To evaluate the need for surgical biopsy in patients diagnosed with radial scars without atypia by percutaneous biopsy.
Material and methodsIn this retrospective observational study, we selected patients with a histological diagnosis of radial scar in specimens obtained by percutaneous biopsy during an 8-year period. The statistical analysis was centered on patients with radial scar without atypia (we assessed the radiologic presentation, the results of the percutaneous biopsy, and their correlation with the results of surgical biopsy and follow-up) and we added the patients with atypia and cancer in the elaboration of the diagnostic indices.
ResultsWe identified 96 patients with radial scar on percutaneous biopsy; 54 had no atypia, 18 had atypia, and 24 had cancer. Among patients with radial scar without atypia, there were no statistically significant differences between patients who underwent imaging follow-up and those who underwent surgical biopsy (p>0.05). The rate of underdiagnosis for percutaneous biopsy in patients without atypia was 1.9 per cent. The rates of diagnosis obtained with percutaneous biopsy in relation to follow-up and surgical biopsy in the 96 cases were sensitivity 92.3 per cent, specificity 100 per cent, positive predictive value 100 per cent, negative predictive value 97.2 per cent, and accuracy 97.9 per cent. The area under the ROC curve was 0.96 (p<0.001), and the kappa concordance index was 0.95 (p<0.001).
ConclusionsWe consider that it is not necessary to perform surgical biopsies in patients with radial scars without atypia on percutaneous biopsies because the rate of underestimation is very low and the concordance between the diagnosis reached by percutaneous biopsy and the definitive diagnosis is very high.
Evaluar la necesidad de biopsia quirúrgica en pacientes diagnosticadas por biopsia percutánea de cicatriz radial sin atipia.
Material y métodosRealizamos un estudio observacional retrospectivo y seleccionamos las pacientes con diagnóstico histológico en biopsia percutánea de cicatriz radial durante un periodo de 8 años. El análisis estadístico principal se centró en pacientes con cicatriz radial sin atipia (valoramos la presentación radiológica, los resultados de la biopsia percutánea y su correlación con la biopsia quirúrgica y seguimiento) y añadimos a las pacientes con atipia y cáncer en la elaboración de índices diagnósticos.
ResultadosIdentificamos 96 pacientes con cicatriz radial en biopsia percutánea. Cincuenta y cuatro no presentaban atipia, 18 asociaban algún tipo de atipia y 24, cáncer. No hubo diferencias estadísticas significativas al comparar las pacientes en seguimiento radiológico con aquellas que se sometieron a biopsia quirúrgica en el grupo sin atipia (p>0,05). La tasa de infraestimación de la biopsia percutánea en pacientes sin atipia fue del 1,9%. Los índices diagnósticos obtenidos para la biopsia percutánea en relación con el seguimiento y la biopsia quirúrgica en los 96 casos fueron: sensibilidad, 92,3%; especificidad, 100%; valor predictivo positivo, 100%; valor predictivo negativo, 97,2%; y exactitud, 97,9%. El área bajo la curva ROC fue de 0,96 (p<0,001) y el índice de concordancia kappa de 0,95 (p<0,001).
ConclusiónConsideramos que no es necesario realizar biopsia quirúrgica en pacientes diagnosticadas de cicatriz radial sin atipia en biopsia percutánea, ya que la tasa de infraestimación es muy baja y existe un elevado grado de concordancia entre la biopsia percutánea y el diagnóstico definitivo.
The radial scar (RS) is a histological entity characterized by one fiber-elastic center continuing glandular elements from which ductal and lobular structures in a “starry sky” configuration irradiate.1–3 Sizes >1cm are called “complex sclerosing lesions”,2 although this term and the term radial scar are used interchangeably.
The radiological appearance of RS is undistinguishable from invasive carcinomas.4 When it is seen in the mammogram, it is common to see a distortion of the architecture (radiolucent core and spiculae irradiating from this core and towards the periphery).5 However, it may also appear as nodules, microcalcifications, or focal asymmetries.6 Its prevalence is around 2 per cent in the percutaneous biopsies performed to non-palpable lesions.7
Benign breast lesions may be categorized into: non-proliferative, proliferative without atypia, and proliferative with atypia. RS may be associated with proliferative lesions without atypia, and proliferative lesions with atypia, cancer.8,9
The increased risk of cancer in proliferative lesions with atypia has been well documented in numerous studies, up to 4.4 times higher than that of the general population.1,10 Quantifying the risk of breast cancer in proliferative lesions without atypia is a common issue, which is why the management of patients diagnosed with RS without atypia through a percutaneous biopsy is still controversial.6 Some authors believe that it is a benign lesion with no risk of malignant transformation,3,11,12 while others believe that it is a pre-malignant lesion precursor of breast cancer.13,14
The usual management of RS without atypia diagnosed through a percutaneous biopsy has been the surgical biopsy for some time now.4,5,15,16 However, recent evidence suggests that surgical resection is not necessary if the RS does not associate epithelial atypia.17,18
The main goal of this study is to assess the need for surgical biopsy after the diagnosis of RS without atypia through a percutaneous biopsy, in order to be able to determine if the surgical intervention may be avoided.
