician with over 10 years of experience, in a prospective way and by consensus. The data provided by the CT and the PET were merged in the working station using the operating system Syngo™ software system (Siemens Medical Imaging, Forchheim, Bavaria, Germany). The regions of interest (ROI) were manually placed on all lung tumors and damaged nodes, while the maximum glucose standard uptake values (SUVmax) were registered.
Surgical-pathological stagingAll surgeries were performed by the same team of thoracic surgeons. Systematic mediastinal lymph node dissections were performed in each case. The mediastinal and hilar lymph nodes removed were tagged and sent to the Anatomic Pathology Service separately in lymph node stations, based on the lymph node map proposed by the International Association for the Study of Lung Cancer.24 Also, the intrapulmonary lymph nodes (stations 12, 13 and 14) were removed with the surgical piece.
Seven out of the 113 patients underwent one endoscopic can prior to the endoscopic ultrasound-guided (EUS) surgery, and 13 patients underwent one mediastinoscopy; both modalities assessed the positive findings of the 18F-FDG PET-CT scan. When data were gathered to conduct this work, the endobronchial ultrasound-guided (EBUS) technique was not available in our center.
All tumors resected were examined by experienced pathologists in lung examination who did not know the results from the 18F-FDG PET-CT scans. The number of lymph nodes in each station studied was informed separately,24 as well as the number of lymph nodes with tumor damage. Both the 2004 World Health Organization classification25 and the 2011 adenocarcinoma classification26 were followed for the histological classification of primary lung tumors.
Statistical analysisThe capacity of the 18F-FDG PET-CT scan was assessed by calculating its sensitivity and specificity indexes, positive predictive value (PPV), negative predictive value (NPV), diagnostic precision and positive likelihood ratio (PLR) and negative likelihood ratio (NLR). Ninety-five per cent confidence intervals (95 per cent CI) were calculated. The logistic regression models were adjusted to assess the differences between true positives (TP) and FP, true negatives (TN) and FN, after performing one univariate analysis using the chi square test. The null hypothesis was rejected with p<0.05. The analysis was performed using the statistical software SPSS 15.0.
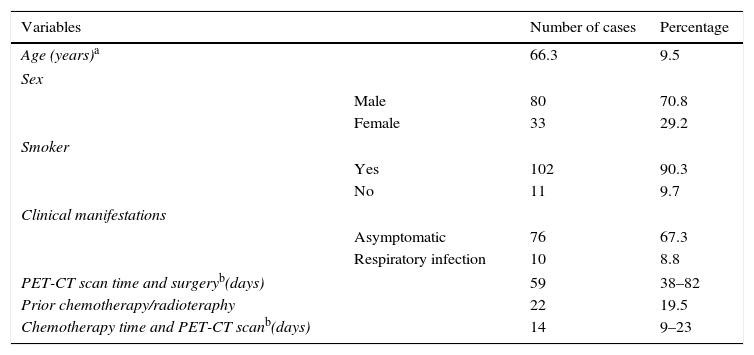
ResultsPatients120 NSCC and 528 lymph node stations of 113 patients were analyzed. Metastatic mediastinal or hilar lymph nodes were found in 26 of the 120 tumors (21.7 per cent) and in 41 of the 528 lymph node stations (7.7 per cent). The clinical characteristics of the 113 patients can be seen in Table 1.
Description of studied patients (n=113).
| Variables | Number of cases | Percentage | |
|---|---|---|---|
| Age (years)a | 66.3 | 9.5 | |
| Sex | |||
| Male | 80 | 70.8 | |
| Female | 33 | 29.2 | |
| Smoker | |||
| Yes | 102 | 90.3 | |
| No | 11 | 9.7 | |
| Clinical manifestations | |||
| Asymptomatic | 76 | 67.3 | |
| Respiratory infection | 10 | 8.8 | |
| PET-CT scan time and surgeryb(days) | 59 | 38–82 | |
| Prior chemotherapy/radioteraphy | 22 | 19.5 | |
| Chemotherapy time and PET-CT scanb(days) | 14 | 9–23 | |
The average age of patients was 66.3 years. 70.8 per cent were men and 90.3 per cent were smokers or former smokers. 67.3 per cent were asymptomatic at the moment of diagnosis. Twenty-two (22) patients (19.5 per cent) had received neoadjuvant therapy prior to surgery. The median time elapsed between the PET-CT and the surgery was 58 days.
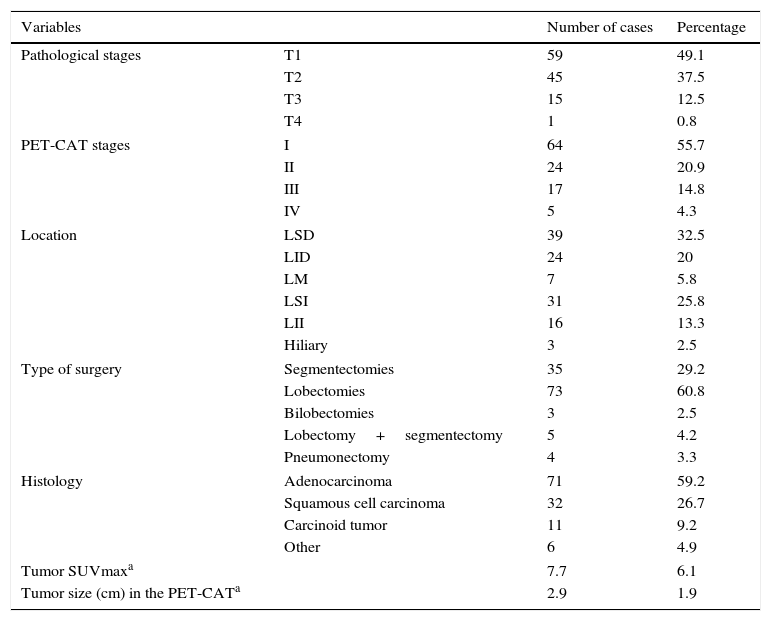
Among the 120 NSCCs, the most common histological subtype was the adenocarcinoma (71/120, 59.2 per cent), the average size was 2.9cm and the average SUVmax, 7.7. The TNM clinical stages determined through the PET-CT scan in the 120 tumors were: stage I in 64 tumors, stage II in 24, stage IIIA in 17, stage IIIB in five tumors (two by T4N2 and the other two by N3 damage; in the latter three, lymph node damage was ruled out through mediastinoscopy), and stage IV in five tumors (metastatic damage was not confirmed in any of them). In two cases we could not perform any clinical staging because the tumor was not detected on the PET-CT scan due to a complete response after the induction therapy. In other three cases, the clinical staging could not be performed adequately because patients had very nonspecific mediastinal and hilar pathological uptake; one of the patients underwent one mediastinoscopy that tested negative, and none of the three patients’ nodes tested positive in the pathological study. Table 2 shows the characteristics of lung tumors.
