Most of the patients who overcome the SARS-COV-2 infection do not present complications and do not require a specific follow-up, but a significant proportion (especially those with moderate/severe clinical forms of the disease) require clinicalradiological follow-up. Although there are hardly any references or clinical guidelines regarding the long-term follow-up of post-COVID-19 patients, radiological exams are being performed and monographic surveillance consultations are being set up in most of the hospitals to meet their needs. The purpose of this work is to share our experience in the management of the post-COVID-19 patient in two institutions thathave had a high incidence of COVID-19 and to propose general follow-uprecommendations from a clinical and radiological perspective.
La mayor parte de los pacientes que superan la infección por SARS-COV-2 no presentan complicaciones ni requieren un seguimiento específico, pero una proporción significativa (especialmente aquellos con formas clínicas moderadas/graves de la enfermedad) necesitan un seguimiento clínico-radiológico. Aunque apenas existen referencias o guías clínicas sobre el seguimiento a largo plazo de estos pacientes post-COVID-19, se están realizando pruebas radiológicas y constituyendo consultas monográficas de vigilancia en la mayor parte de los centros hospitalarios para atender sus necesidades. El propósito de este trabajo es compartir nuestra experiencia en el manejo del paciente post-COVID-19 en dos instituciones que han tenido una elevada incidencia de la COVID-19 y proponer unas recomendaciones generales de seguimiento desde una perspectiva clínica y radiológica.
In this article in the Radiology and COVID-19 series, we review some general recommendations for the follow-up of post-COVID-19 patients with a dual clinical and radiological approach, being aware of the limited scientific literature on the matter and the lack of standardised protocols. We focus on the monitoring of lung complications (pulmonary fibrosis and pulmonary thromboembolism), the most common in post-COVID-19 patients, while recognising that there may be other non-respiratory sequelae which also require some type of ongoing care.1,2 Most of the patients who overcome the SARS-CoV-2 infection do not develop complications or require specific aftercare, but a significant proportion (especially those with moderate/severe clinical forms of the disease or who have required mechanical ventilation) need some type of clinical/radiological follow-up.3 Although there are virtually no references or clinical guidelines on the long-term follow-up of these post-COVID-19 patients, radiological tests are being performed and dedicated surveillance clinics are being set up in most hospitals to provide the care they need.4 The aim of this article is to share our experience in the management of post-COVID-19 patients at two hospitals that have had a high incidence of the disease, and to propose general follow-up recommendations from a clinical and radiological perspective. The lack of solid and robust scientific evidence on the management of post-COVID-19 patients means we must be flexible and requires each centre to be prepared to modify their existing clinical/radiological follow-up protocols, or propose alternative ones.
Post-COVID-19 patient follow-up: the lung specialist’s perspectiveOutpatient assessment after hospital dischargeThe long-term clinical consequences of COVID-19 have not yet been fully identified.3 Some patients may have significant sequelae and a resulting increase in morbidity and mortality.5 Due to the complexity of this viral infection and the potential involvement of multiple organs and systems, a multidisciplinary holistic assessment is needed, made up of different medical services such as primary care, radiology, rehabilitation, psychology/psychiatry and internal and respiratory medicine (to mention but a few).4 A broad, multifaceted approach of this type would enable optimal patient progress monitoring and the best possible management.
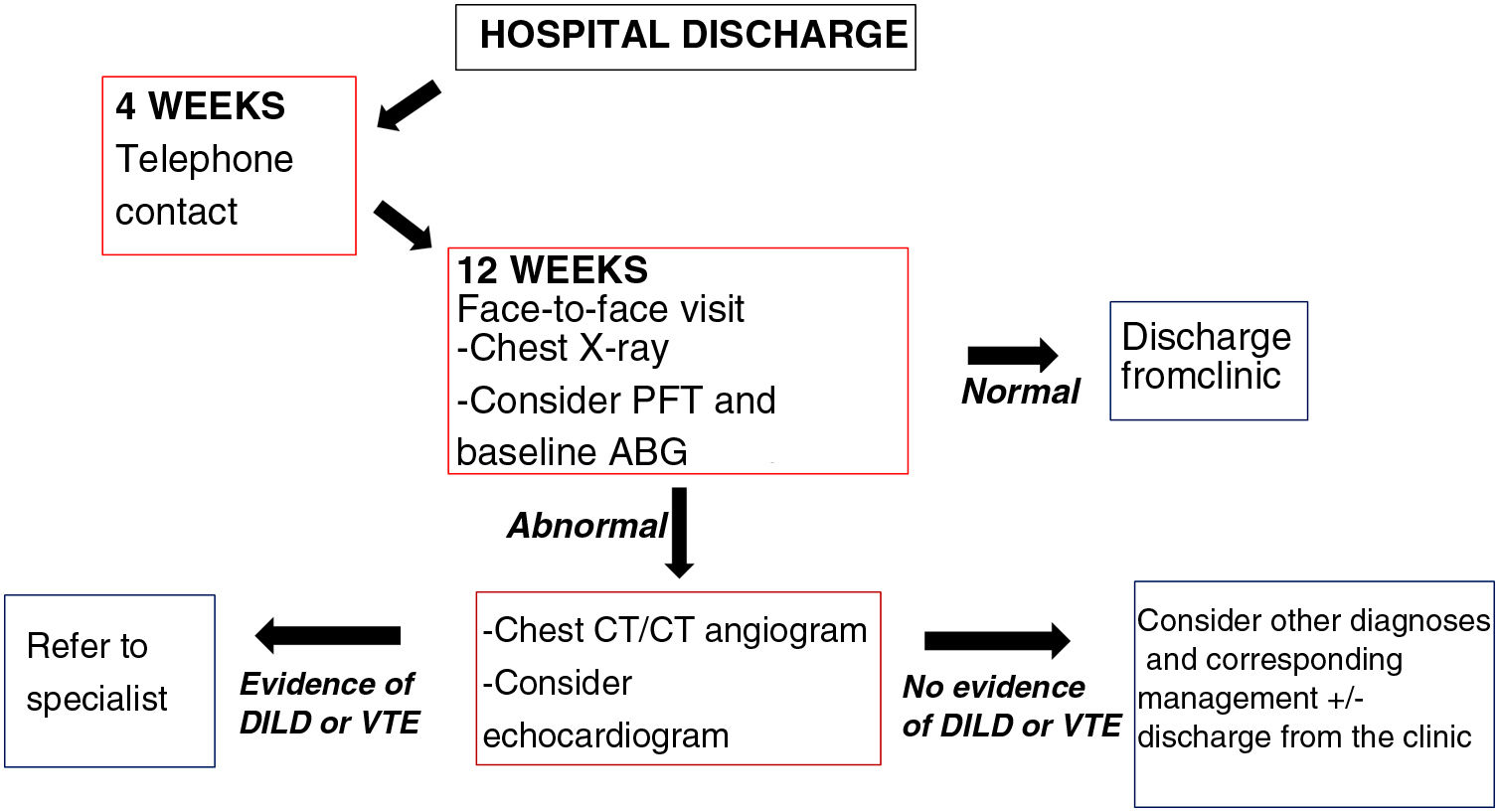