Material and methodsWe conducted one retrospective observational trial after obtaining the approval from our center ethics committee. We selected those patients whose anatomopathological report of the percutaneous biopsies performed showed radial scars, sclerosing radial lesions, or complex sclerosing lesions in order to identify cases of RS during a period of 8 years (from 2005 through 2012). The main statistical analysis was performed with the group of patients without atypia. Patients with signs of malignancy and atypia only, and patients without atypia were included in the study while conducting the concordance analyses and diagnostic indices.
Patients diagnosed with RS through the percutaneous biopsy, and those who did not undergo surgical biopsies, or were not followed were excluded.
All those mammographic findings based on the Breast Imaging Reporting and Data System (BIRADS) – 5th edition19 in nodules, architectural distortion, focal asymmetry, or microcalcifications were assessed.
The percutaneous biopsy was performed while guided by an ultrasound or stereotaxy and depending on the visibility of the lesion and preference of the radiologist. During the first years, the core needle biopsy (CNB) (Acecut biopsy needle, TSK, Japan) was used, but it would be substituted by the vacuum-assisted biopsy (VAB) with one 9 or 10G needle (Mammotome, stereotactic guided breast biopsy system Eviva, Hologic, UK and Stereotactic/Ultrasound Encor Probe, SenoRx Inc., USA). The percutaneous biopsy used to be repeated in the absence of concordance between the radiological and histological findings, or if the pathologist recommended taking more samples. The minimum experience of the radiologists and pathologists who participated in the study was 10 years.
In the case of ultrasound-guided biopsies, there was not such a thing as a standard number of samples, and the final amount depended on the radiologist who performed it (between 3 and 6). In the stereotaxy-guided VAB, the minimum quantity was 12.
All patients with cancer diagnosed by the percutaneous biopsy received therapy. Surgical biopsies were recommended to patients who underwent percutaneous biopsies before 2006 and whose results were RS with or without atypia, because it was the recommended practice at the time. From 2006 through 2012, the common practice was performing one VAB if the CNB findings were RS. The surgical biopsy was only performed in the presence of atypia, or in the absence of concordance between the radiological findings and the anatomopathological findings.
Patients who did not undergo surgical biopsies underwent clinical-radiological follow-up. At least during the first two years, the very first follow-up after the biopsy was conducted after 6 months, and then followed by annual controls. In the remaining cases, follow-up was annual.
The data collected for the study were: age of the patient, type of lesion, size of the lesion, method of percutaneous biopsy used, if needed method of second percutaneous biopsy used, follow-up time of those patients who did not undergo surgical biopsies, and anatomopathology of the percutaneous biopsies and the surgical biopsy. We included the variable of presence of cancer, or not, as definitive diagnosis, with the understanding that it was definitive as long as the patients showed cancer in the surgical biopsy or at some point during follow-up. These data were collected retrospectively using the computing system of radiological reports (PDI), the imaging storage server (PACS, Carestream), and our hospital system for the management of anatomopathological reports.
The statistical analysis was conducted using the IBM SPSS software (version 10.0). We conducted descriptive studies of patients with RS and without atypia. We assessed the differences among the different variables between the follow-up groups and the surgical biopsy group (chi square statistic test, Fisher's exact test, or Student's t test). The underestimation rate of the percutaneous biopsy in both groups was assessed (Fisher's exact test). We defined underestimation as a benign finding in the percutaneous biopsy, and as a malignant finding in the definitive diagnosis. Findings were expressed as absolute numbers, and/or percentages in the qualitative variables, and as mean, standard deviation (SD), and range in the quantitative variables.
Finally we included all patients without atypia, with atypia, and with cancer in order to correlate the findings from the percutaneous biopsy (presence of cancer, or not) with the definitive findings using 2×2 contingency tables, and then proceeded to estimate the diagnostic indices with their corresponding 95 per cent confidence intervals (CI) (sensitivity, specificity, positive predictive value, negative predictive value, and accuracy), and their graphic representation with ROC curves. We used these same variables to conduct the concordance analysis using Cohen's kappa coefficient. p-Value<0.05 was considered a significant value.
