The increasing precision of multiparametric magnetic resonance imaging of the prostate, together with greater experience and standardization in its interpretation, has given this technique an important role in the management of prostate cancer, the most prevalent non-cutaneous cancer in men. This article reviews the concepts in PI-RADS version 2.1 for estimating the probability and zonal location of significant tumors of the prostate, using a practical approach that includes current considerations about the prerequisites for carrying out the test and recommendations for interpreting the findings. It emphasizes benign findings that can lead to confusion and the criteria for evaluating the probability of local spread, which must be included in the structured report.
La creciente precisión de la resonancia magnética multiparamétrica de próstata, en combinación con una mayor experiencia y estandarización en su interpretación, han conferido a esta técnica un papel actual sustancial en el manejo del cáncer de próstata, neoplasia no cutánea más prevalente en el varón. Revisamos los conceptos del sistema PI-RADS versión 2.1 para la estimación de la probabilidad y localización zonal de tumores significativos de próstata, con un enfoque práctico que incluye consideraciones actuales sobre los requisitos previos de la prueba y recomendaciones para su interpretación. Se hace hincapié en hallazgos benignos que pueden llevar a confusión y los criterios de valoración de la probabilidad de extensión local de la enfermedad, que deben también formar parte de un informe estructurado.
Prostate carcinoma (PC) is the most prevalent non-skin neoplasm in men and the second leading cause of cancer deaths after bronchopulmonary neoplasm.1 Its management is particularly complex as indolent tumours must be distinguished from aggressive or clinical significant tumours.2 The latter may be defined as tumours with a Gleason histologic grade ≥7 and/or a tumour volume ≥0.5cm3 and/or extraglandular extension.3 The use of multiparametric magnetic resonance imaging (mpMRI) of the prostate has seen exponential growth in the detection and estimation of the risk of clinically significant prostate cancer (CSPC), biopsy guidance, and focal treatment and follow-up of patients.4–6 Many studies with a high level of evidence support its use for diagnosing PC, showing a significant improvement in diagnosis compared to non-targeted systematic transrectal prostate biopsy.7–16 The latest guidelines even recommend mpMRI before the first biopsy in patients with high prostate-specific antigen (PSA) levels, a pathological digital rectal examination or both.12,17,18 Proper use of mpMRI requires keeping constantly up to date on standards for its use, interpretation and radiological reporting so as to optimise its implementation and clinical acceptance.4,19
In March 2019, the Prostate Imaging Report and Data System (PI-RADS) Steering Committee published the current version of this document, called the PI-RADSv2.1,4 which includes a number of clarifications and adjustments to resolve some inconsistencies and ambiguities detected in the previous version.20–23
The primary objective of this study is to provide a practical guide to facilitate the reading and structured reporting of MRI of the prostate according to the PI-RADSv2.1 guidelines. To do this, we review the anatomy of the prostate, basic concepts and some novel or misleading aspects of the PI-RADS, anatomical structures and benign findings that may generate confusion, and criteria for extraprostatic extension (EPE) of a tumour, which should also be assessed as a part of the study.
Anatomy of the prostateThe morphology of the prostate is similar to an inverted cone. Four different histologically different areas of the prostate are distinguished, as described by McNeal:24
- 1
Peripheral zone (PZ): this contains 70%-80% of the glandular tissue, with ducts radiating posterolaterally from the distal urethra to the verumontanum.
- 2
Central zone (CZ): this contains 20% of the glandular tissue; it surrounds the ejaculatory ducts in the posterosuperior part of the gland, beneath the seminal vesicles (SVs).
- 3
Transition zone (TZ): this contains 5% of the glandular tissue and forms two periurethral lobes proximal to the verumontanum with small ducts, which in adulthood develop benign prostatic hyperplasia (BPH).
- 4
Anterior fibromuscular stroma (AFMS): this does not contain glandular tissue.3,25–29
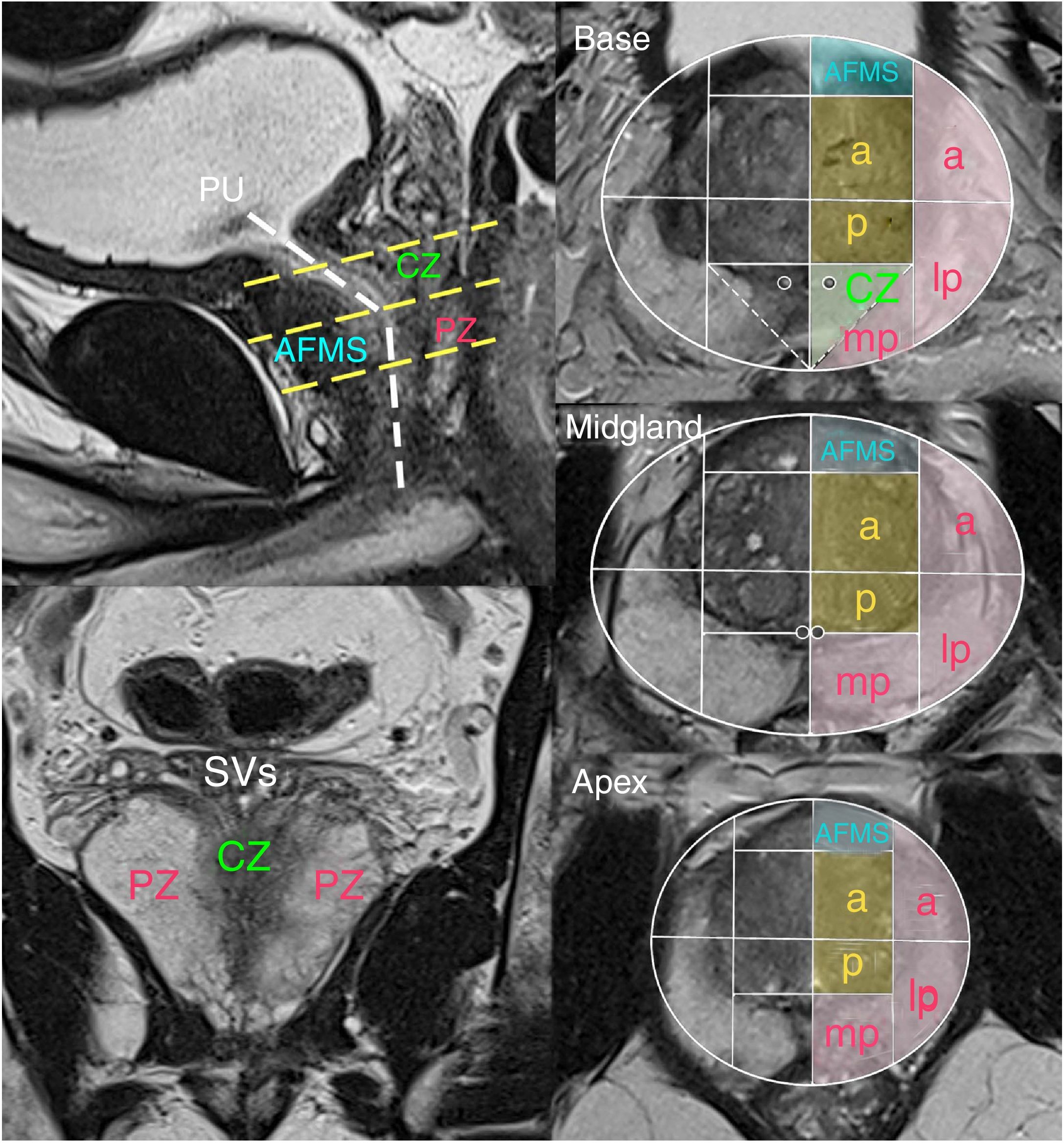
The model of anatomical segmentation adopted in the PI-RADS v2.1, which should be used to specify lesion location, divides the prostate into two lobes (left and right), three levels on an up and down axis (base, midgland and apex) and several sectors in each McNeal zone, totalling 41 segments (including the two SVs and the membranous urethra)3 (Fig. 1).
Sectoral anatomy of the prostate according to PI-RADSv2.1. The sectoral diagram (PZ in pink, TZ in orange, CZ in green and AFMS in blue) is identical at all three levels apart from the two small additional sectors at the base of the CZ. In front of the prostatic urethra (PU) is the AFMS, and behind it are the CZ in the superior half and the medioapical PZ in the inferior part. The normal CZ is symmetrical with a V shape on coronal planes (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article).
AFMS: anterior fibromuscular stroma; CZ: central zone; PZ: peripheral zone; TZ: transition zone.