How and when?Patients who have overcome COVID-19 require continuing healthcare after hospital discharge and a number of follow-up strategies have been proposed (Fig. 1)4:
Proposal for outpatient follow-up of post-COVID-19 patients who required admission for severe pneumonia in our centre (Hospital Universitario Ramón y Cajal, Madrid, Spain). Adapted from George et al.4.
DILD: diffuse interstitial lung disease; VTE: venous thromboembolic disease; ABG: arterial blood gas; PFT: pulmonary function test.
Patients admitted for severe pneumonia: in general, a first telephone assessment is recommended four weeks after hospital discharge. Subsequently, at 12 weeks, a face-to-face assessment is preferable with a follow-up chest X-ray. Depending on their availability, pulmonary function tests (PFTs) are recommended at this point and baseline arterial blood gas analysis, in particular to assess the possible need for home oxygen therapy after hospital discharge. If no abnormalities are found in these tests and the patient has made a good recovery with resolution of their symptoms, the follow-up can be terminated. If any of these tests do show abnormalities or the patient is not recovering as they should, a high-resolution chest computed tomography (CT) scan or a CT angiogram of the pulmonary arteries should be requested (depending on clinical suspicions) to rule out interstitial lung and/or vascular involvement. However, if any abnormalities are confirmed, the patient should be referred to the specific specialised unit.4
Patients with mild/moderate pneumonia: the first assessment should be at 12 weeks following the schedule described above. In mild cases, a repeat chest X-ray should be considered if abnormalities are detected at the first check-up before moving on to high-resolution chest CT/pulmonary artery CT angiogram.
At our centre (Hospital Universitario Ramón y Cajal, Madrid, Spain) we base our clinical management on that proposed by George et al.4, which essentially involves collaboration between primary care and internal and respiratory medicine. In order to avoid patients being lost to follow-up, we have an alert system whereby the radiology department reports all follow-up chest X-rays in which significant radiological sequelae are noted to the respiratory medicine department, who they will then request a high-resolution chest CT/CT angiogram of the pulmonary arteries or repeat chest X-ray as necessary on an individual basis, as well as any other tests required to further investigate each patient.
Pulmonary function tests (PFTs): significant respiratory function abnormalities are being found in the follow-up of post-COVID-19 patients. A recent study of 110 post-COVID-19 patients showed that, when discharged from hospital, 47% had a decrease in the diffusing capacity of the lungs for carbon monoxide (DLCO), this being directly proportional to the severity of their pneumonia.6 Another recent study showed that up to 25% of post-COVID-19 patients had abnormal PFTs three months after hospital discharge and that the D-dimer level can be an effective predictor of impaired DLCO.7 These data are consistent with what was observed in the follow-up in 2003 of patients with severe acute respiratory syndrome (SARS), in whom impaired DLCO was found in 15.5%–43.6% of the cases.8 Another less common abnormality is the decrease in total lung capacity (TLC), in other words, pulmonary restriction.6
Post-COVID-19 consequences and sequelaeA significant proportion of patients who survive acute SARS-CoV-2 infection go on to suffer a deterioration in their health. Respiratory symptoms persist in more than half of patients after hospital discharge, particularly those who had to be treated in an intensive care unit (ICU) and those discharged but requiring home oxygen therapy. Clinical complications after the acute phase go beyond the respiratory sphere and can be very varied. Renal, cardiac, neurological, gastrointestinal, ocular and psychological complications have all been described.3,4 This article examines the most important respiratory sequelae: venous thromboembolic disease (VTE) and pulmonary fibrosis.