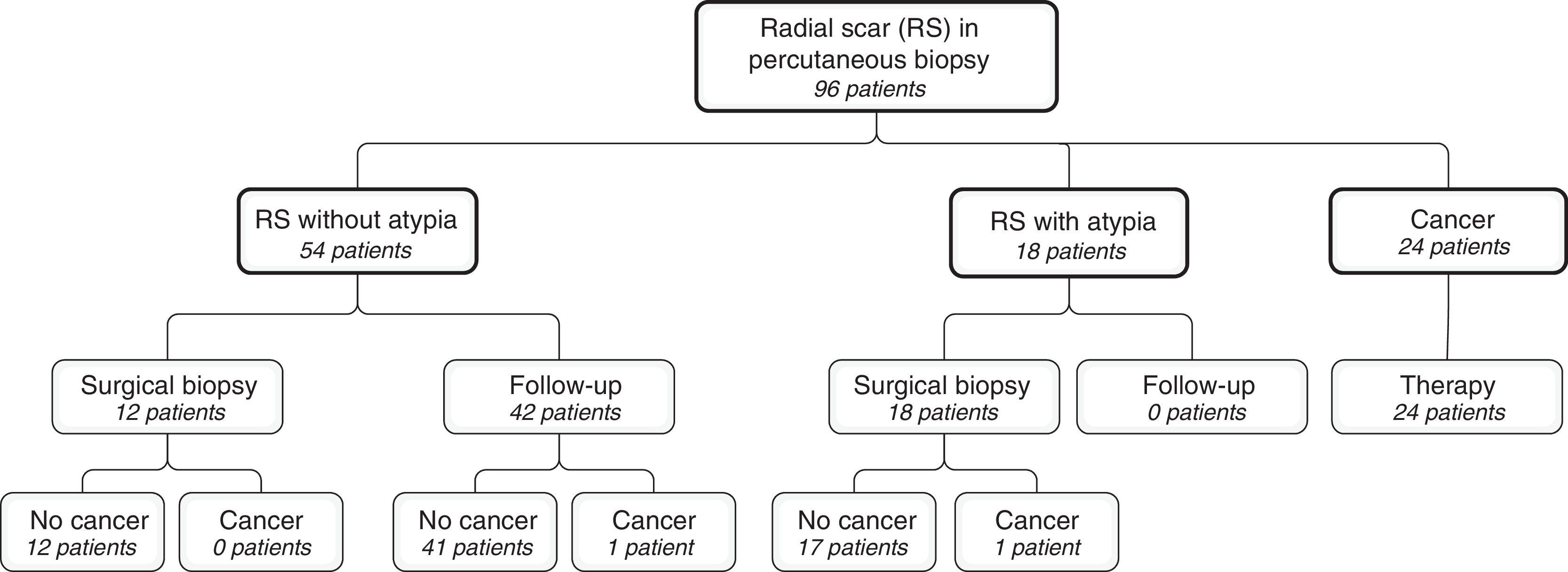
ResultsWe identified a total of 96 patients with histological diagnoses of RS after performing the percutaneous biopsy (Fig. 1). The study central group was established with 54 patients without atypia (56.3 per cent).
The age range of patients from this group was between 24 and 71 years old (an average 49.1±10.2 years old).
The most common mammographic finding was the distortion of breast architecture in a total of 32 cases (59.3 per cent), followed by the nodule in 17 patients (31.5 per cent), microcalcifications in 3 patients (5.6 per cent), and focal asymmetry in 2 of these patients (3.7 per cent). The average size was 17.8±11.8mm (5–60mm).
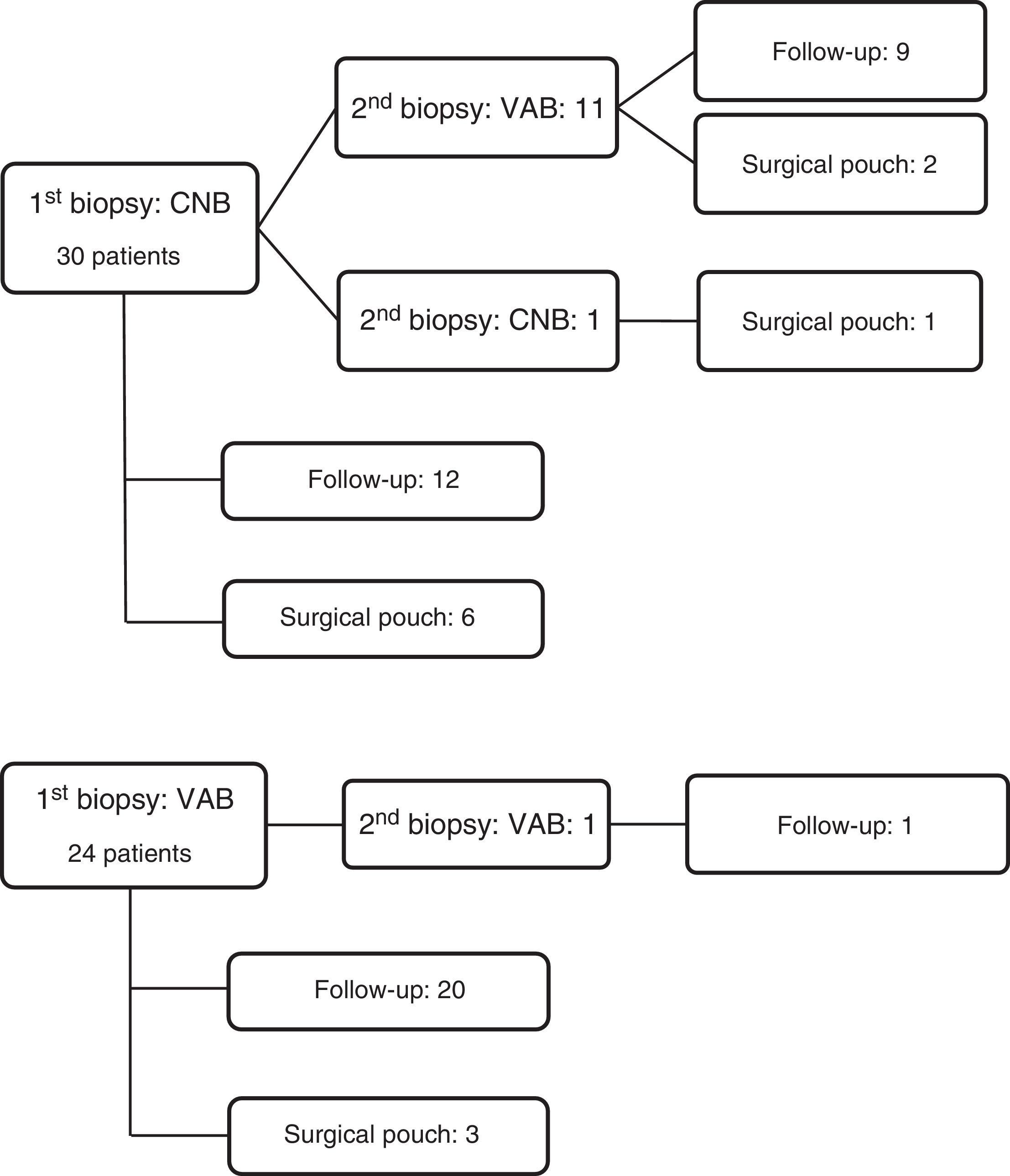
Forty-one patients underwent one percutaneous biopsy only. In the remaining 13 cases, two percutaneous biopsies were needed. Data are shown in Fig. 2.
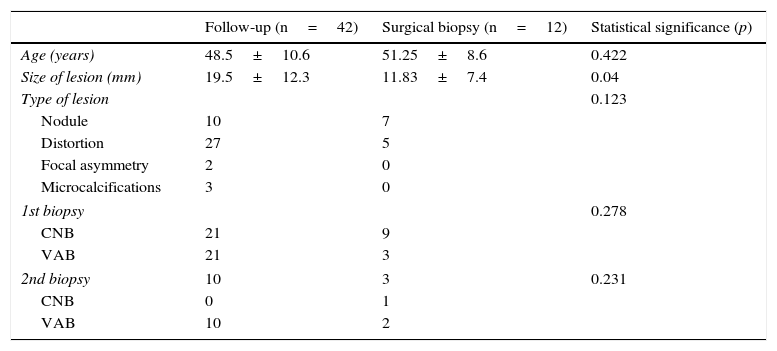
Twelve patients underwent surgical biopsies, and 42 were referred to follow-up. The average follow-up time was 71.4 months (24–113 months). Table 1 shows the variables included in the study comparing the follow-up patients with the surgical biopsy group. We only found statistically