Approximately 70%- 75% of cases of PC originate in the PZ, and 20%-30% originate in the TZ. Cancers that originate in the CZ are uncommon and are usually secondary to extension from tumours of the PZ.3
The normal PZ is hyperintense, and the normal CZ and AFMS are hypointense. BPH presents systematically at around age 50 and becomes more accentuated with age. Hyperplastic nodules result from mixed proliferation of glandular tissue and fibromuscular stroma in the TZ. Nodules that are predominantly glandular are hyperintense on T2, and nodules that are predominantly stromal are hypointense, with more restricted diffusion and early contrast enhancement.26
The prostate is delimited on T2-weighted sequences by a hypointense line created by superficial stromal condensation at its interface with periprostatic fat which does not represent a true capsule.30,31 Hence, it is more correct to indicate that a lesion shows or does not show signs of EPE, not extracapsular signs or signs of capsule infiltration. The apex of the prostate lacks this structure altogether and therefore represents one of the regions prone to EPE of a tumour.25,32
PI-RADSv2.1Technical considerationsmpMRI of the prostate is a tool that is highly dependent on imaging quality, based on a combination of pulse sequences, high-resolution multiplanar T2-weighted sequences, diffusion-weighted imaging (DWI) sequences and dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) sequences. These would have a low yield if used in isolation.13,33
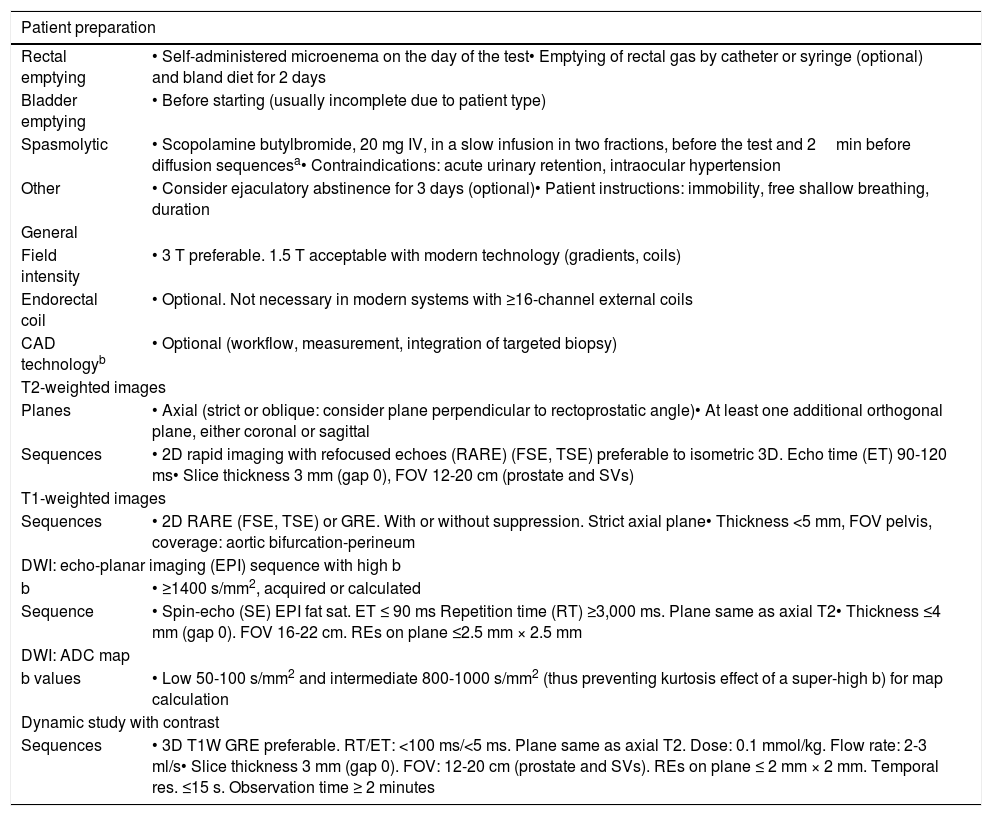
Table 1 summarises the main principles of patient preparation mentioned in the PI-RADS, without specifying regimens due to a lack of consensus, and the essential technical requirements for image acquisition.3 The level of evidence and degree of recommendation of both types of guidelines, analysed in a recent review by the PI-RADS committee, are generally moderate apart from those referring to the optimal combination of receptor antennae (with level 1 A): spinal integrated into the table and multichannel phased-array external, with no need for an endorectal coil, which may even be counterproductive.33
| Patient preparation | |
|---|---|
| Rectal emptying | • Self-administered microenema on the day of the test• Emptying of rectal gas by catheter or syringe (optional) and bland diet for 2 days |
| Bladder emptying | • Before starting (usually incomplete due to patient type) |
| Spasmolytic | • Scopolamine butylbromide, 20 mg IV, in a slow infusion in two fractions, before the test and 2min before diffusion sequencesa• Contraindications: acute urinary retention, intraocular hypertension |
| Other | • Consider ejaculatory abstinence for 3 days (optional)• Patient instructions: immobility, free shallow breathing, duration |
| General | |
| Field intensity | • 3 T preferable. 1.5 T acceptable with modern technology (gradients, coils) |
| Endorectal coil | • Optional. Not necessary in modern systems with ≥16-channel external coils |
| CAD technologyb | • Optional (workflow, measurement, integration of targeted biopsy) |
| T2-weighted images | |
| Planes | • Axial (strict or oblique: consider plane perpendicular to rectoprostatic angle)• At least one additional orthogonal plane, either coronal or sagittal |
| Sequences | • 2D rapid imaging with refocused echoes (RARE) (FSE, TSE) preferable to isometric 3D. Echo time (ET) 90-120 ms• Slice thickness 3 mm (gap 0), FOV 12-20 cm (prostate and SVs) |
| T1-weighted images | |
| Sequences | • 2D RARE (FSE, TSE) or GRE. With or without suppression. Strict axial plane• Thickness <5 mm, FOV pelvis, coverage: aortic bifurcation-perineum |
| DWI: echo-planar imaging (EPI) sequence with high b | |
| b | • ≥1400 s/mm2, acquired or calculated |
| Sequence | • Spin-echo (SE) EPI fat sat. ET ≤ 90 ms Repetition time (RT) ≥3,000 ms. Plane same as axial T2• Thickness ≤4 mm (gap 0). FOV 16-22 cm. REs on plane ≤2.5 mm × 2.5 mm |
| DWI: ADC map | |
| b values | • Low 50-100 s/mm2 and intermediate 800-1000 s/mm2 (thus preventing kurtosis effect of a super-high b) for map calculation |
| Dynamic study with contrast | |
| Sequences | • 3D T1W GRE preferable. RT/ET: <100 ms/<5 ms. Plane same as axial T2. Dose: 0.1 mmol/kg. Flow rate: 2-3 ml/s• Slice thickness 3 mm (gap 0). FOV: 12-20 cm (prostate and SVs). REs on plane ≤ 2 mm × 2 mm. Temporal res. ≤15 s. Observation time ≥ 2 minutes |
Also of note are the usefulness of spasmolytic agents in reducing intestinal motility and resulting blurriness on T2-weighted images, the superiority of a self-administered precleansing micro-enema in rectal aspiration of gas using a catheter to prevent artefacts on diffusion, and the reasonable positive effect of ejaculatory abstinence for three days on the visibility of lesions in the PZ and tumour invasion of the SVs.33
The orientation of planes advised in this study, another ambiguous point in the PI-RADSv2.1, is parallel and perpendicular to the rectoprostatic angle for the coronal and axial slices, respectively. Coronal planes show the gland with a "heart" shape and a good representation of possible seminal impairment, and axial planes show better correlation with transverse sections of prostatectomy specimens. Diffusion and perfusion planes should be identical in terms of angulation and geometry to the axial T2-weighted sequences selected, for optimal synchronised interpretation of the study.33
Regarding b values for diffusion, the PI-RADSv2.1 recommends at least one low value, in the range of 0−100s/mm2, and one high value that is less than 1000 (800-1,000) to calculate the apparent diffusion coefficient (ADC) map, thus preventing vascular and kurtosis effects, respectively, in addition to a value of at least 1400s/mm2 for the DWI sequence, whether it is calculated, or better, acquired.3,33
DCE imaging should have a temporal resolution of less than 15s. Despite its limited role in tumour detection, it retains its value as a "support technique" for observers with less expertise, tumour staging, unsuitability of other sequences due to artefacts, and cases with strong clinical suspicion and a negative prior MRI.3,33
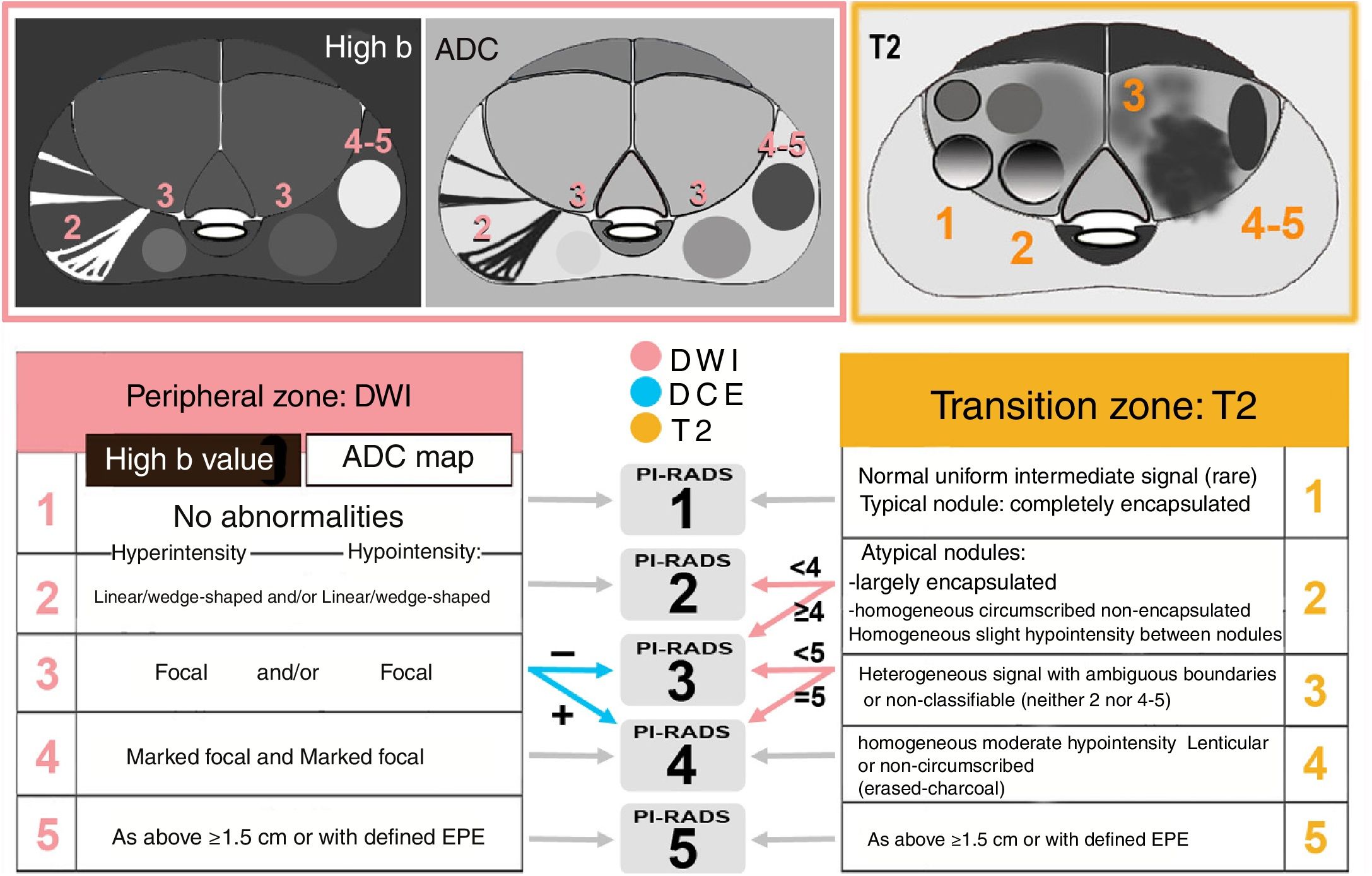
Keys to interpretationPI-RADS is a risk assessment method that predicts the likelihood of CSPC (from 1, very low, to 5, very high) by means of standardised, weighted reading by anatomical zones of the sequences that comprise mpMRI (Fig. 2). A dominant sequence with greater weight in the definitive score for the lesion is established in each zone: diffusion (jointly assessing DWI with high b and ADC map) in the PZ, and T2 in the TZ3,34 (Fig. 3).