Venous thromboembolic diseaseThe actual incidence of VTE associated with COVID-19 is unknown, but could well be high. Several studies have found variable prevalences (between 18% and 42%) of VTE among patients with SARS-CoV-29–14 infection.9–14 Different hypotheses have been put forward about the underlying pathophysiological mechanism, one of which is proliferative involvement of the vascular endothelium.10 The role of D-dimer should also be mentioned. Although this marker of the degree of activation of the coagulation cascade has been shown to be a good indicator of disease severity, it is also elevated in many other disease processes present in these patients, making it difficult to determine a cut-off point for it to be useful in detecting VTE in this patient profile.15 The need for anticoagulant prophylaxis during outpatient follow-up after the acute phase of the disease is the subject of debate. A recent consensus document from the Sociedad Española de Cardiología [Spanish Society of Cardiology] considered it prudent to prolong the use of low molecular weight heparin in prophylactic doses from 7 to 10 days after hospital discharge (during the home convalescence phase, especially in patients with reduced mobility).16 Data from patients with COVID-19 are now being incorporated into the international Registro Informatizado de pacientes con Enfermedad Tromboembólica (RIETE) [Computerized Registry of Patients with Venous Thromboembolism], which should definitely help us better understand the relationship between VTE and this viral infection. In any event, we must be sure to always suspect pulmonary thromboembolic disease in patients who continue to have respiratory symptoms, primarily dyspnoea, on their post-COVID-19 follow-up visit. The thromboembolic complications of COVID-19 have been discussed more fully in other articles in this series.
Pulmonary fibrosisThe other major respiratory complication after the acute phase of COVID-19 is the development of pulmonary fibrosis. Again, the mechanisms by which this occurs are not fully understood. It seems to be more common after overcoming severe forms of the disease, especially in patients who required ICU care17, a longer hospital stay and/or had a greater inflammatory burden measured by analytical parameters.18 However, uncertainties remain about the extent of the role attributable to the virus itself and to adjuvant factors such as superinfection, drug toxicities or even mechanical ventilation. Nevertheless, we do know that some of the elements present in the inflammatory cascade (mainly in the severe forms of COVID-19) are also found in the profibrotic response of the archetypal and most common fibrotic lung disease, idiopathic pulmonary fibrosis (IPF).19 Clinical trials are therefore being set up to establish whether or not the antifibrotic drugs used to treat IPF (pirfenidone and nintedanib) may be useful in post-COVID-19 pulmonary fibrosis.20