significant differences in the size of the lesion (19.5mm versus 11.8mm; p-value=0.04). Table 2 shows the number of cancer cases detected by the percutaneous biopsy in the follow-up group compared to the surgical biopsy group. There were not any statistically significant differences (p=0.778). The overall rate of percutaneous biopsy underestimation in patients without atypia was one case out of a total of 54 patients (1.9 per cent).
Characteristics of patients with radial scar without atypia in the groups of follow-up and surgical biopsy. CNB: core needle biopsy; VAB: vacuum-assisted biopsy.
| Follow-up (n=42) | Surgical biopsy (n=12) | Statistical significance (p) | |
|---|---|---|---|
| Age (years) | 48.5±10.6 | 51.25±8.6 | 0.422 |
| Size of lesion (mm) | 19.5±12.3 | 11.83±7.4 | 0.04 |
| Type of lesion | 0.123 | ||
| Nodule | 10 | 7 | |
| Distortion | 27 | 5 | |
| Focal asymmetry | 2 | 0 | |
| Microcalcifications | 3 | 0 | |
| 1st biopsy | 0.278 | ||
| CNB | 21 | 9 | |
| VAB | 21 | 3 | |
| 2nd biopsy | 10 | 3 | 0.231 |
| CNB | 0 | 1 | |
| VAB | 10 | 2 | |
Quantitative variables expressed in mean and standard deviation (SD); qualitative variables expressed in absolute numbers.