Diagram of keys to categorisation in the PI-RADSv2.1: dominant sequences by zone and final PI-RADS. In the PZ, diffusion is the dominant sequence, and a dynamic study with contrast is considered if the PI-RADS on diffusion is 3. In the TZ, T2 is the dominant sequence, and diffusion is considered if the PI-RADS on T2 is 2 or 3. A focal lesion in the PZ maintains a score of 3 on diffusion, even if hyperintensity on DWI or hypointensity on ADC is marked, not both at once. If the signal is marked on both, the final score is 4.
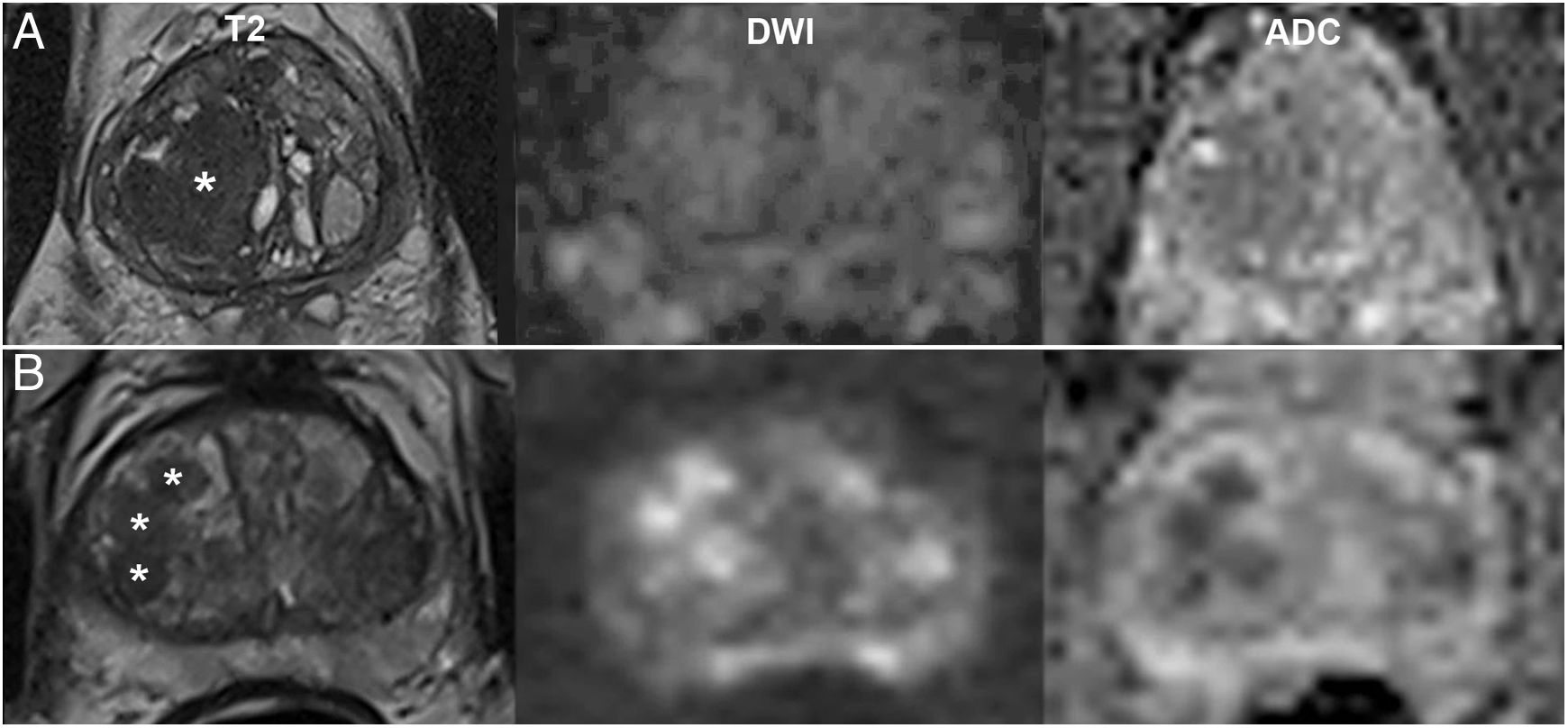
Examples of PI-RADS v2.1 categories determined almost exclusively by the dominant sequence. A) PI-RADS 1 in the TZ: typical hyperplastic nodule. B) PI-RADS 5 in the TZ: non-circumscribed homogeneous hypointensity ("erased-charcoal sign") larger than 1.5cm in the anterior sectors of both lobes on a medial level. C) PI-RADS 2 in the PZ: wedge-shaped hyperintensity on DWI and hypointensity on ADC. The T2-weighted sequence, though not dominant, aids in assessing its morphology. D) PI-RADS 4 in the PZ: focus of restriction marked both on DWI with b1400 and on the ADC map, smaller than 1.5cm.
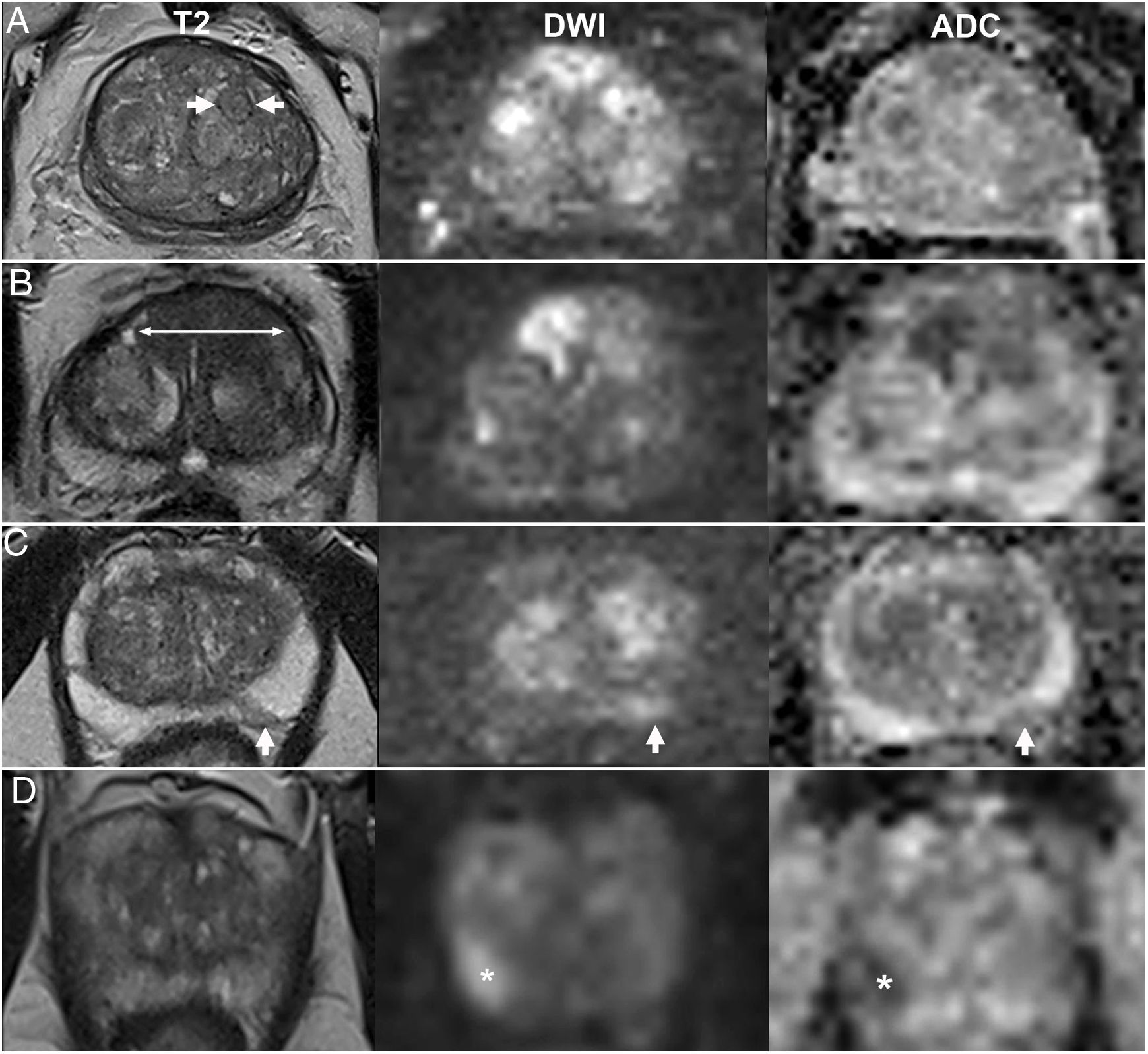
Non-dominant sequences impact the final score of a finding. Generally, they maintain or even increase its definitive category by a point in cases of low or indeterminate probability on the dominant sequence. For example, BPH nodules are considered typical and, according to the PI-RADSv2.1, receive a definitive score of 1 when completely encapsulated. By contrast, partially encapsulated lesions, even circumscribed ones, are considered atypical and are assigned a provisional score of 2 (Fig. 4A) that increases to 3 if, as is often the case, they exhibit markedly restricted diffusion3 (Fig. 4B).