Characteristics of tumors (n=120).
| Variables | Number of cases | Percentage | |
|---|---|---|---|
| Pathological stages | T1 | 59 | 49.1 |
| T2 | 45 | 37.5 | |
| T3 | 15 | 12.5 | |
| T4 | 1 | 0.8 | |
| PET-CAT stages | I | 64 | 55.7 |
| II | 24 | 20.9 | |
| III | 17 | 14.8 | |
| IV | 5 | 4.3 | |
| Location | LSD | 39 | 32.5 |
| LID | 24 | 20 | |
| LM | 7 | 5.8 | |
| LSI | 31 | 25.8 | |
| LII | 16 | 13.3 | |
| Hiliary | 3 | 2.5 | |
| Type of surgery | Segmentectomies | 35 | 29.2 |
| Lobectomies | 73 | 60.8 | |
| Bilobectomies | 3 | 2.5 | |
| Lobectomy+segmentectomy | 5 | 4.2 | |
| Pneumonectomy | 4 | 3.3 | |
| Histology | Adenocarcinoma | 71 | 59.2 |
| Squamous cell carcinoma | 32 | 26.7 | |
| Carcinoid tumor | 11 | 9.2 | |
| Other | 6 | 4.9 | |
| Tumor SUVmaxa | 7.7 | 6.1 | |
| Tumor size (cm) in the PET-CATa | 2.9 | 1.9 | |
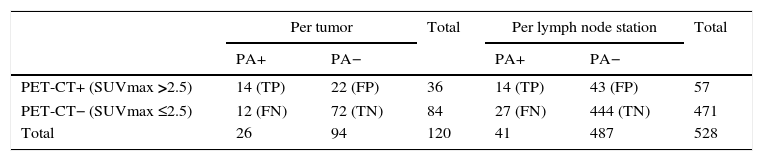
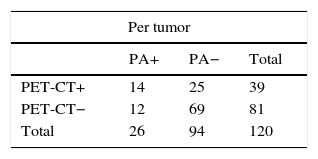
This modality correctly identified lymph node stage in 86 out of 120 tumors (71.6 per cent), overestimated lymph node stage in 22 tumors (FP, 22/36 [61.1 per cent]) and underestimated lymph node stage in 12 tumors (FN, 12/84 [14.2 per cent]) (Table 3).
Value of the PET-CT in lymph node staging.
| Per tumor | Total | Per lymph node station | Total | |||
|---|---|---|---|---|---|---|
| PA+ | PA− | PA+ | PA− | |||
| PET-CT+ (SUVmax >2.5) | 14 (TP) | 22 (FP) | 36 | 14 (TP) | 43 (FP) | 57 |
| PET-CT− (SUVmax ≤2.5) | 12 (FN) | 72 (TN) | 84 | 27 (FN) | 444 (TN) | 471 |
| Total | 26 | 94 | 120 | 41 | 487 | 528 |
PA: pathological anatomy; FN: false negative; FP: false positives; TN: true negatives; TP: true positives.
In the study per tumor, the 18F-FDG PET-CT scan showed these values: 53.8 per cent sensitivity (95 per cent CI: 34.7–73.0), 76.6 per cent specificity (95 per cent CI: 68.0–85.2), 38.9 per cent PPV (95 per cent CI: 23.0–54.8), 85.7 per cent NPV (95 per cent CI: 78.2–93.2), 71.7 per cent diagnostic precision (95 per cent CI: 63.6–79.7), 2.30 PLR (95 per cent CI: 1.38–3.83), 0.60 NLR (95 per cent CI: 0.38–0.95), and 21.7 per cent prevalence.
One sensitivity analysis was carried out in order to assess whether taking as a positive criterion on the 18F-FDG PET-CT scan the qualitative or visual analysis of lymph nodes (Table 4) or jointly one SUVmax value >2.5 or one short-axis diameter of the node >1cm (Table 5) the results previously obtained on the semiquantitative assessment of a SUVmax >2.5 would be modified. Similar values were obtained without any statistically significant differences.
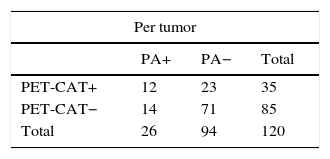
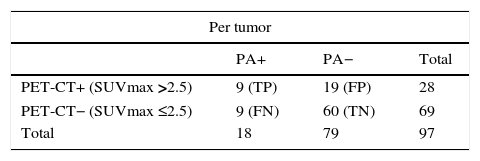
Likewise, the sensitivity, specificity, PPV, NPV and diagnostic precision of the 18F-FDG PET-CT scan in lymph node staging in the group of 97 tumors that had not been treated with neoadjuvant chemotherapy prior to surgery were determined, and no statistically significant changes were detected when they were compared to the total group of 120 tumors (p >0.05) (Table 6).
Value of the PET-CT in lymph node staging in patients with no prior neadjuvant therapy.
| Per tumor | |||
|---|---|---|---|
| PA+ | PA− | Total | |
| PET-CT+ (SUVmax >2.5) | 9 (TP) | 19 (FP) | 28 |
| PET-CT− (SUVmax ≤2.5) | 9 (FN) | 60 (TN) | 69 |
| Total | 18 | 79 | 97 |
PA: pathological anatomy; FN: false negative; FP: false positives; TN: true negatives; TP: true positives.