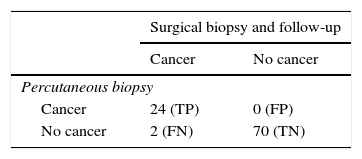
Comparison between follow-up and surgical biopsy groups with respect to the definitive diagnosis in patients without atypia in the percutaneous biopsy.
| Definitive diagnosis | Total | ||
|---|---|---|---|
| Cancer | No cancer | ||
| Follow-up | 1 (2.4 per cent) | 41 (97.6 per cent) | 42 (100 per cent) |
| Surgical biopsy | 0 (0 per cent) | 12 (100 per cent) | 12 (100 per cent) |
When correlating the study variables with the presence of cancer in the definitive diagnosis we found no differences at all (p>0.05). However, the differences in size between both groups were close to being statistically significant, with an average of 40mm in the cancer group, and an average of 17mm in the cancer-free group (p=0.057).
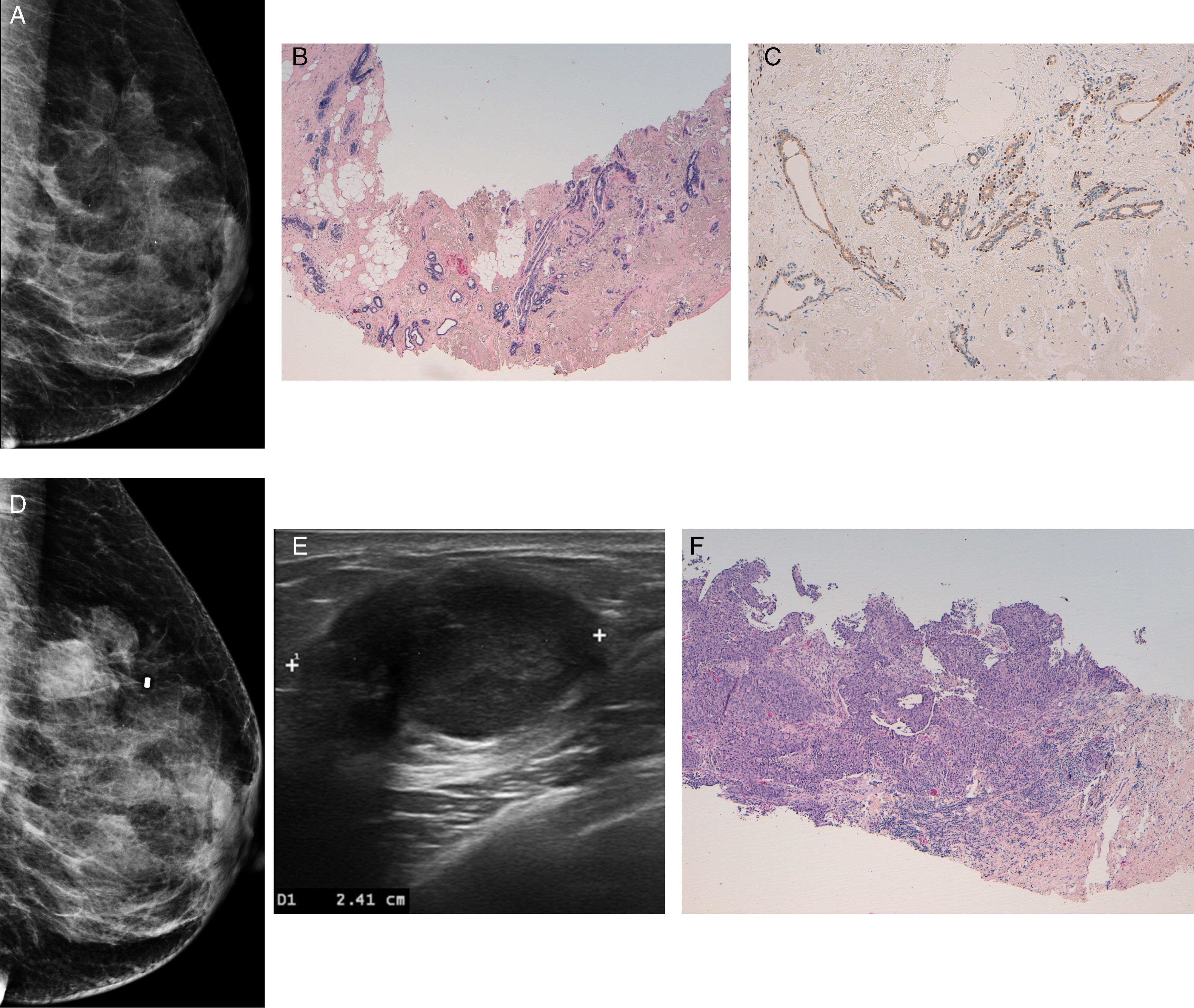
The only patient with cancer during follow-up underwent one CNB procedure with a diagnosis of RS without atypia. Ordered by the pathologist, the same percutaneous biopsy was performed through one VAB procedure with the same finding. After two years, one nodule adjacent to the lesion was found with a diagnosis of infiltrating duct carcinoma (IDC) through the CNB (Fig. 3).