Value of diffusion in the TZ as a non-dominant sequence. A) PI-RADS 2: partially encapsulated nodule (asterisk) with non-marked abnormal diffusion (3) on DWI with b1400 and ADC map (maintains a definitive score of 2). B) PI-RADS 3: atypical, hypointense, non-encapsulated nodules (asterisks), with a score of 4 on diffusion (2 on T2, increasing definitively to 3). In these cases, diffusion also facilitates detection of areas requiring a more detailed morphological analysis on T2.
This indeterminate result, frequently seen in the TZ, is one of the limitations inherent to the current PI-RADS for detecting CSPC in this area, which is more complex than in the PZ and is based on disruption of the "organised chaos" of BPH due to poorly defined lesions or lesions with a non-nodular form.3,34
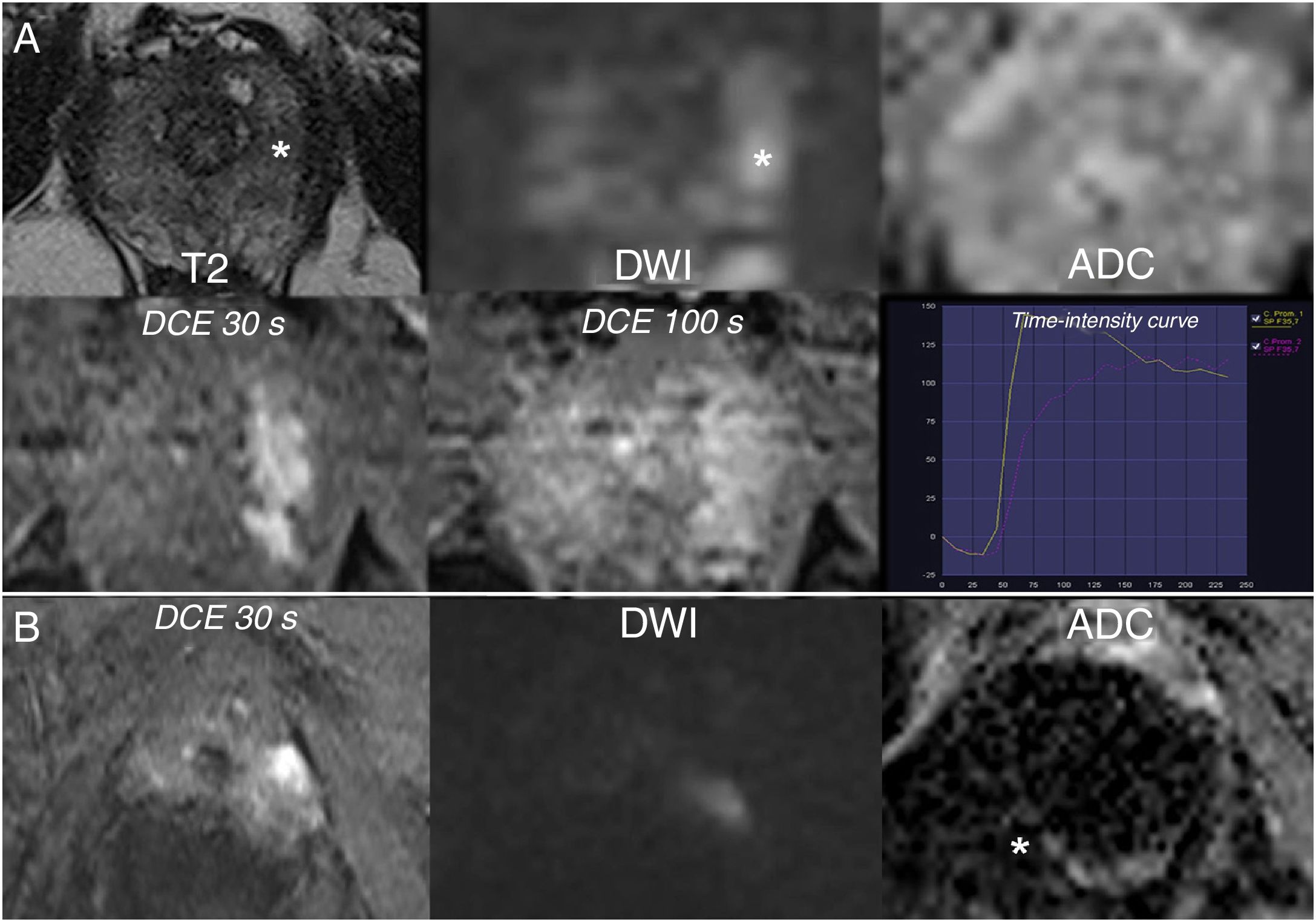
The role of DCE imaging is limited to assessment of PZ lesions with a score of 3 on diffusion, the dominant sequence in the zone (Fig. 5). For this, a binary (positive or negative) criterion is used, with focal enhancement in relation to adjacent tissue considered positive, whether it is early or simultaneous, corresponding to the suspicious finding on diffusion and/or T2. Negative results include the absence of this enhancement, non-correlation with a visible focal lesion on another sequences or correlation with a nodule typical of BPH.3
Value of DCE imaging. A) PI-RADS 3 on diffusion in the PZ increased to 4 following DCE imaging: non-marked focal restriction on at least one type of diffusion image (slight hyperintensity on high-b DWI, undetectable on ADC), with positive DCE: early focal enhancement corresponding to the suspicious finding. B) Unsuitable dominant sequence: ADC map cannot be assessed because of artefacts due to a right hip prosthesis. Focal hyperintensity on DWI with positive DCE imaging: PI-RADS 4.
It is advisable to use a dedicated software program and a workstation that displays the entire study at a glance, suitably adapted for evaluating the quality of the study and the presence or absence of haemorrhage, adjusting levels of shine-through and contrast, as needed, and measuring prostate volume, as initial steps.35
Next, it is useful to have a screen configuration that synchronously displays the three fundamental axial sequences (axial T2-weighted, high-b DWI and ADC map), along with a longitudinal, preferably coronal, T2-weighted sequence as a reference for the cut-off point analysed. Any sequence is valid for detection of lesions as a prior step in their characterisation, which always requires simultaneous assessment of all of them. Agreement between the position of a finding and determination of its corresponding zone is crucial.
The remainder of the series is to be added when necessary depending on lesion type, e.g. for a triplanar morphological analysis on T2. Although the PI-RADSv2.1 allows it, in practice it is difficult to abandon one of the two longitudinal planes: the sagittal plane is perhaps less essential as it does not represent glandular symmetry, though it does represent SV invasion and the morphology of anatomical components, and it is the ideal plane for measuring the longitudinal and anteroposterior diameters to calculate prostate volume. In DCE imaging, perfusion is assessed visually using continuous plane displacement in cine mode, with or without the help of colour-coded parametric maps or regional time-intensity curves. The behaviour of PC is highly variable, and few published data support the clinical usefulness of classification of curves or analysis of pharmacokinetic models of enhancement.3
Lesions should be measured on the dominant sequence and axial plane, unless they are found to be much better delimited on another sequence or plane, in which case this must be specified.3 It is also recommended that the minimum numeric values for the ADC in the suspicious foci be recorded.
Assignment of PI-RADS categories based on observed abnormalities in morphology in signal intensity is a basically qualitative process in which precise terminology is key. For this, the PI-RADS has an appendix with a lexicon, wherein "circumscribed" is defined as synonymous with "well-defined" and the opposite of "ill-defined", "indistinct" or "blurred". Hyperintensity and hypointensity are defined in relation to the background prostate tissue or other reference tissue or structure: "markedly hypointense" is a "signal intensity lower than expected for normal or abnormal tissue of the reference type, e.g., when involved with calcification or blood or gas". Focal means localised at a focus, distinct or different from the background pattern, as opposed to diffuse, continuous, non-localised or disseminated. Regional denotes an intermediate extension between focal and diffuse such as a sextant, zone or lobe of the prostate.3
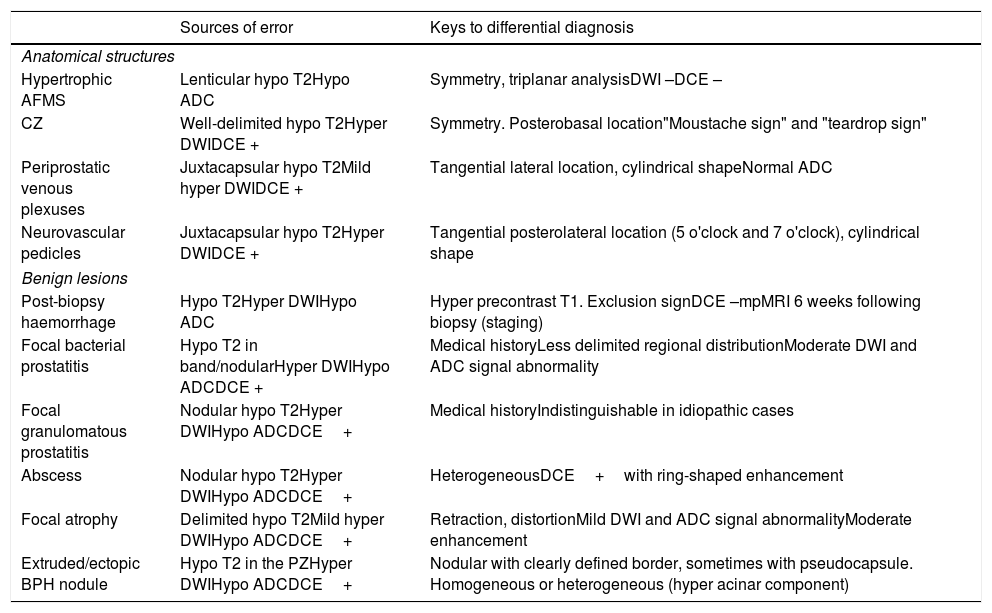
Benign findings that may lead to diagnostic errorDespite standardisation in mpMRI, certain anatomical structures that are normal or show paraphysiological changes and certain benign lesions simulate cancer.27,35–40 They may lead to false positives, but also false negatives. Sources of error that should be borne in mind and keys to preventing resulting confusion in applying anatomical knowledge, clinical data and PI-RADS criteria with particular rigour are summarised in Table 2.