Post-COVID-19 patient follow-up: the radiologist’s perspectiveTypes of study/modalitiesChest X-rayWe still do not know what course the recovery process will follow in these patients from a radiological perspective, but we can expect similarities with that of patients who suffered infection by other coronaviruses such as SARS in 2002–2003 or Middle East Respiratory Syndrome (MERS) in 2012.21
In a study with SARS survivors, 36% had residual abnormalities on chest X-ray 12 weeks after discharge which were still present at six months in 30%, the most common being parenchymal opacities and reticulation.22 Similarly, in a study of MERS survivors, at six weeks of follow-up 36% of patients had residual abnormalities on chest X-ray, most of them related to fibrotic changes.23,24
Most patients will have abnormalities on chest X-ray when discharged from hospital and it has yet to be determined when is the best time to perform follow-up X-rays and assess the resolution of this condition. In our setting, follow-up X-rays for community-acquired pneumonia are performed at four weeks, mainly to rule out the possibility of a primary bronchial tumour having contributed to the pneumonia.25 In patients with COVID-19, the features of the parenchymal involvement (multiple and patchy ground-glass and alveolar opacities) are not suspected of being cancer-related, so a longer time interval (12 weeks is proposed) could be appropriate to confirm the resolution of opacities or for early detection of complications.4
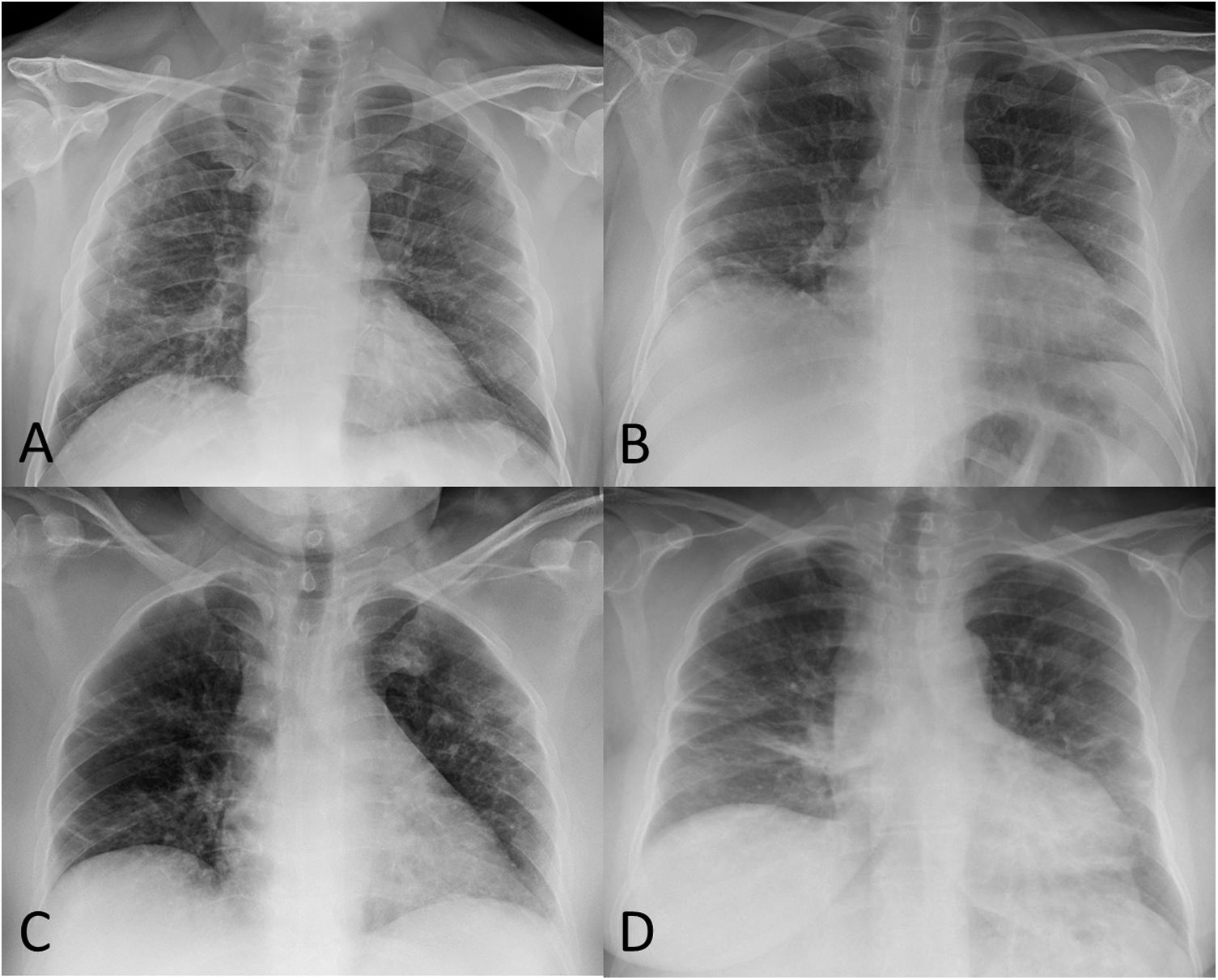
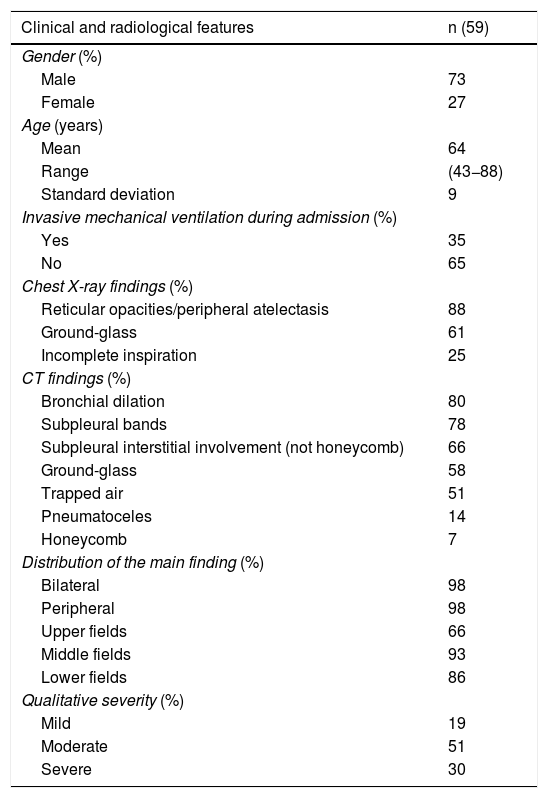
In a review of 59 patients with SARS-CoV-2 pneumonia admitted to our centre (Hospital Universitario Ramón y Cajal, Madrid, Spain) who had abnormalities on chest X-ray at hospital discharge, none of the X-rays performed at 8–12 weeks were normal (Table 1). Findings included reticular opacities/peripheral atelectasis in 88% of cases and ground-glass opacities in 61%. Remarkably, 25% of the examinations showed poor inspiratory effort (Fig. 2). One recent publication describes a lower rate (12%) of radiological sequelae in 110 post-COVID-19 patients at 8–12 weeks after hospital admission.26 We think the discrepancy between that article and our review of 59 patients in terms of radiological sequelae may in part be due to the method of quantifying lung involvement, and because our series reflects the outcomes of more seriously ill patients. In any event, analysis of multicentre series with more cases is needed to help provide more solid conclusions about the prevalence of late radiological sequelae in post-COVID-19 patients.