Fifty-two year-old-patient with a distortion in the external upper quadrant of her left breast (A). The HE×40 histological examination after vacuum-assisted biopsy (B) shows breast tissue with hyalinization and areas of central elastosis that capture and distort the ducts. The immunohistochemistry [p63×100 (C)], confirms the presence of myoepithelial cells surrounding the ducts–indicative of their non-invasive nature, and all of it compatible with one radial scar. The mammogram (D) and ultrasound (E) of the same patient performed two years after the first diagnosis show one nodule with poorly-defined edges, that is solid in the ultrasound, in the external upper quadrant of her left breast. In the analysis performed using one core needle biopsy [HE×40 (F)] the diagnosis of poorly differentiated infiltrating ductal carcinoma in the area of prior radial scar is confirmed.
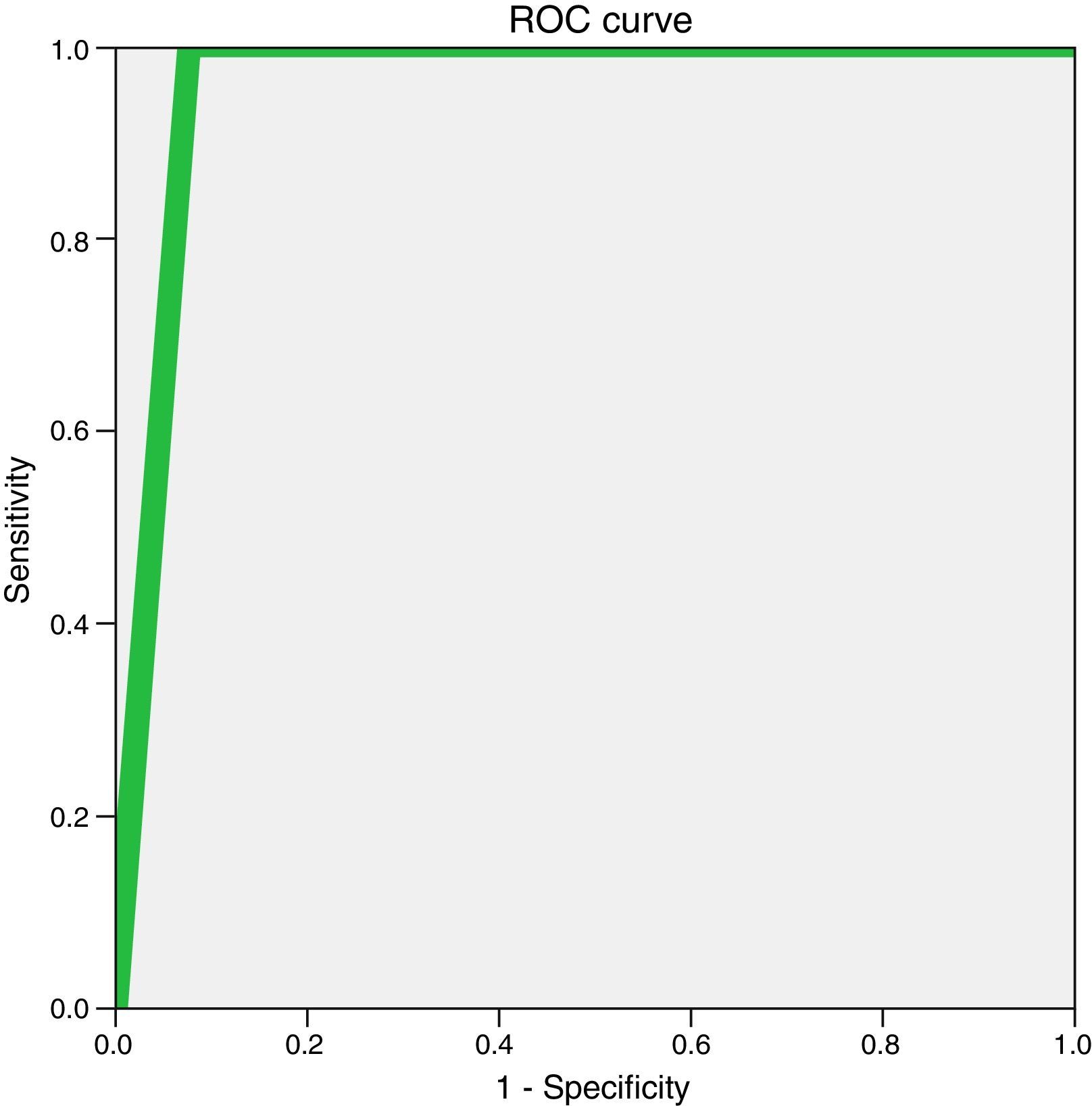
Table 3 shows the findings made through percutaneous biopsies and their correlation with the definitive diagnosis achieved in the 96 cases (patients without atypia, with atypia, and cancer), from which the following diagnostic indices with their corresponding 95 per cent CI were obtained: sensitivity, 92.3 per cent (75.9–97.9 per cent); specificity, 100 per cent (94.8–100 per cent); positive predictive value, 100 per cent (95–100 per cent); negative predictive value, 97.2 per cent (90.4–99.2 per cent), and accuracy, 97.9 per cent (92.7–99.4 per cent). Fig. 4 shows one ROC curve where these data are graphically represented. The area under the curve was 0.96 (I95 per cent CI; 0.9–1) (p<0.001).