Findings that may lead to diagnostic error in MRI of the prostate and recommendations for its prevention.27,35–52.
| Sources of error | Keys to differential diagnosis | |
|---|---|---|
| Anatomical structures | ||
| Hypertrophic AFMS | Lenticular hypo T2Hypo ADC | Symmetry, triplanar analysisDWI –DCE – |
| CZ | Well-delimited hypo T2Hyper DWIDCE + | Symmetry. Posterobasal location"Moustache sign" and "teardrop sign" |
| Periprostatic venous plexuses | Juxtacapsular hypo T2Mild hyper DWIDCE + | Tangential lateral location, cylindrical shapeNormal ADC |
| Neurovascular pedicles | Juxtacapsular hypo T2Hyper DWIDCE + | Tangential posterolateral location (5 o'clock and 7 o'clock), cylindrical shape |
| Benign lesions | ||
| Post-biopsy haemorrhage | Hypo T2Hyper DWIHypo ADC | Hyper precontrast T1. Exclusion signDCE –mpMRI 6 weeks following biopsy (staging) |
| Focal bacterial prostatitis | Hypo T2 in band/nodularHyper DWIHypo ADCDCE + | Medical historyLess delimited regional distributionModerate DWI and ADC signal abnormality |
| Focal granulomatous prostatitis | Nodular hypo T2Hyper DWIHypo ADCDCE+ | Medical historyIndistinguishable in idiopathic cases |
| Abscess | Nodular hypo T2Hyper DWIHypo ADCDCE+ | HeterogeneousDCE+with ring-shaped enhancement |
| Focal atrophy | Delimited hypo T2Mild hyper DWIHypo ADCDCE+ | Retraction, distortionMild DWI and ADC signal abnormalityModerate enhancement |
| Extruded/ectopic BPH nodule | Hypo T2 in the PZHyper DWIHypo ADCDCE+ | Nodular with clearly defined border, sometimes with pseudocapsule. Homogeneous or heterogeneous (hyper acinar component) |
AFMS: anterior fibromuscular stroma; BPH: benign prostatic hyperplasia; CZ: central zone.
Hypertrophic AFMS and pseudonodular thickening of the surgical capsule of the transition zone adenoma are possible sources of error due to their hypointensity on T2, despite a lack of shine-through on DWI: this sequence along with their characteristic topography and symmetry enable them to be distinguished from a neoplasm or other lesion (Fig. 6A and 6B).27,31,36,41,42 DCE imaging is less useful.27,43–45
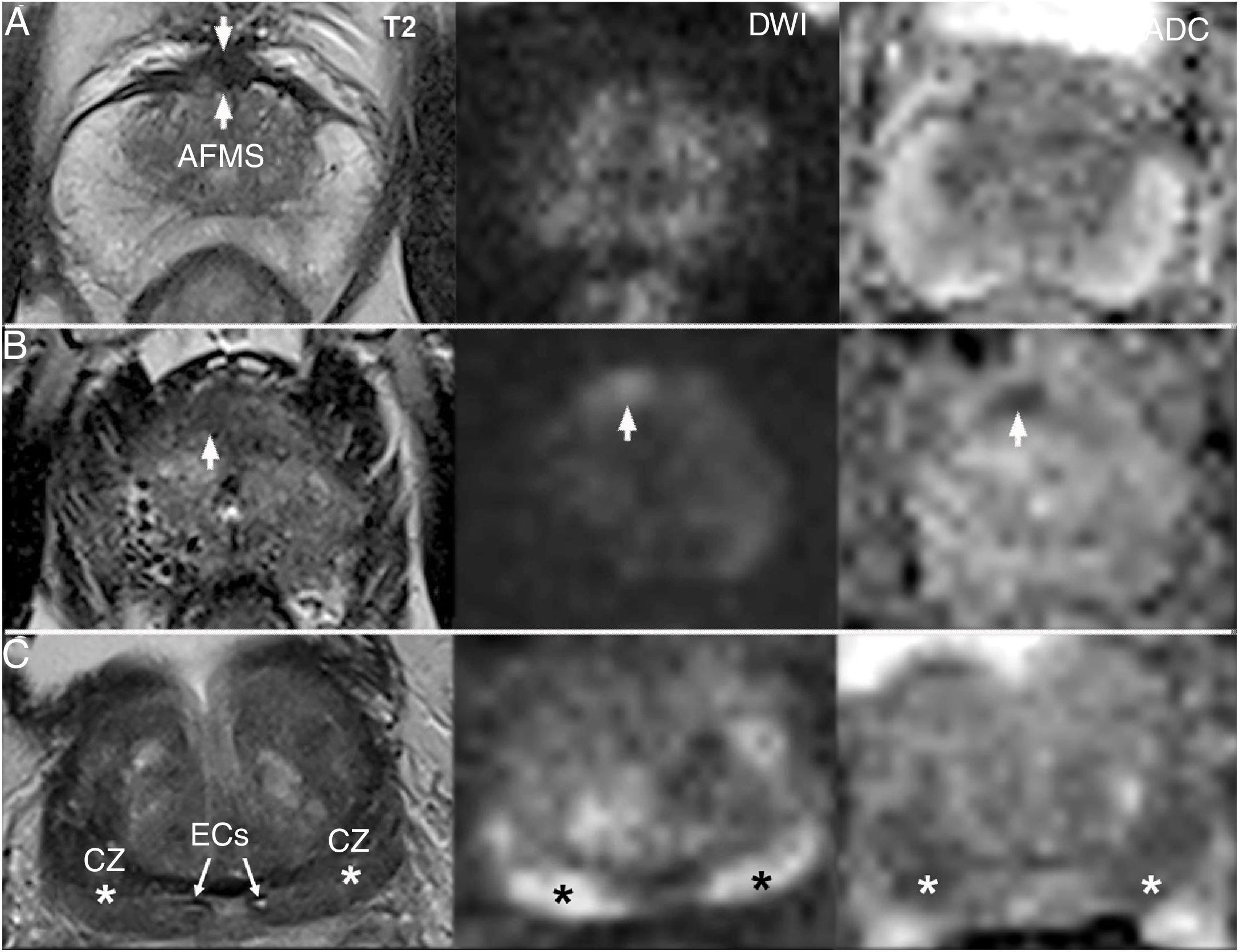
Sources of error by anatomical structure. A) Normal AFMS: hypointense and symmetrical on T2, without shine-through on DWI. B) PI-RADS 4 lesion which should be differentiated from the AFMS: very anterior asymmetrical hypointensity in the right lobe with increased signal on DWI and hyposignal on ADC. Chronic prostatitis on biopsy following MRI. C) Normal CZ (asterisks): hypointense on T2 and markedly hyperintense on DWI, with a symmetrical appearance on all sequences ("moustache sign"). Systematic biopsy prior to MRI, negative for malignancy: adenomyomatous hyperplasia with stromal predominance in all basal cylinders.
Distortion and compression of the CZ against the PZ, secondary to BPH and sometimes associated with periductal fibrosis around the ejaculatory ducts, result in ovoid foci with well-delimited homogeneous hypointensity in both lobes of the prostate, with signal abnormality on both DWI and ADC (Fig. 6C). Their differentiation from PC depends on a triplanar morphological analysis, with symmetry being a key trait.26,37,39,46 The "moustache sign" refers to the appearance of these rejected foci towards the posterobasal margin of the gland, on both axial and coronal planes. The "teardrop sign" is seen when the position is more medial and vertical on the coronal plane, convergent and thinned distally with a nodular appearance at the midline on axial planes, always proximal to the verumontanum.36
Both the CZ and the AFMS should not be scored when normal;3, while signal asymmetry of these on any of the sequences is suggestive of PC.27,31,41 In some cases, it is impossible to distinguish an asymmetrical CZ from PI-RADS 4 focus. This diagnostic uncertainty should be recorded in the report and, being aware of the limitations of the technique, a targeted biopsy should be proposed.27,31,41,44 PC that presents in the CZ (7%) is rare, but tends to be more aggressive, with a higher grade and likelihood of EPE and SV invasion.26,45
The venous plexuses and periprostatic neurovascular bundles produce juxtacapsular hypointensities with more or less restricted diffusion and intense vascular enhancement, likely to simulate PC with EPE.4,41,42 Their respective lateral and posterolateral location, and cylindrical shape tangential to the prostate, should be sufficient to distinguish them.32,42
Benign lesions that mimic cancerPost-biopsy haemorrhage is common, especially in the PZ and SVs, and shows characteristics similar to PC on T2-weighted, DWI and ADC map images that may simulate or obscure a neoplasm, as well as its EPE. The absence of early enhancement on contrast images with subtraction aids in distinguishing them. However, it is vital to have a pre-contrast T1-weighted sequence, on which they appear as hyperintense foci that usually spare the region where the tumour is located, in case of coexistence.3,27,37,47 The "haemorrhage exclusion sign" (Fig. 7A) describes this absence of bleeding in tumour tissue due to its low citrate content (endogenous anticoagulant that helps preserve semen) in comparison to normal prostate tissue, which contains more citrate and therefore is more prone to presenting bleeding. Usually, where there is no haemorrhage, there is no tumour.36,37
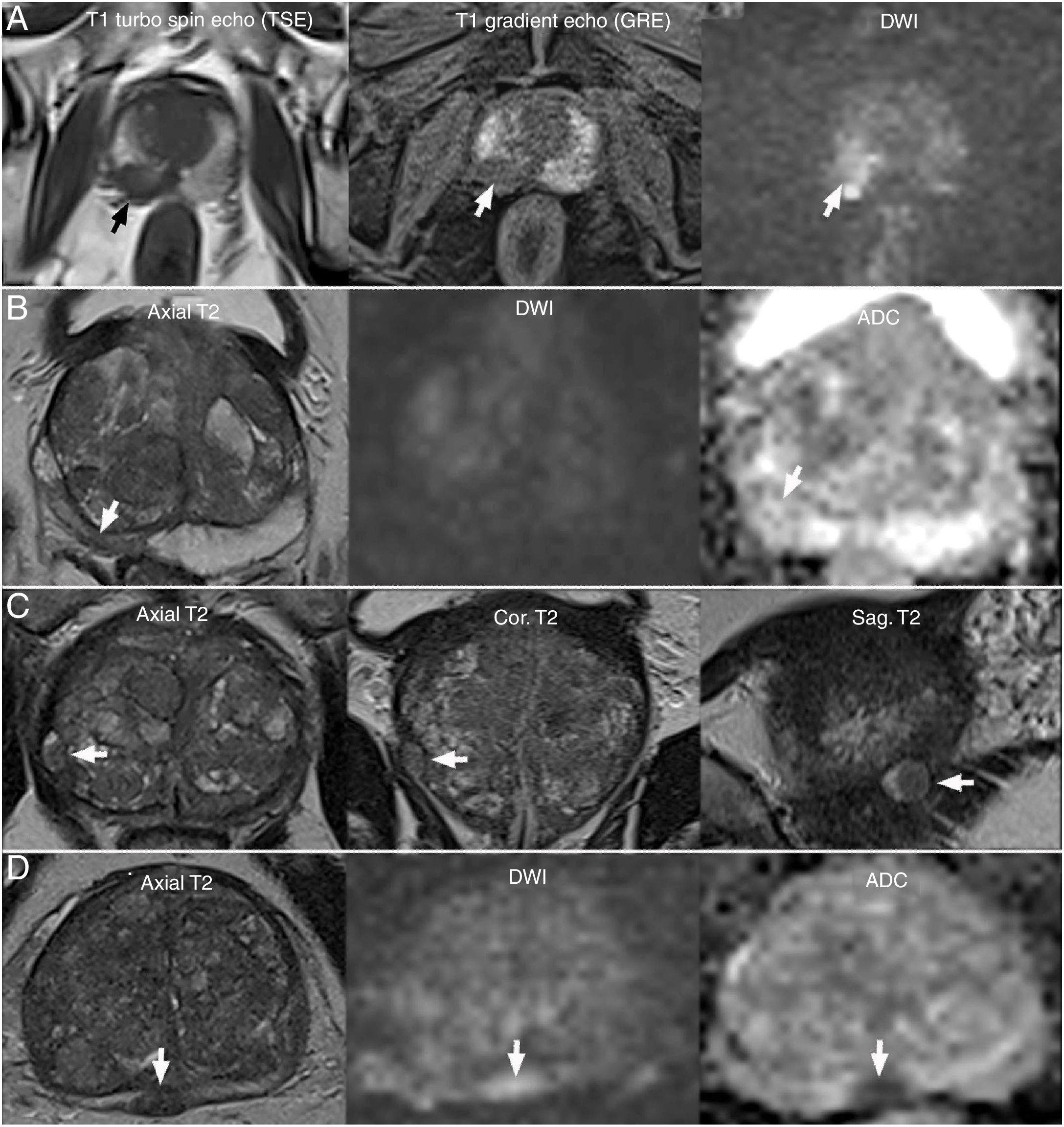
Sources of error due to benign lesions. A) Haemorrhage exclusion sign: Extensive bleeding delimiting a tumour focus in the right lobe as a bleeding defect (arrows). B) Atrophy secondary to chronic prostatitis: focal hypointensity in the peripheral zone (PZ) (arrow) with negative DWI, slight hypointensity on ADC and retraction: PI-RADS 2. C) Extruded encapsulated hyperplastic nodule in the PZ (arrow): PI-RADS 2 due to its location, in spite of its typical morphology. D) Non-encapsulated ectopic hyperplastic nodule: small capsular bulging (arrow) due to a circumscribed nodule with PI-RADS 4 on diffusion, suggestive of a BPH nodule, which was recommended for biopsy given its location and atypical characteristics, yielding a negative result for malignancy.