Clinical and radiological characteristics of the post-COVID-19 patients reviewed (8-12 weeks after hospital discharge with abnormal chest X-ray).
| Clinical and radiological features | n (59) |
|---|---|
| Gender (%) | |
| Male | 73 |
| Female | 27 |
| Age (years) | |
| Mean | 64 |
| Range | (43−88) |
| Standard deviation | 9 |
| Invasive mechanical ventilation during admission (%) | |
| Yes | 35 |
| No | 65 |
| Chest X-ray findings (%) | |
| Reticular opacities/peripheral atelectasis | 88 |
| Ground-glass | 61 |
| Incomplete inspiration | 25 |
| CT findings (%) | |
| Bronchial dilation | 80 |
| Subpleural bands | 78 |
| Subpleural interstitial involvement (not honeycomb) | 66 |
| Ground-glass | 58 |
| Trapped air | 51 |
| Pneumatoceles | 14 |
| Honeycomb | 7 |
| Distribution of the main finding (%) | |
| Bilateral | 98 |
| Peripheral | 98 |
| Upper fields | 66 |
| Middle fields | 93 |
| Lower fields | 86 |
| Qualitative severity (%) | |
| Mild | 19 |
| Moderate | 51 |
| Severe | 30 |
A) 52-year-old male post-COVID-19 whose follow-up chest X-ray shows subpleural reticular opacities, more evident in the right lung. B) 51-year-old male post-COVID-19 whose chest X-ray shows significant volume loss in both lungs and residual involvement in the form of linear subpleural opacities in the right lung. C) 56-year-old female post-COVID-19 whose follow-up chest X-ray shows a loss of volume in both lungs, peripheral laminar atelectasis in the right lung and some subpleural ground-glass opacities in the left upper lobe. D) 58-year-old female post-COVID-19 whose follow-up chest X-ray shows bilateral basal "band" opacities in relation to laminar atelectasis.
In general, we do not recommend follow-up chest X-rays in patients who have had no signs of pneumonia on imaging tests or in whom complete resolution of lung parenchymal involvement was already documented in tests performed during hospital admission. For patients whose condition was mild or moderate (not requiring ICU care), if there is obvious clinical improvement at the first follow-up visit, we think repeating the chest X-ray may be considered without the need for other more complex imaging tests. Where persistent abnormalities on the chest X-ray and/or clinical deterioration are identified at the first check-up, the tests should be completed with a high-resolution chest CT or CT-angiogram of the pulmonary arteries if thromboembolic complications are suspected.
Chest computed tomographyThe medium-to-long-term abnormalities detectable by CT in patients who have had SARS-CoV-2 pneumonia have yet to be precisely determined. What we do have is a good number of reports of long-term sequelae in patients with a history of pneumonia due to other coronaviruses (such as SARS or MERS), with evidence that 20%–60% of these patients will show deterioration in respiratory function and fibrotic changes on imaging tests.21,24,27,28 Most of the studies focus on the impact on PFTs and the clinical repercussions,8,28 with very few discussing the visible sequelae on CT. In a study of 71 SARS patients followed up for 15 years, 38% had CT abnormalities in the form of ground-glass opacity and band-like consolidation.29 The researchers showed that the percentage of lung affected decreased significantly in the first year of follow-up (from 2003 to 2004), but remained stable from 2004 until the end of the study in 2018. From this experience, we would expect that patients with a history of pneumonia due to SARS-CoV-2 who have respiratory symptoms and/or abnormal PFT results may well have fibrotic changes visible on imaging tests.30
In the case of SARS-CoV-2 pneumonia, the indication for on whom and when to perform follow-up chest CT is also yet to be precisely defined. As discussed above, the British Thoracic Society (BTS) recommends performing CT scans in patients who, 12 weeks after discharge from hospital, have abnormal chest X-ray and/or PFT findings, and propose that CT is performed with high-resolution reconstructions and with contrast with pulmonary embolism (PE) protocol.4,31 Other authors recommend a high-resolution baseline study without contrast, repeat CT at 6 and 12 months, and if fibrotic abnormalities persist, also at 24 and 36 months.32 A reasonable indication would be to perform CT scans in patients with persistent respiratory symptoms and abnormal PFT and/or chest X-ray findings three months after discharge or clinical resolution of pneumonia, at which point acute lesions would be resolved and so any visible abnormalities could be considered chronic.
Ideally, the chest CT scan should be performed with a high-resolution volumetric technique (with a slice thickness ≤1.5mm and a high-frequency reconstruction algorithm for the lung parenchyma). Intravenous contrast may be administered in cases with a history of PE, but in most cases it can be performed without. Due to the not-insignificant frequency with which we have detected air trapping, it might be advisable to include slices in expiration, at least in the first study. Although the protocol should always be individualised for each patient according to their body habitus and the clinical situation, in the subsequent check-ups, low-dose radiation techniques can be used and the acquisition in expiration dispensed with.