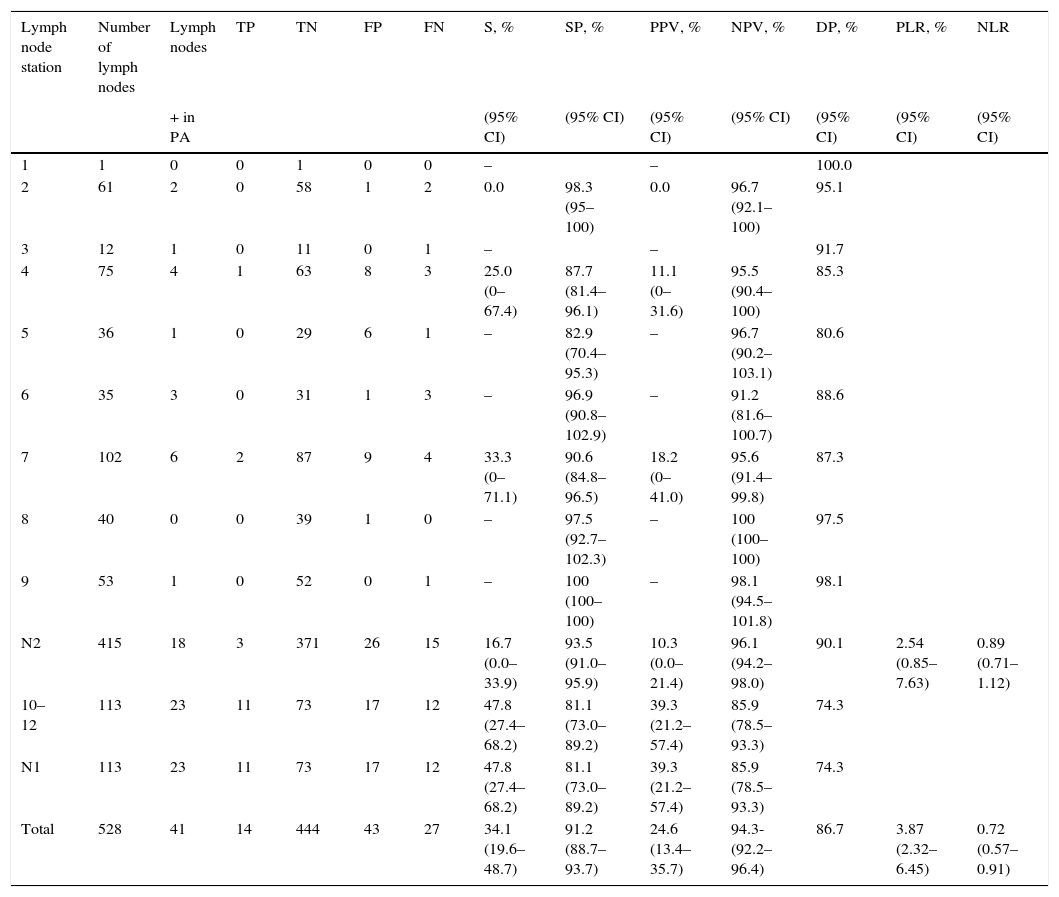
528 lymph node stations were evaluated: 113 were N1 and 415 were N2 (Table 7). The PET-CT correctly identified metastases in 14 of the 41 lymph node stations (34.1 per cent) (Fig. 1) and ruled out lymph node tumor damage in 444 of 487 (91.1 per cent) (Table 7). It correctly assessed lymph node damage in 458 of the 528 stations (86.7 per cent), overestimated 43 of 57 lymph node stations (75.4 per cent FP) and underestimated 27 of 471 lymph node stations (5.7 per cent FN) (Table 3).
Diagnosis per lymph node station, effectiveness of the 18F-FDG PET-CT scan.
| Lymph node station | Number of lymph nodes | Lymph nodes | TP | TN | FP | FN | S, % | SP, % | PPV, % | NPV, % | DP, % | PLR, % | NLR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + in PA | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | ||||||
| 1 | 1 | 0 | 0 | 1 | 0 | 0 | – | – | 100.0 | ||||
| 2 | 61 | 2 | 0 | 58 | 1 | 2 | 0.0 | 98.3 (95–100) | 0.0 | 96.7 (92.1–100) | 95.1 | ||
| 3 | 12 | 1 | 0 | 11 | 0 | 1 | – | – | 91.7 | ||||
| 4 | 75 | 4 | 1 | 63 | 8 | 3 | 25.0 (0–67.4) | 87.7 (81.4–96.1) | 11.1 (0–31.6) | 95.5 (90.4–100) | 85.3 | ||
| 5 | 36 | 1 | 0 | 29 | 6 | 1 | – | 82.9 (70.4–95.3) | – | 96.7 (90.2–103.1) | 80.6 | ||
| 6 | 35 | 3 | 0 | 31 | 1 | 3 | – | 96.9 (90.8–102.9) | – | 91.2 (81.6–100.7) | 88.6 | ||
| 7 | 102 | 6 | 2 | 87 | 9 | 4 | 33.3 (0–71.1) | 90.6 (84.8–96.5) | 18.2 (0–41.0) | 95.6 (91.4–99.8) | 87.3 | ||
| 8 | 40 | 0 | 0 | 39 | 1 | 0 | – | 97.5 (92.7–102.3) | – | 100 (100–100) | 97.5 | ||
| 9 | 53 | 1 | 0 | 52 | 0 | 1 | – | 100 (100–100) | – | 98.1 (94.5–101.8) | 98.1 | ||
| N2 | 415 | 18 | 3 | 371 | 26 | 15 | 16.7 (0.0–33.9) | 93.5 (91.0–95.9) | 10.3 (0.0–21.4) | 96.1 (94.2–98.0) | 90.1 | 2.54 (0.85–7.63) | 0.89 (0.71–1.12) |
| 10–12 | 113 | 23 | 11 | 73 | 17 | 12 | 47.8 (27.4–68.2) | 81.1 (73.0–89.2) | 39.3 (21.2–57.4) | 85.9 (78.5–93.3) | 74.3 | ||
| N1 | 113 | 23 | 11 | 73 | 17 | 12 | 47.8 (27.4–68.2) | 81.1 (73.0–89.2) | 39.3 (21.2–57.4) | 85.9 (78.5–93.3) | 74.3 | ||
| Total | 528 | 41 | 14 | 444 | 43 | 27 | 34.1 (19.6–48.7) | 91.2 (88.7–93.7) | 24.6 (13.4–35.7) | 94.3-(92.2–96.4) | 86.7 | 3.87 (2.32–6.45) | 0.72 (0.57–0.91) |
PA: pathological anatomy; NLR: negative likelihood ratio; PLR: positive likelihood ratio; SP: specificity; FN: false negative; FP: false positive; 95% CI: 95 per cent confidence; DP: diagnostic precision; TN: true negative; TP: true positive; NPV: negative predictive value; PPV: positive predictive value; S: sensitivity.