Focal bacterial prostatitis, whether acute or chronic, causes an elevation in PSA that typically fluctuates in response to antibiotic treatment. Local and systemic inflammatory symptoms are more minor in the chronic form, which may be asymptomatic or present only with lower urinary tract symptoms. These cases of indolent prostatitis with persistently elevated PSA prompt mpMRI studies and may coexist with a tumour.30–32,41 Signal abnormalities particular to prostatitis are: hypointensity in T2, restricted diffusion and hypervascularity in the PZ — all of which are less intense and more localised in general than in PC.26,36,38,48–50
It is useful to quantitatively measure the ADC in a region of interest (ROI) traced in the centre of the lesion. Even with overlap, 0.9 mm2/s is considered a threshold, below which the lesion is suspected to be neoplastic, while inflammation values are generally higher.3,49–51 The finding of a ring-shaped enhancement pattern around a heterogeneous lesion may under certain clinical circumstances suggest a diagnosis of abscess.36
Granulomatous prostatitis, which is uncommon, may be idiopathic or, rarely, secondary to intravesical instillation of Bacillus Calmette-Guerin (BCG) in bladder cancer, genitourinary tuberculosis or prostate surgery. It is considered a great simulator in terms of signs and symptoms and PC imaging, since both conditions may feature a firm nodule detected on digital rectal examination and findings that might even simulate EPE of a tumour, due to inflammatory infiltration of periprostatic fat. Where mycobacterial prostatitis is suspected, there is the option of evaluating, in the short term, response to specific treatment. This condition manifests with areas of necrosis on the contrast study (necrotising prostatitis).37–39
Focal or regional atrophy of the prostate (Fig. 7B) is a common condition generally resulting from chronic inflammation, ischaemia, radiation or antiandrogen therapy. It is characterised by hypointensities on T2 associated with thinning of the PZ and architectural distortion, with more defined margins and less diffusion abnormalities than in other conditions, generally enabling its identification.39,42,52
Isolated hyperplastic BPH nodules are often found in the PZ. They may be extruded or ectopic depending on whether they are more or less connected to the TZ. They may be recognised as such when they are encapsulated (Fig. 7C), in which case they receive a score of 2, even if they are hypointense on ACD — that is to say, without applying the TZ or PZ criteria. Sometimes they contain bright inner foci with an acinar component. Round nodules that have a clearly defined border but are not encapsulated raise a diagnostic dilemma in the PZ (Fig. 7D). Observer experience in recognising BPH traits and precise analysis of all planes and sequences, especially T2-weighted sequences, are critical.
Local staging of prostate cancerLocal staging of PC is governed by the 2017 (8th) edition of the TNM system from the American Joint Committee on Cancer (AJCC). MRI contributes above all to distinguishing between a primary tumour confined to the gland or extended outside of it (T2 and T3 categories), but has more limitations in assessing its intraglandular extension (T2 subcategories), which it tends to underestimate. This is because it identifies the index lesion, or most aggressive lesion, in a high proportion of cases, but is more sensitive for detecting other foci of PC, which is often multifocal.53
The T descriptor, combined with serum PSA and Gleason grade on biopsy, determine, according to different classifications, risk groups with shared subsequent diagnostic management, treatment options and prognosis. Category T3 (tumour with EPE) represents high-risk disease, which does not rule out radical treatment with intention to cure, though it does modify the approach and intensity of treatment. In this case, oncological safety prevails over other considerations (preservation of neurovascular bundles, lymph node dissection associated with prostatectomy, duration of hormone therapy adjuvant to radiotherapy, etc.).54–57 Prostatectomy is rarely indicated in stage T4.53
The reliability of MRI for detection of EPE is highly variable and depends on technical factors, reader experience and the diagnostic threshold adopted.58 In general, mpMRI is more specific than it is sensitive: a 2015 meta-analysis found a specificity of 91% for detection of EPE.59 The final section of the PI-RADSv2.1 mentions signs of EPE and recommends evaluating them,3 though it does not assign them an EPE probability grading, as the ESUR did propose, based on the relationship of the tumour to the prostate surface or "capsule".60 However, the effectiveness and consistency of the ESUR scale have been called into question, as that scale lacks an adequate semiological definition and, more importantly, overlooks the length of capsular contact of the tumour, a strong predictor of extension according to various authors.59,61–64
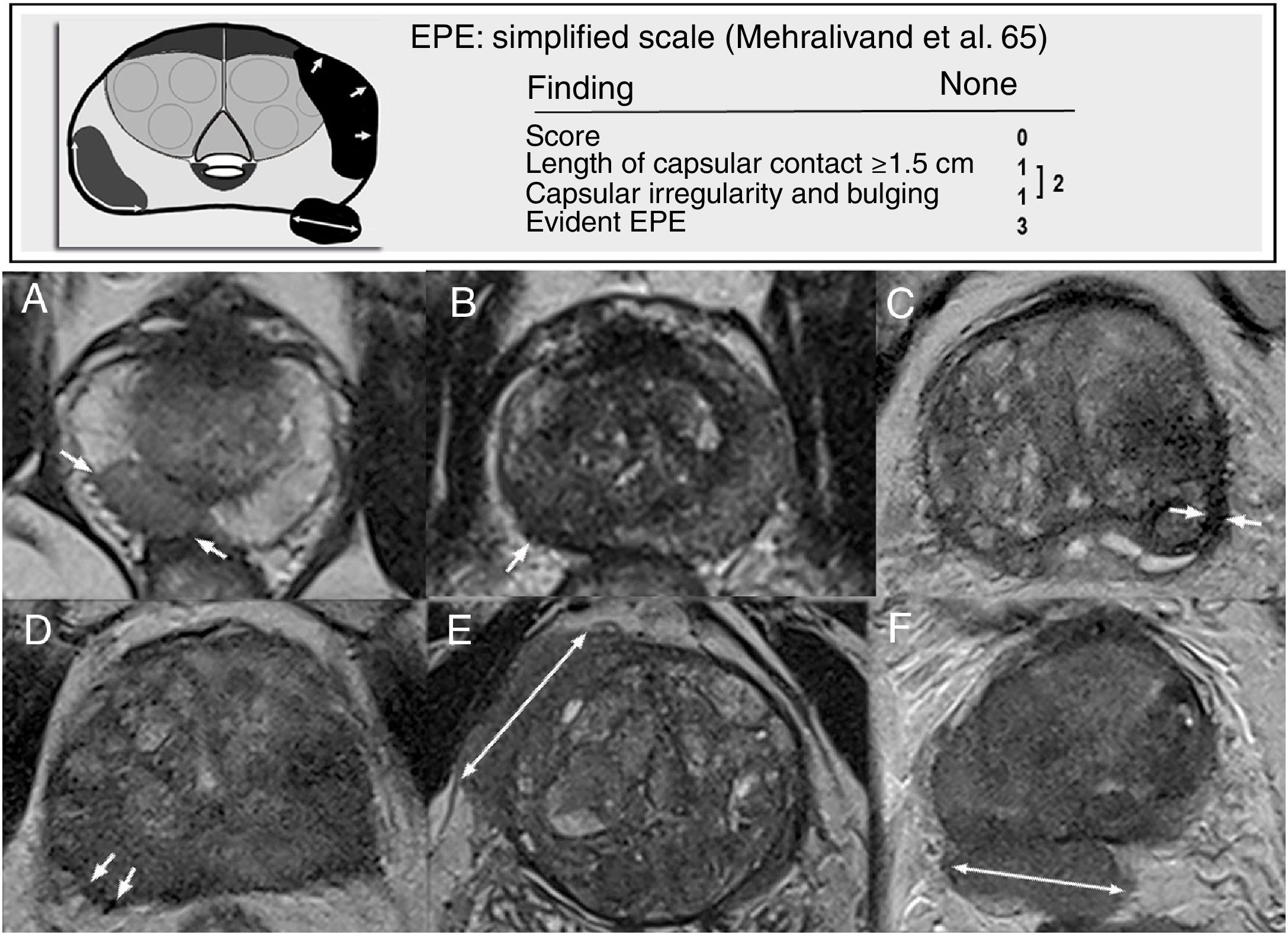
Mehralivand et al. proposed a new scoring system (from 0 to 3) that reduces subjectivity and improves precision in MRI, based on two findings: length of capsular contact exceeding 1.5cm and capsular irregularity with bulging. The presence of one of these results in a score of 1, and the presence of both results in a score of 2; a score of 3 corresponds to manifest capsular disruption. Asymmetry of neurovascular bundles and obliteration of the rectoprostatic angle are considered indicators of late EPE that are not very sensitive and are dependent on the tumour location. They are excluded from this scoring system, as its main advantage is its simplicity65 (Fig. 8).