There are very few published descriptions of the CT findings of patients with post-COVID-19 pulmonary sequelae7,32–36; most cover a maximum follow-up period of one month from hospital discharge, and several of them are case reports.34–36 Certain factors have been suggested as predictors of the development of fibrosis.17,18 It is more common in older patients, in those who had higher levels of C-reactive protein and interleukin-6, those who had received more steroid pulses and/or antiviral therapy for longer, those who required a longer hospital stay,17 and those who had a greater degree of involvement on CT during the acute phase of the infection.18
The most frequently reported findings are parenchymal bands, irregular interfaces, coarse reticular pattern and bronchial dilation.17,37,38 The development of honeycombing is very rare, with only a few reports of isolated cases.35 The most common CT finding is of fibrotic changes without honeycombing, but with histological evidence of fibrosis, as demonstrated in the autopsy described by Schwensen et al.36 The fibrotic changes coincide with the areas where there was previously ground-glass opacity during the acute episode of pneumonia.34,35,37,38 This is to be expected, as they correspond to the areas of greatest lung inflammation. As in acute SARS-CoV-2 pneumonia, fibrotic changes are most often bilateral and predominate in the peripheral region of the lower lobes.
A transient increase in the extension of ground-glass opacification with a decrease in density has been described three to four weeks after the acute phase of pneumonia, known as the "tinted" sign, associated with bronchovascular bundle distortion, which has been attributed to the gradual resolution of inflammation with re-expansion of alveoli. However, this remains to be demonstrated and histological correlation studies are therefore required for confirmation.38
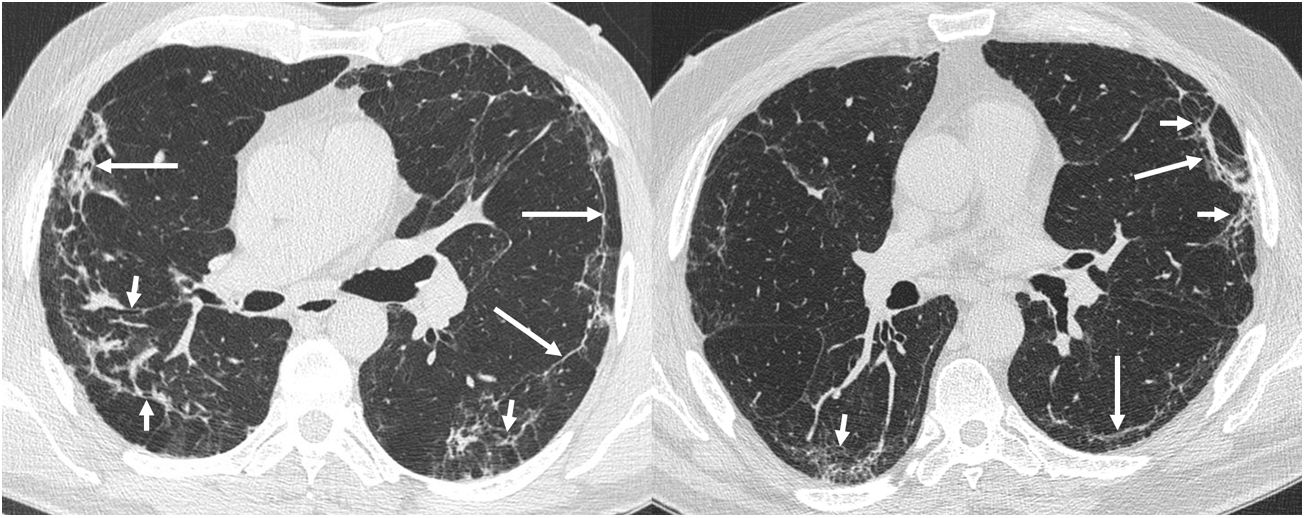
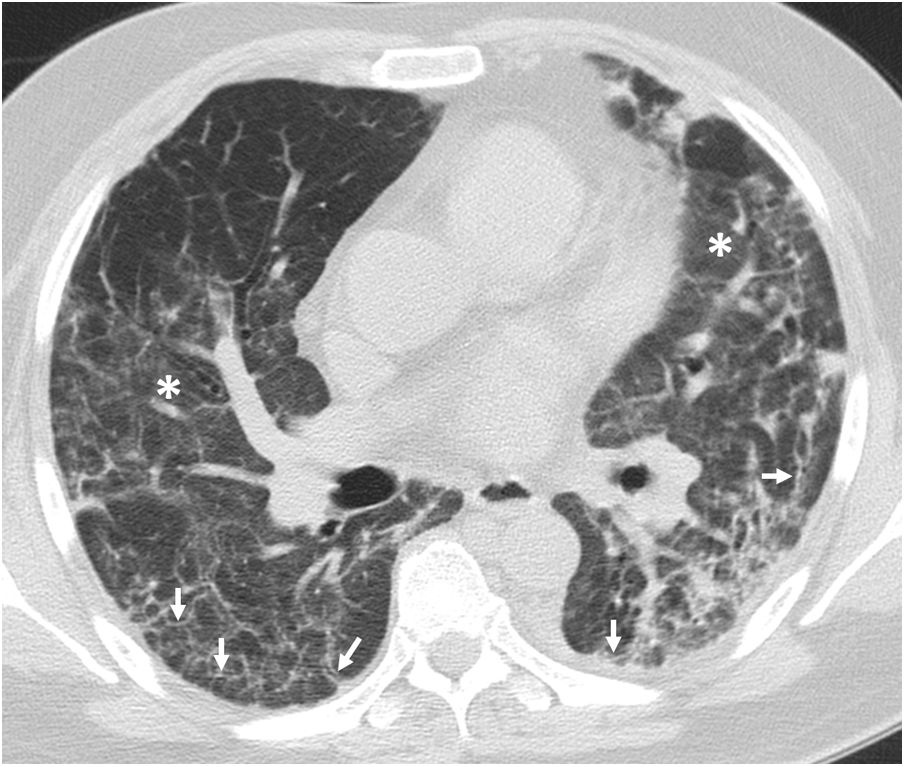