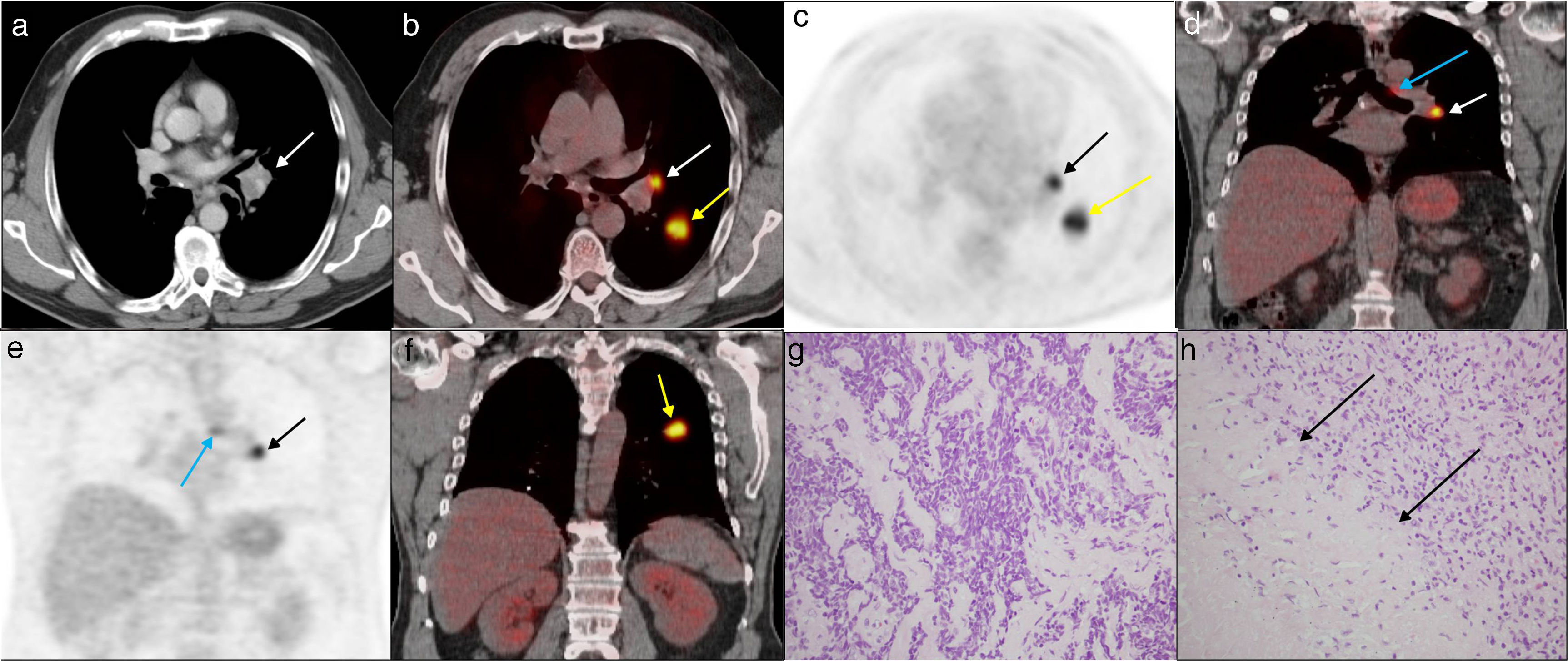
Fifty-six year-old male with NSCC (the diagnostic fine needle puncture aspiration of the primary tumor was indicative of adenocarcinoma). Case of trur positive. The images a, b and c on the axial plane of the staging 18F-FDG PET-CT scan show one left hilar adenopathy of 1.5cm, with a SUVmax of 16.3 (white and black arrows), and the primary tumor in the left lower lobe with a SUVmax of 21.5 (yellow arrows). In images d, e and f, on the coronal plane, we can see the 18-FDG uptake of the primary tumor, the left hilar adenopathy and of one <1cm-adenopathy in the aorto-pulmonary window (blue arrows) that did not show tumor infiltration and with a negative EUS. In the histology, tumor infiltration was detected in a left hilar lymph node (image g, hematoxylin-eosin staining [HE] 40×) and damage due to tuberculosis in another left hilar lymph node (image h, HE staining 40×) showing one large tuberculous granuloma with abundant necrosis (arrows). The Zhiel staining procedure tested positive.
Per lymph node station, the sensitivity of the 18F-FDG PET-CT scan was 34.1 per cent (95 per cent CI: 19.6–48.7), specificity 91.2 per cent (95 per cent CI: 88.7–93.7), PPV 24.6 per cent (95 per cent CI: 13.4–35.7), NPV 94.3 per cent (95 per cent CI: 92.2–96.4), diagnostic precision 86.7 per cent (95% CI: 83.8–89.6), PLR 3.87 (95 per cent CI: 2.32–6.45) and NRL 0.72 (95 per cent CI: 0.57–0.91), and prevalence, 7.8 per cent.