Signs and probability grading of extraprostatic extension (EPE) according to Mehralivand et al.65 A) Capsular contact exceeding 1.5cm (arrows). B-D) Capsular irregularity with bulging. E) and F) Evident EPE. These would correspond respectively on the old European Society of Urogenital Radiology (ESUR) scale to: capsular contact, irregularity, thickening, engulfment of neurovascular bundles, bulging and measurable EPE.
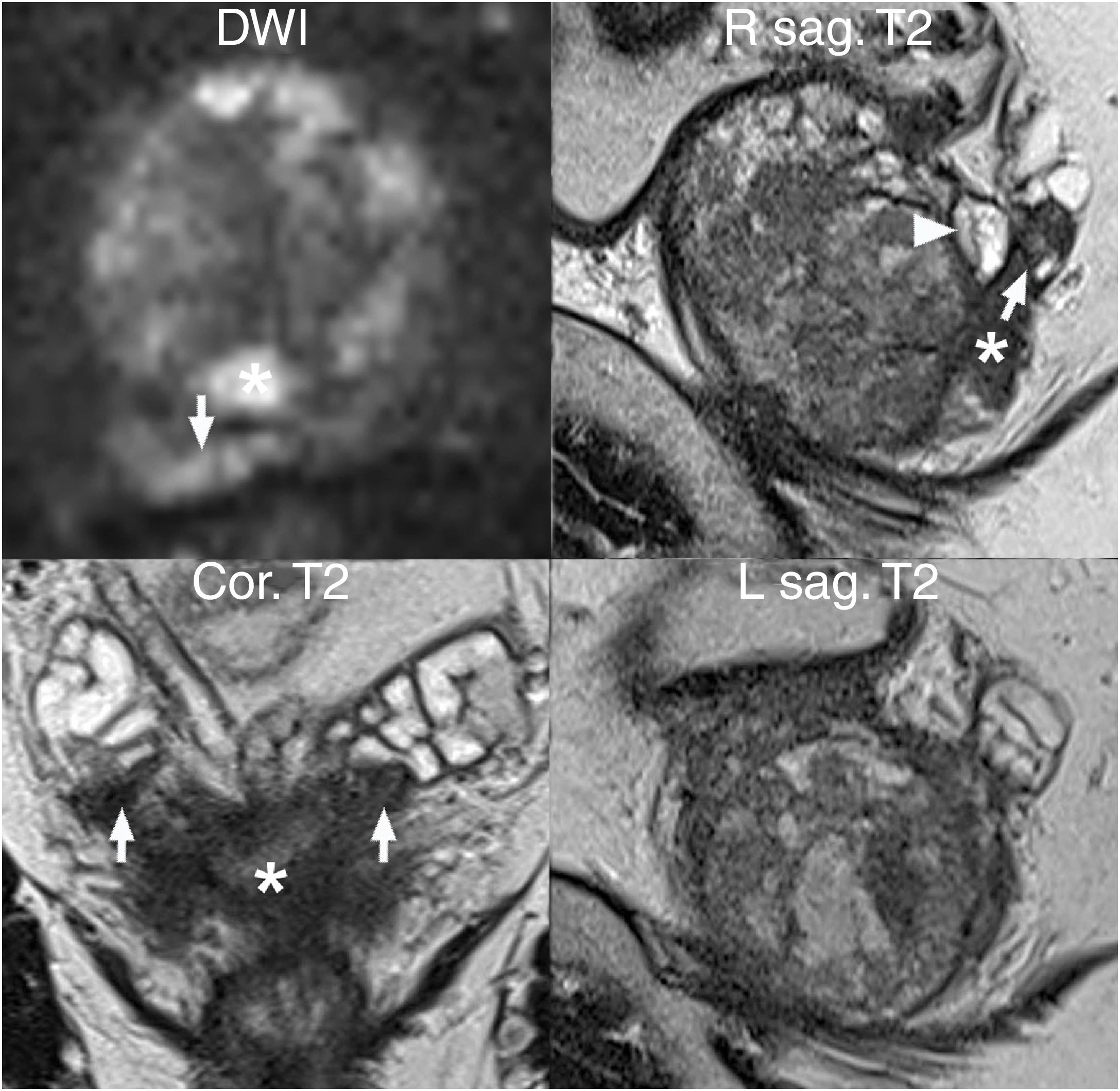
SV invasion (T3b) (Fig. 9) manifests primarily with foci of hypointensity on T2, which should be analysed on the three planes for better detection of instances of asymmetry.55,61,66 DWI and DCE imaging are also useful in assessing SVs and, in general, the extension of the primary tumour (T), with added value for the yield of the technique with respect to the isolated T2-weighted sequence, a sequence that is essential in staging.55,59,61,67
SV invasion (local stage T3b). Value of DWI and T2-weighted multiplanar images: multifocal tumour, with index lesion in the peripheral area of both lobes (asterisk) invading both SVs (arrows), with greater extension on the right as shown by the comparative diffusion-weighted imaging and parasagittal T2-weighted sequences. An extruded nodule typical of BPH is also visible at the base of the right peripheral area (arrow head).
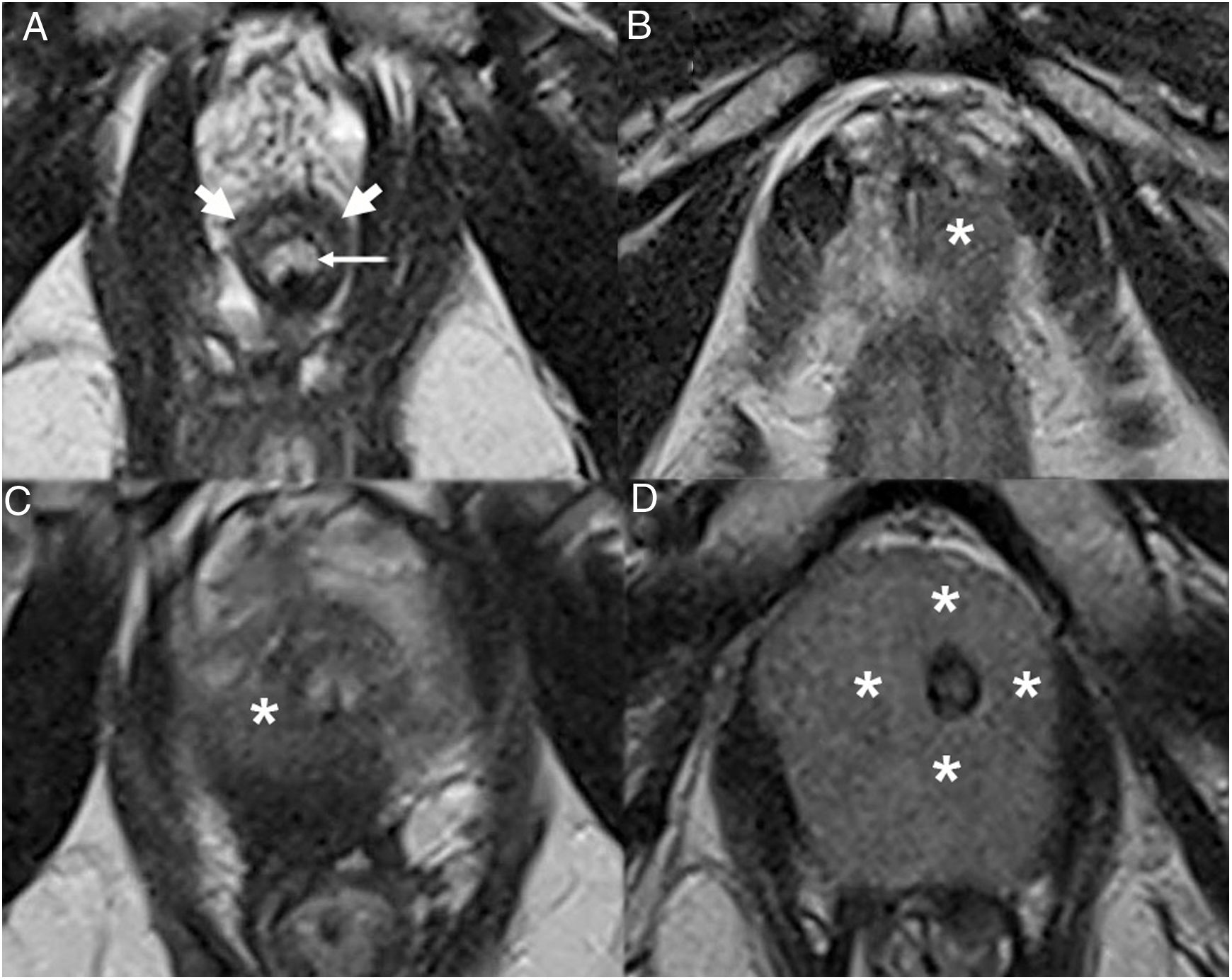
The location of the index lesion is a significant piece of information in staging. While basal tumours are prone to seminal extension, the importance of the apex lies in its lack of capsule and its proximity to the external urethral sphincter (EUS), the integrity of which is key to preserving urinary continence. The EUS appears on T2 as a hypointense half-moon structure anterolaterally surrounding the membranous urethra. Sphincter signal abnormality, associated with PI-RADS 4 or 5 apical lesions (Fig. 10), should raise suspicion of possible invasion which contraindicates surgery (category T4).53
Axial T2-weighted images of a normal EUS and the EUS in several cases of prostate carcinoma. A) Normal EUS (thick arrows) surrounding the membranous urethra (thin arrow) from the front. B-D) Infiltration of the EUS. Apical tumours (asterisk) with a different degree of contact with the EUS, a key finding in decision-making and planning with respect to surgery, even in the absence of clear signs of infiltration.