Four thoracic radiologists from Hospital Universitario Ramón y Cajal (AUV, JJMP, LGS and JJAR) reviewed the chest CT findings for 59 patients with a history of SARS-CoV-2 pneumonia with persistent respiratory symptoms, fibrotic-like lesions on chest X-ray and/or abnormal PFT results. The chest CT scans were performed within a maximum period of three months from hospital discharge, with a high-resolution volumetric technique (1mm slices), without intravenous contrast, and including an expiratory phase. The results are shown in Table 1, where we see a clear predominance of males (73%) over females (27%), reflecting the demonstrated greater severity of pneumonia in males. The most common CT findings were bronchial dilation (80%, primarily peripheral) and parenchymal bands (78%), consisting of elongated opacities 1−3mm thick in the lung parenchyma, subpleural in location or extending up to the pleural surface, frequently with distortion of the lung architecture (Fig. 3). Other findings identified in more than half of the cases were: 1) coarse subpleural reticulation with irregular interfaces due to architectural distortion, but with no honeycombing, which we have called subpleural interstitial involvement without honeycombing (66%); 2) areas of ground-glass opacity (58%) (Fig. 4); and 3) a mosaic pattern due to air trapping demonstrated in the expiratory phase of the scan (51%) (Fig. 5). In 14% of the patients we demonstrated the existence of pneumatoceles, the development of which is probably related to mechanical ventilation, which 75% of our patients with these lesions had required. The least common finding was honeycombing (4%) (Fig. 6). In practically all patients, the involvement was bilateral (98%) and peripheral (98%), with a predominance of middle (93%) and lower (86%) fields, in line with what has been reported in other articles.17,33,34,37
The most common findings on chest computed tomography (lung parenchyma window) in post-COVID-19 patients with radiological sequelae are subpleural parenchymal bands ("band opacities" and "subpleural lines", long arrows) with distortion of the lung architecture and secondary bronchial dilation (short arrows).
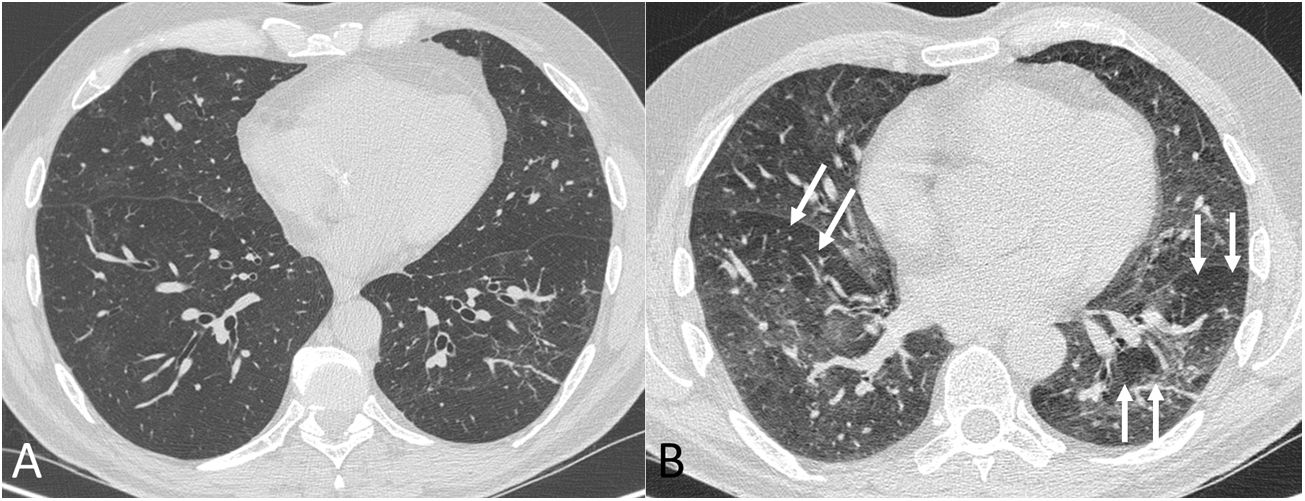
Chest computed tomography (lung parenchyma window) in inspiration (A) and expiration (B) in a post-COVID-19 patient with dyspnoea. In the expiratory phase of the study (B) a mosaic pattern is revealed (arrows). This pattern, which means the presence of areas of trapped air, is barely perceptible in the inspiratory phase.