Cases of false negatives in the 18F-FDG PET-CT scan in the tumor study (n=120)One rate of FNs of around 14.2 per cent (12 tumors) was achieved. In all of them, hilar lymph node damage N1 could be found (in three of them only intrapulmonary pathological lymph nodes could be found, and they were resected with the surgical piece), and in five, in addition, N2 damage. In 7 of the 12 cases (58.3 per cent) lymph node staging went from N0 to N1, and in 5 of the 12 (41.6 per cent) from N0 to N2. The diameter of the metastatic node was ≤1cm in all (Fig. 2). Six tumors were adenocarcinomas slightly differentiated from the lung and one was an atypical carcinoid tumor. Five patients were diabetic, but their blood glucose levels were normal before the PET-CT scan.
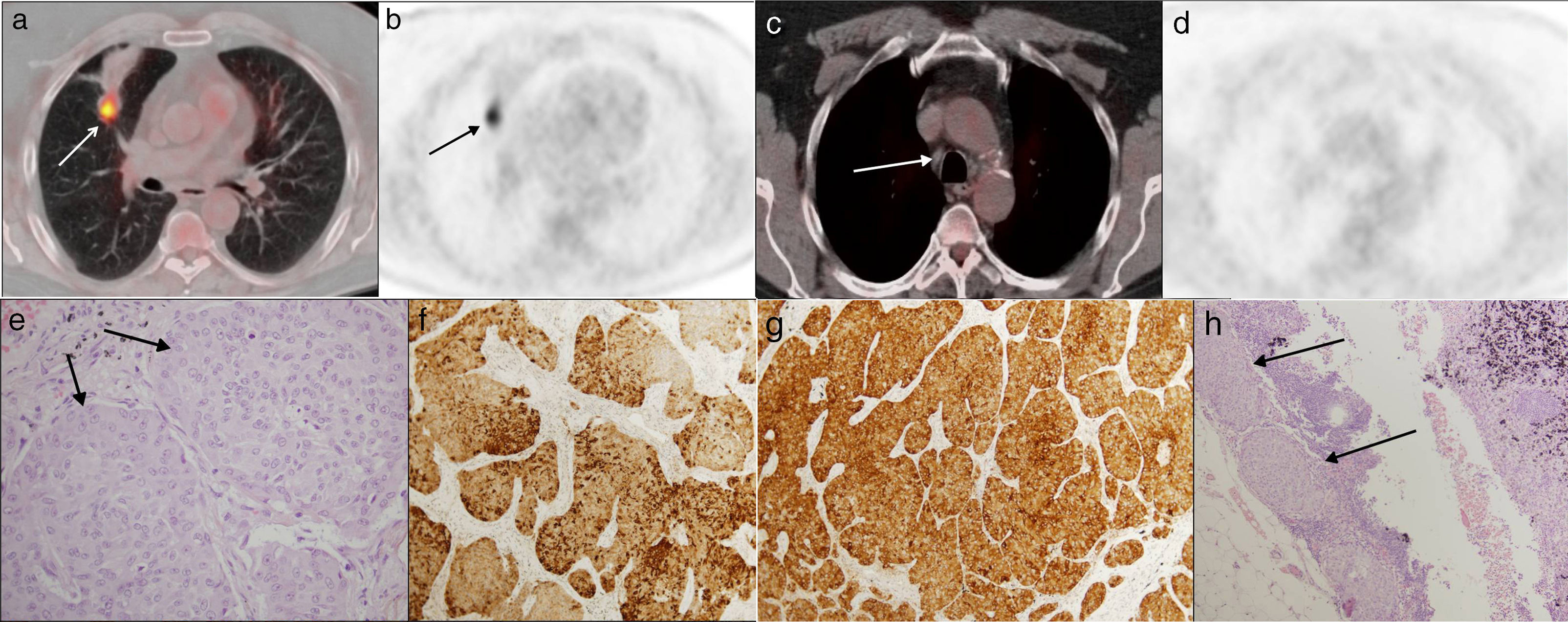
Fifty-seven year-old male. Incidental finding of one lymph node in the chest Rx: atypical carcinoid tumor. Case of false negative. In images a and b, on the axial plane of the staging 18F-FDG PET-CT scan, one primary tumor can be seen in the middle lobe, showing 18-FDG uptake (arrows) and distal atelectasis with no uptake. In axial images c and d one <1cm (white arrow) lower right paratracheal lymph node can be seen without 18-FDG uptake. Image e (hematoxylin–eosin staining [HE] 40×) shows the histology of the primary tumor, with cell nests with wide eosinophil cytoplasm and discrete nucleoli that are stained using the synaptophysin technique (image f, 10×) and the chromogranin technique (image g, 10×). Image h shows the histology of the lymph node with HE staining (10×), with metastatic cell nests of the carcinoid tumor (arrows).
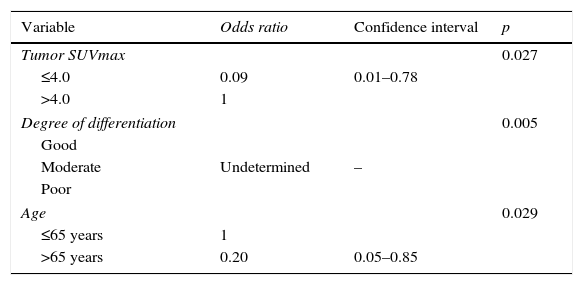
The causes for the FN on the PET-CT scan were analyzed as well as their association with age (under or over 65 years), the existence or not of tuberculous disease or intercurrent infection, the location of the primary tumor, histologic type, degree of cellular differentiation, primary tumor size (larger than or ≤3cm), SUVmax (larger or ≤4) and lymph node size (larger or ≤1cm). The multivariate logistic regression analysis showed that a moderate degree of differentiation of the primary tumor (p=0.005), age of the patient ≤65 years (p=0.029) and primary tumor SUVmax >4 (p=0.027) were all factors associated with FN when compared to TN (Table 8).