Correlation between the percutaneous biopsy findings and the definitive diagnosis in patients without atypia, with atypia, and cancer.
| Surgical biopsy and follow-up | ||
|---|---|---|
| Cancer | No cancer | |
| Percutaneous biopsy | ||
| Cancer | 24 (TP) | 0 (FP) |
| No cancer | 2 (FN) | 70 (TN) |
FN: false negative; FP: false positive; TN: true negative; TP: true positive.
The concordance using the Cohen's kappa coefficient between the percutaneous biopsy and the definitive diagnosis was 0.95 (95 per cent CI; 0.87–1) (p<0.001).
DiscussionThe radiological definition of RS was established by Tabár and Dean, and in a mammogram it requires the presence of, at least, three of the following criteria: radiolucent core, spiculae irradiating from the radiotransparent center, variable appearance in the different projections, and absence of a palpable lesion, or cutaneous changes.4,20 However, histological tubular carcinomas may present as a lesion similar to the one reported by these authors, which is the reason why it is always necessary to perform a biopsy to confirm diagnosis.2
The RS is present in between 8 per cent, and 16 per cent of all women and goes up to 42 per cent in the contralateral breast in patients with a history of IDC, according to studies based on autopsies.11 Other studies estimate its incidence in autopsies at around 28 per cent, and in biopsized patients due to the presence of radiological anomalies, its incidence remains around 7 per cent.21
The larger number of RS detection during the last few years has been attributed to the introduction of new more sensitive imaging modalities capable of detecting more of these lesions that before used to be misdiagnosed.5
Our study shows a low rate of percutaneous biopsy underestimation, and excellent diagnostic indices derived from percutaneous biopsies. Equally important is the lack of a statistically significant association between age, type/size of lesion, or type/number of percutaneous biopsies performed and the risk of cancer, and the non-statistically significant differences between the cases underestimated in the follow-up group and the surgical biopsy group in patients without atypia.
In the medical literature we reviewed, we could not find consensus on what cases are truly eligible for surgical biopsy when in the presence of one proliferative lesion after percutaneous biopsy. There is a great variability on the prevalence of carcinomas associated with RS after performing one surgical biopsy, that goes from 0 per cent to 40 per cent.12,22 When a diagnosis of RS with atypical ductal hyperplasia, lobular neoplasm, or papilloma is achieved through one percutaneous biopsy, the frequency of malignancy after surgery is around 26 per cent, number that drops to 7 per cent whenever the RS does not associate this type of proliferative lesion.12
In our study, the underestimation rate of percutaneous biopsies in the group of patients with RS without atypia was very low (1.9 per cent) and these data are consistent with those reported by other authors such as Li et al. (0.9 per cent),12 Elizabeth Min (1.6 per cent),22 Conlon et al. (2 per cent),4 and Rajan et al. (4.5 per cent).23 All of them conclude that the development of cancer close to a region where there already existed one RS is really an incidental finding. This hypothesis is backed by the fact that patients followed-up after RS surgery do not have a higher risk of malignancy than women in the general population. Also, when comparing patients diagnosed with breast cancer with women without cancer, the history of surgery for RS is the same in both groups.24,25 In our case, there were not any statistically significant differences when comparing cases of cancer between followed patients and patients who underwent surgical biopsies (considered as the best option for a long time).
Some authors claim that the age of the patients, and the size of the lesion may have something to do with the chances of having cancer regions associated with one RS. Thus, lesions <6–7mm in women under 40 do not pose a higher risk of cancer, but in patients over 50 and lesions >2cm, the risk of cancer is higher.17,26 In our case, the differences in size between patients with and without cancer in the definitive diagnosis were not statistically significant, although they were close to being statistically significant.