A diffusion study with field of view (FOV) extended to the lesser pelvis is sensitive for detection of pelvic lymph nodes, but non-specific for diagnosis of tumour infiltration thereof (category N1). The latter should be based on morphological characteristics, which are similar on MRI and computed tomography (CT): round shape, short axis exceeding 8mm, spiculated or hyperenhancing border on DCE imaging.3 Non-regional metastatic lymph nodes (common iliac or retroperitoneal lymph nodes) are considered M1a.43
Finally, examination of all other bone and visceral structures included in the study must not be overlooked so as to detect possible metastases and primary neoformations in the rectum, bladder and other structures.
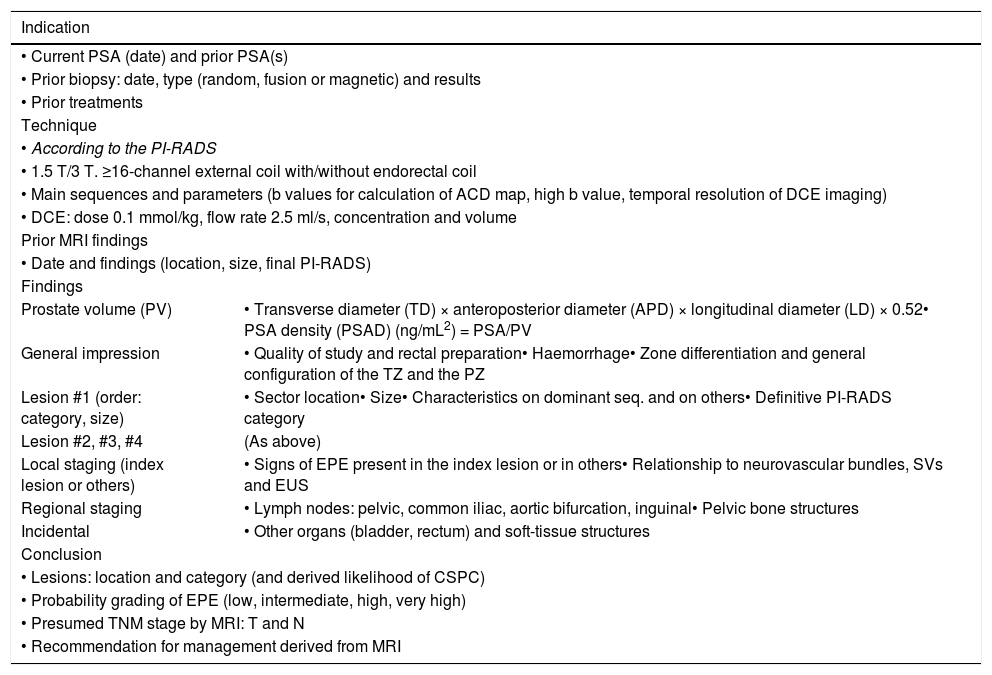
Report recommendationsThe current version of the PI-RADS provides a structured report template, which we present with slight modifications in Table 3, in order to facilitate patient management based on the score obtained in the mpMRI for up to 4 lesions, ordered by their importance.
Structured report template, adapted from the PI-RADS 2.1.3.
| Indication | |
|---|---|
| • Current PSA (date) and prior PSA(s) | |
| • Prior biopsy: date, type (random, fusion or magnetic) and results | |
| • Prior treatments | |
| Technique | |
| • According to the PI-RADS | |
| • 1.5 T/3 T. ≥16-channel external coil with/without endorectal coil | |
| • Main sequences and parameters (b values for calculation of ACD map, high b value, temporal resolution of DCE imaging) | |
| • DCE: dose 0.1 mmol/kg, flow rate 2.5 ml/s, concentration and volume | |
| Prior MRI findings | |
| • Date and findings (location, size, final PI-RADS) | |
| Findings | |
| Prostate volume (PV) | • Transverse diameter (TD) × anteroposterior diameter (APD) × longitudinal diameter (LD) × 0.52• PSA density (PSAD) (ng/mL2) = PSA/PV |
| General impression | • Quality of study and rectal preparation• Haemorrhage• Zone differentiation and general configuration of the TZ and the PZ |
| Lesion #1 (order: category, size) | • Sector location• Size• Characteristics on dominant seq. and on others• Definitive PI-RADS category |
| Lesion #2, #3, #4 | (As above) |
| Local staging (index lesion or others) | • Signs of EPE present in the index lesion or in others• Relationship to neurovascular bundles, SVs and EUS |
| Regional staging | • Lymph nodes: pelvic, common iliac, aortic bifurcation, inguinal• Pelvic bone structures |
| Incidental | • Other organs (bladder, rectum) and soft-tissue structures |
| Conclusion | |
| • Lesions: location and category (and derived likelihood of CSPC) | |
| • Probability grading of EPE (low, intermediate, high, very high) | |
| • Presumed TNM stage by MRI: T and N | |
| • Recommendation for management derived from MRI | |
CSPC: clinically significant prostate cancer; DCE: dynamic contrast-enhanced imaging; EPE: extraprostatic extension; EUS: external urethral sphincter; PSA: prostate specific antigen; PZ: peripheral zone; SVs: seminal vesicles; TZ: transition zone.
The report should contain: indication, technique, findings and impression. The impression should explicitly state not only the definitive score for the lesions, but also their significance (likelihood of CSPC).54,68
In the body of the report, lesions should always be located on the sector map and described on each imaging sequence with the resulting overall category; according to the PI-RADSv2.1, indicating their particular category on each sequence is optional.3 It is useful to add key images to the report or at least permanently identify them in the picture archiving and communication system (PACS).
Findings relevant for staging, or the absence thereof, should be expressly stated, especially length of capsular contact, capsular bulging or irregularity, and the distance or relationship of the index lesions to the neurovascular bundles, SVs and EUS.
The overall estimated likelihood of EPE may be considered in terms of a scale with four grades (low, intermediate, high and very high) corresponding to levels 0, 1, 2 and 3 on the simplified scale.65 It is recommended, but not required before histological diagnosis, that the presumed stage be specified.
The PI-RADSv.2.1 does not include recommendations for management. Therefore, the impression must, especially where there are misleading results, be accompanied by a recommendation that includes available imaging characteristics and clinical data: age, PSA history and density (cut-off point >0.15ng/mL2), prior biopsies and MRIs, digital rectal examination, inflammatory symptoms, patient expectations and standards of care.69–74 It is a good idea for indeterminate cases and cases with a discrepancy between signs and symptoms, imaging, and anatomical pathology to be discussed as a team in multidisciplinary committees.
ConclusionmpMRI, a technique in constant development, has attained high levels of standardisation and clinical use, rendering it a very valuable tool for the radiologist. This study offers a review of the set of current recommendations for its performance, with a particular emphasis on practical considerations and possible causes of error or confusion that should be taken into consideration in its interpretation. Understanding and assimilating the PI-RADS guidelines is an initial requirement that, along with other training resources, helps improve the quality of the radiological information from the mpMRI and its value for the diagnosis and initial management of prostate cancer in different settings.
AuthorshipResponsible for the integrity of the study: RSO, JTN and GMS.
Study concept: RSO and JTN.
Study design: RSO, JTN, GMS, QGO and MB.
Data acquisition: not applicable.
Data analysis and interpretation: not applicable.
Statistical processing: not applicable.
Literature search: RSO, JTN and QGO.
Drafting of the study: RSO, JTN, GMS, QGO and MB.
Critical review of the manuscript with intellectually relevant contributions: RSO, JTN, GMS, QGO and MB.
Approval of the final version: RSO, JTN, GMS, QGO and MB.
FundingThis review received no specific grants from public agencies, the commercial sector or non-profit organisations.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The authors would like to thank the specialist radiology and nursing teams of the Diagnostic Imaging Department at Hospital General de Teruel [Teruel General Hospital], as well as: Dr E.M. Alonso-Muñoz, Dr E. García Martínez, Dr SP Alandete Germán and Dr M.A. Meseguer Ripollés.
Please cite this article as: Sánchez-Oro R, Torres Nuez J, Martínez-Sanz G, Grau Ortega Q, Bleila M. Resonancia magnética de próstata: guía práctica de interpretación e informe según PI-RADS versión 2.1. Radiología. 2020;62:437–451.