Multivariate analysis of factors associated with false negatives when compared to true negatives.
| Variable | Odds ratio | Confidence interval | p |
|---|---|---|---|
| Tumor SUVmax | 0.027 | ||
| ≤4.0 | 0.09 | 0.01–0.78 | |
| >4.0 | 1 | ||
| Degree of differentiation | 0.005 | ||
| Good | |||
| Moderate | Undetermined | – | |
| Poor | |||
| Age | 0.029 | ||
| ≤65 years | 1 | ||
| >65 years | 0.20 | 0.05–0.85 | |
A false negative rate of 61.1 per cent was found (22 tumors). In 7 of the 22 (31.8 per cent) there was more than one lymph node territory affected. In 11 of 22 cases, lymph node staging went from N1 to N0, and in the remaining 11 tumors it went from N2 to N0. The lymph node diameter was >1cm in 50 per cent of the cases, and lymph node SUVmax was ≤3 in 9 of the 22 cases. Histology was squamous-cell carcinoma in 11 cases. Three patients had clinical manifestations of respiratory infection at the moment of diagnosis. Four patients were administered neoadjuvant treatment prior to the surgery. In two patients, silico-anthracotic nodes were found in the pathological study, and in another patient, a sarcoid-type granulomatous reaction was identified (Fig. 3).
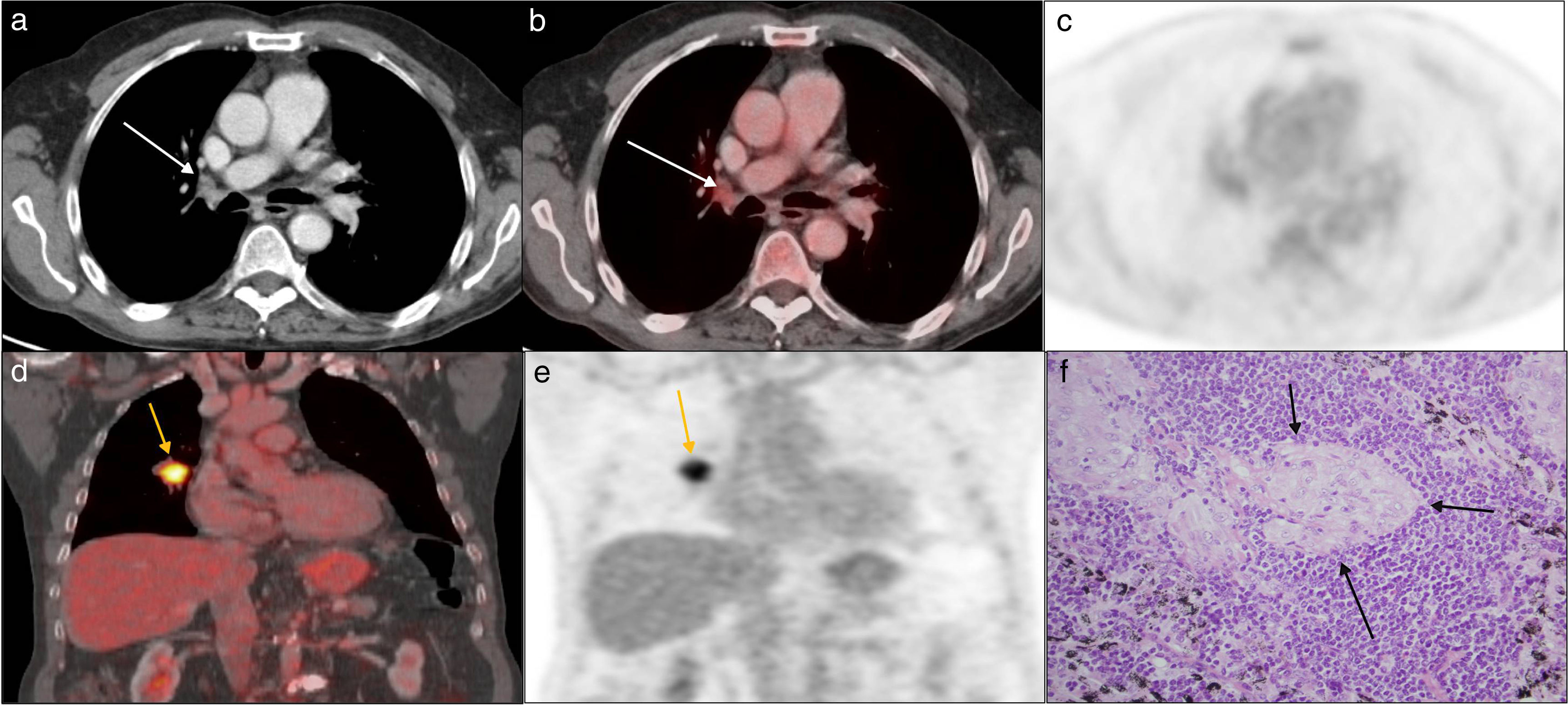
Seventy-eight year-old asymptomatic male. Incidental finding of one right hilar lung lymph node in presurgical chest X ray: lung adenocarcinoma. Case of false positive. In images a, b, and c, on the axial plane of the staging 18F-FDG PET-CT scan, one <1cm-right hilar lymph node can be seen, with a SUVmax of 3.5 (arrows); coronal images d and e show the core primary tumor, with a SUVmax of 17. In image f, corresponding to the histology of the lymph node (hematoxylin–eosin staining 40×), no tumor infiltration was found, yet several sarcoid granulomas were seen with epithelioid histiocytes intermingled with lymphocytes (arrows).
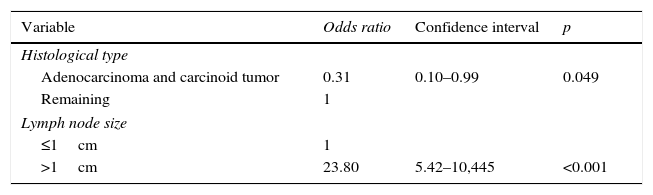
The causes for the cases of FP on the PET-CT scan were analyzed as well as their association with the same variables than in the case of FN. In the multivariable logistic regression analysis, when comparing FP with TP, the data showed the existence of an obvious association with lymph node sizes >1cm (p<0.001) and with histological types different from adenocarcinomas and carcinoid tumors (p=0.049) (Table 9).