This study has some limitations. Although it had access to a numerous population given the circumstances of our center during a long period of time, the number of patients included is not too high, which may lead to inconsistent findings. The type of biopsies performed, the number of samples obtained, and the concordance, or not, between the histological and the radiological findings were not standardized and depended on the radiologist responsible for the patient. Also we did not have the breast cancer family history either, or the personal risk for developing breast cancer. In addition, not all the patients were followed for the same amount of time, since the criterion for referral at discharge was not standardized and depended on other additional factors unforeseen by this analysis.
The surgical biopsy is not free from risks (pain, hematomas, suture dehiscence, infections, risks derived from anesthesia, etc.), which adds to the costs of the intervention, and the anxiety it generates in the patient. The development of multidisciplinary teams, the advances of imaging modalities, and the advances of percutaneous biopsies make follow-up, occasionally, a better option than surgical interventions. This is why we believe that patients diagnosed with RS without atypia through a percutaneous biopsy are eligible for radiological follow-up, and it is not necessary to perform any surgical biopsies since the rate of cancer detected is low and the concordance, sensitivity, and specificity of the percutaneous biopsy for the diagnosis of malignant lesions in one RS is very high. Although the underestimation rate of percutaneous biopsies in patients with RS and atypia has been lower than expected, we think it necessary to make analyses that target this type of lesions in order to confirm the findings reported.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed abide by the ethics and regulations of the Human Research Committee, the World Health Organization and the Declaration of Helsinki.
Confidentiality of dataThe authors declare that they have followed the protocols from their centers on the disclosure of data from patients.
Right to privacy and informed consentThe authors have obtained prior written informed consent from the aforementioned patients. This document belongs to the corresponding author.
Authors contribution- 1.
Manager of the integrity of the study: JMQ.
- 2.
Study Idea: MCG, JLRP, SRM, JMQ, MMP, and AML.
- 3.
Study Design: JMQ, SRM, JLRP, and MCG.
- 4.
Data Mining: MCG, MMP, JLRP, JMQ, SRM, and AML.
- 5.
Data Analysis and Interpretation: JMQ, SRM, JLRP, and MCG.
- 6.
Statistical Analysis: JMQ, and SRM.
- 7.
Reference: JMQ, and SRM.
- 8.
Writing: JMQ.
- 9.
Critical review of the manuscript with intellectually relevant remarks: JLRP, MCG, SRM, MMP, and AML.
- 10.
Approval of final version: JLRP, SRM, MCG, AML, MMP, and JMQ.
The authors declare no conflict of interests associated with this article whatsoever.
Please cite this article as: Mesa-Quesada J, Romero-Martín S, Cara-García M, Martínez-López A, Medina-Pérez M, Raya-Povedano JL. Cicatriz radial sin atipia en biopsia percutánea. ¿Puede evitarse la biopsia quirúrgica? Radiología. 2017;59:523–530.







![Fifty-two year-old-patient with a distortion in the external upper quadrant of her left breast (A). The HE×40 histological examination after vacuum-assisted biopsy (B) shows breast tissue with hyalinization and areas of central elastosis that capture and distort the ducts. The immunohistochemistry [p63×100 (C)], confirms the presence of myoepithelial cells surrounding the ducts–indicative of their non-invasive nature, and all of it compatible with one radial scar. The mammogram (D) and ultrasound (E) of the same patient performed two years after the first diagnosis show one nodule with poorly-defined edges, that is solid in the ultrasound, in the external upper quadrant of her left breast. In the analysis performed using one core needle biopsy [HE×40 (F)] the diagnosis of poorly differentiated infiltrating ductal carcinoma in the area of prior radial scar is confirmed. Fifty-two year-old-patient with a distortion in the external upper quadrant of her left breast (A). The HE×40 histological examination after vacuum-assisted biopsy (B) shows breast tissue with hyalinization and areas of central elastosis that capture and distort the ducts. The immunohistochemistry [p63×100 (C)], confirms the presence of myoepithelial cells surrounding the ducts–indicative of their non-invasive nature, and all of it compatible with one radial scar. The mammogram (D) and ultrasound (E) of the same patient performed two years after the first diagnosis show one nodule with poorly-defined edges, that is solid in the ultrasound, in the external upper quadrant of her left breast. In the analysis performed using one core needle biopsy [HE×40 (F)] the diagnosis of poorly differentiated infiltrating ductal carcinoma in the area of prior radial scar is confirmed.](https://static.elsevier.es/multimedia/21735107/0000005900000006/v1_201711202321/S2173510717300514/v1_201711202321/en/main.assets/thumbnail/gr3.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)