DiscussionWe achieved 54 per cent sensitivity in the PET-CT scan in lymph node staging in patients who underwent surgery for NSCC (in the study per tumor). The rate of FNs (14.2 per cent) is consistent what has been published in the medical literature,27 but the rate of FPs found is high. Recent meta-analyses28–30 have achieved a 67–81.3 per cent overall sensitivity in 18F-FDG PET-CT scans for the detection of lymph node metastases in patients with NSCC and a 79.4–90 per cent specificity, suggestive that this modality is more specific and less sensitive for the staging of lymph node metastases.
It has been reported that the sensitivity of the 18F-FDG PET-CT scan for the detection of lymph node metastasis is lower in regions where tuberculosis is endemic,31 since bilateral and symmetric uptakes of 18F-FDG in mediastinal and hilar lymph nodes are much more common in these regions, and metastatic lymph nodes can go misdiagnosed between the inflammation and the infection. In our series, six of the 113 patients had had tuberculosis and only in one patient without known prior history, the histological study found tuberculous and metastatic hilar adenopathies.
The incidence of hidden lymph node metastases in patients with NSCC who do not exhibit uptake in the 18F-FDG PET-CT scan ranges between 7 per cent and 16 per cent.19,20 Even in patients with clinical stage INSCC, the rate of FNs is 6–7 per cent.32 In our series, the rate of FNs was 14.2 per cent. The FNs on the PET-CT scan are related mainly with tumor infiltration of small-sized nodes.33 All of our FNs occurred in lymph nodes ≤1cm. Lymph node micrometastases are not usually detected with the existing imaging modalities because of today's PET-CT machines limited spatial resolution (5–7mm).34
The slower the cellular growth rate of a tumor, the less glucose activity it will exibit, therefore, greater chances of a FN on the PET image. The diagnostic performance of a 18F-FDG PET-CT scan may be low in lung tumors such as well-differentiated carcinoid tumors or adenocarcinomas, especially in the mucinous subtype.35 Pattenden et al.36 describe low sensitivities (33 per cent) and high specificities (94 per cent) in 18F-FDG PET-CT scans in the lymph node staging of 207 patients with lung carcinoid tumors. In our series there is a 9.2 per cent of carcinoid tumors. Among the FNs (12 cases) there was one atypical carcinoid tumor and six adenocarcinomas with moderate differentiation (two predominantly solid with mucin production).
Several factors are associated with a greater risk of hidden lymph node metastases such as the central location of primary tumor, its larger size (>3cm according to some authors), poorly-differentiated tumors, the adenocarcinoma histologic subtype and a higher SUVmax of primary tumor (>4 according to some authors).37,38 In our study, we found a correlation between FNs and primary tumor SUVmax >4, as well as a moderate degree of tumor differentiation, when compared to cases of TNs. It is well known that the greater the metastatic potential of tumor cells really is, the lower the degree of tumor differentiation is, and higher the incidence of lymph node damage. Also, poorly-differentiated tumors exhibit greater glucose uptake, with higher SUVmax than well- or moderately differentiated tumors. This could be one of the reasons for our findings of FNs. On the other hand, the medical literature shows a clear correlation between a high SUVmax of the primary tumor and the incidence of hidden metastases in mediastinal lymph nodes.37,38 In our series, and in all cases except one, the SUVmax of the primary tumor was >4.
In 58.3 per cent of the FNs found, lymph node staging went from N0 to N1, and in 41.6 per cent it went from N0 to N2; that is, surgery could have been prevented in 41.6 per cent of all the cases of FNs if after using the 18F-FDG PET-CT scan the staging had been correct. However, today the true clinical importance of underestimating patients with hidden N2 lymph node damage before surgery is unknown. It has not been demonstrated that neoadjuvant therapy and not surgery followed by adjuvant chemotherapy, in this group of patients, improves survival.38
There is not such a thing as a universally accepted criterion for the classification of mediastinal or hilar lymph nodes as positive when performing a 18F-FDG PET-CT scan. Criterion changes from series to series, but the one that is more commonly used is the visual or qualitative criterion, that goes on to compare the uptake in the lymph node with the background mediastinal uptake. Several semiquantitative criteria have been published, but out of all of them, a lymph node SUVmax >2.5 is the criterion that is more often used for the diagnosis of metastatic lymph node damage in patients with lung cancer,39 and is the one we have used in this study, since it has high specificity and high NPV in patients with negative PET-CT in the mediastinum (and so invasive staging can be avoided), but also an increase of FPs due to the inflammatory changes that occur in the lymph nodes, with a low PPV. In the medical literature, statistically significant differences regarding the diagnosis of lymph node damage have not been reported between the use of the semiquantitative objective criterion (SUVmax) and the visual or qualitative criterion.30
In patients with NSCC, a higher rate of FPs has been confirmed in mediastinal lymph nodes, due to an increase of metabolic activity in acute intercurrent pulmonary infections, granulomatous diseases, bronchiectasis, post-obstructive pneumonitis and interstitial pneumonias.21 Also, the uptake has been reported in lymph nodes with follicular hyperplasia in the cortex and histiocytosis in the sinus (infiltration of macrophages), with anthracotic pigmentation or with the formation of fibrotic nodes, with or without calcification, in the marrow.40 In our work, we found a 61.1 per cent rate of FPs. Results show that a lymph node size >1cm is associated with a greater risk of FP (p<0.001), which may be due to the fact that these lymph nodes are affected by inflammatory or intercurrent infectious processes.
In 50 per cent of all FPs, lymph node staging went from N1 to N0, and in the remaining 50 per cent it went from N2 to N0, indicative of the importance of pathological confirmation of positive cases on the PET-CT scan prior to surgery.
Our study has some limitations. First, only patients that underwent surgery due to NSCC were included, and most of them were in the early stages of the disease, which may imply underestimation of both the sensitivity and diagnostic precision of the 18F-FDG PET-CT scan for the detection of lymph node metastases. This is why the results of this study cannot be generalized to the general group of patients with lung carcinoma. Second, it is a retrospective study, although both the PET-CT scan and the pathological anatomy have been assessed independently. Lastly, the mean time elapsed between the PET-CT scan and the surgery was more than a month.
In sum, in our setting, the 18F-FDG PET-CT scan is a modality that has limited sensitivity and PPV, and high specificity, NPV and diagnostic precision for the detection of lymph node metastases in patients with NSCC. The rate of FNs found is similar to that reported previously, and is higher in cases with primary tumor SUVmax values >4 and a moderate degree of tumor differentiation. In cases with negative lymph nodes on the 18F-FDG PET-CT scan, given its high specificity and NPV, the invasive mediastinal staging could be avoided and go directly to surgery while taking all the aforementioned aspects into consideration.
Given the high rate of FPs found, and before precluding the patient from surgery, it is recommended to perform biopsies of the positive lymph nodes with the use of one EBUS, EUS, or mediastinoscopy.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the proceedings followed fully abide by the ethical rules and regulations from the human experimentation committee and are in full compliance with the World Health Organization (WHO) and the Declaration of Helsinki.
Confidentiality of dataThe authors confirm that they have followed their centers protocols on the publication and disclosure of data from patients.
Right to privacy and informed consentThe authors have obtained prior written informed consent from patients and/or individuals referred to in this paper. This document belongs to the corresponding author.
AuthorsManager of the integrity of the study: ABGC, JFD, RDB, CFP and JLCD.
Study idea: ABGC.
Study design: ABGC.
Data mining: BCM, MGGE and ABGC.
Data analysis and interpretation: CFP, ABGC, MGGE, JFD, RDB and JLCD.
Statistical analysis: CFP.
Reference: ABGC, BCM and MGGE.
Writing: ABGC, BCM, MGGE, JFD, RDB and JLCD.
Critical review of the manuscript with intellectually relevant remarks: JFD, RDB, CFP and JLCD.
Approval of final version: ABGC, BCM, MGGE, JFD, RDB, JLCD and CFP.
Conflict of interestThe authors declare no conflict of interest.
Please cite this article as: Bustos García de Castro A, Ferreirós Domínguez J, Delgado Bolton R, Fernández Pérez C, Cabeza Martínez B, García García-Esquinas M, et al. La PET-TC en la estadificación ganglionar prequirúrgica del carcinoma de pulmón de células no pequeñas: implicación de los falsos negativos y falsos positivos. Radiología. 2017;59:147–158.




![Fifty-six year-old male with NSCC (the diagnostic fine needle puncture aspiration of the primary tumor was indicative of adenocarcinoma). Case of trur positive. The images a, b and c on the axial plane of the staging 18F-FDG PET-CT scan show one left hilar adenopathy of 1.5cm, with a SUVmax of 16.3 (white and black arrows), and the primary tumor in the left lower lobe with a SUVmax of 21.5 (yellow arrows). In images d, e and f, on the coronal plane, we can see the 18-FDG uptake of the primary tumor, the left hilar adenopathy and of one <1cm-adenopathy in the aorto-pulmonary window (blue arrows) that did not show tumor infiltration and with a negative EUS. In the histology, tumor infiltration was detected in a left hilar lymph node (image g, hematoxylin-eosin staining [HE] 40×) and damage due to tuberculosis in another left hilar lymph node (image h, HE staining 40×) showing one large tuberculous granuloma with abundant necrosis (arrows). The Zhiel staining procedure tested positive. Fifty-six year-old male with NSCC (the diagnostic fine needle puncture aspiration of the primary tumor was indicative of adenocarcinoma). Case of trur positive. The images a, b and c on the axial plane of the staging 18F-FDG PET-CT scan show one left hilar adenopathy of 1.5cm, with a SUVmax of 16.3 (white and black arrows), and the primary tumor in the left lower lobe with a SUVmax of 21.5 (yellow arrows). In images d, e and f, on the coronal plane, we can see the 18-FDG uptake of the primary tumor, the left hilar adenopathy and of one <1cm-adenopathy in the aorto-pulmonary window (blue arrows) that did not show tumor infiltration and with a negative EUS. In the histology, tumor infiltration was detected in a left hilar lymph node (image g, hematoxylin-eosin staining [HE] 40×) and damage due to tuberculosis in another left hilar lymph node (image h, HE staining 40×) showing one large tuberculous granuloma with abundant necrosis (arrows). The Zhiel staining procedure tested positive.](https://static.elsevier.es/multimedia/21735107/0000005900000002/v1_201704120052/S2173510717300265/v1_201704120052/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Fifty-seven year-old male. Incidental finding of one lymph node in the chest Rx: atypical carcinoid tumor. Case of false negative. In images a and b, on the axial plane of the staging 18F-FDG PET-CT scan, one primary tumor can be seen in the middle lobe, showing 18-FDG uptake (arrows) and distal atelectasis with no uptake. In axial images c and d one <1cm (white arrow) lower right paratracheal lymph node can be seen without 18-FDG uptake. Image e (hematoxylin–eosin staining [HE] 40×) shows the histology of the primary tumor, with cell nests with wide eosinophil cytoplasm and discrete nucleoli that are stained using the synaptophysin technique (image f, 10×) and the chromogranin technique (image g, 10×). Image h shows the histology of the lymph node with HE staining (10×), with metastatic cell nests of the carcinoid tumor (arrows). Fifty-seven year-old male. Incidental finding of one lymph node in the chest Rx: atypical carcinoid tumor. Case of false negative. In images a and b, on the axial plane of the staging 18F-FDG PET-CT scan, one primary tumor can be seen in the middle lobe, showing 18-FDG uptake (arrows) and distal atelectasis with no uptake. In axial images c and d one <1cm (white arrow) lower right paratracheal lymph node can be seen without 18-FDG uptake. Image e (hematoxylin–eosin staining [HE] 40×) shows the histology of the primary tumor, with cell nests with wide eosinophil cytoplasm and discrete nucleoli that are stained using the synaptophysin technique (image f, 10×) and the chromogranin technique (image g, 10×). Image h shows the histology of the lymph node with HE staining (10×), with metastatic cell nests of the carcinoid tumor (arrows).](https://static.elsevier.es/multimedia/21735107/0000005900000002/v1_201704120052/S2173510717300265/v1_201704120052/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)