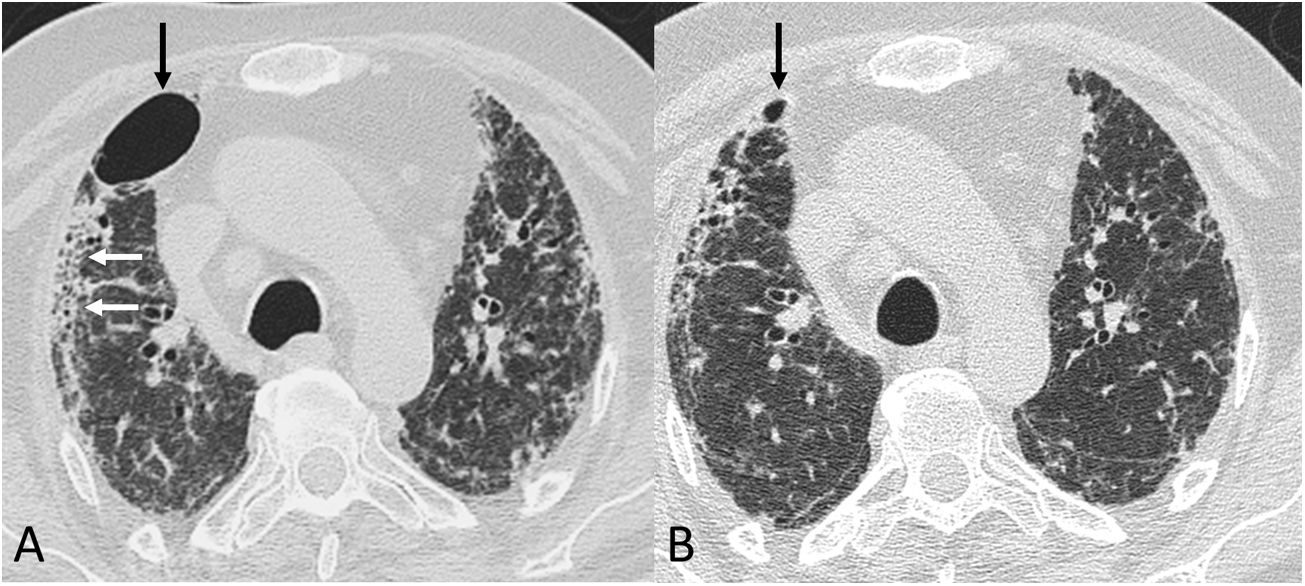
Chest computed tomography (lung parenchyma window) in a post-COVID-19 patient. A) A study from early June 2020 shows a pneumatocele (black arrow), bilateral coarse subpleural reticulation and areas of honeycombing (white arrows). B) In a follow-up study 8 weeks later, the pneumatocele (arrow) has significantly decreased in size.
In summary, it is advisable to perform a CT scan in patients with respiratory symptoms and abnormal PFT and/or chest X-ray approximately three months after hospital discharge, as in our experience these patients will have relevant findings. The most frequent radiological findings are bronchial dilation, parenchymal bands, coarse subpleural reticulation without honeycombing, ground-glass opacity and signs of air trapping (in the expiratory phase of the study), affecting the peripheral region of both lungs (particularly the middle and lower fields). We think that these residual parenchymal abnormalities can in some cases, from a radiological point of view, mimic a pattern of non-specific interstitial pneumonia (especially fibrotic forms) or even a pattern of probable usual interstitial pneumonia, to the point that in the future, post-COVID-19 sequelae may have to be included as a differential diagnosis for these radiological patterns.
The frequency with which follow-up studies should be carried out has yet to be determined, as well as how these abnormalities evolve over time and what their clinical and functional repercussions are. These are factors which will have to be established in the future with long-term follow-up studies in large groups of patients.
Radiological examples of long-term post-COVID-19 progressionA significant percentage of patients with SARS-CoV-2 pneumonia have radiological abnormalities on the follow-up chest X-ray one month after hospital discharge. In these patients we generally recommend/perform a chest CT without intravenous contrast. In our experience, the severity of the clinical picture usually implies a greater radiological involvement of the lung parenchyma after hospital discharge and a slow favourable response on imaging tests (Fig. 7), especially in patients who required prolonged ICU stays. We do not yet know how much time is needed to establish that the residual radiological findings are stable and definitive. However, some patients with mild or moderate pneumonia may achieve complete resolution of the radiological findings in the first months after discharge (Fig. 8). Ground-glass opacities are among the imaging abnormalities that tend to resolve earlier, while subpleural bands, bronchial dilation and subpleural interstitial involvement usually follow a favourable course that progresses slowly (Figs. 9 and 10). There is, therefore, great variability among patients in the time it takes for residual post-COVID-19 radiological findings to resolve or stabilise. The severity of the clinical picture is probably the most determining factor.
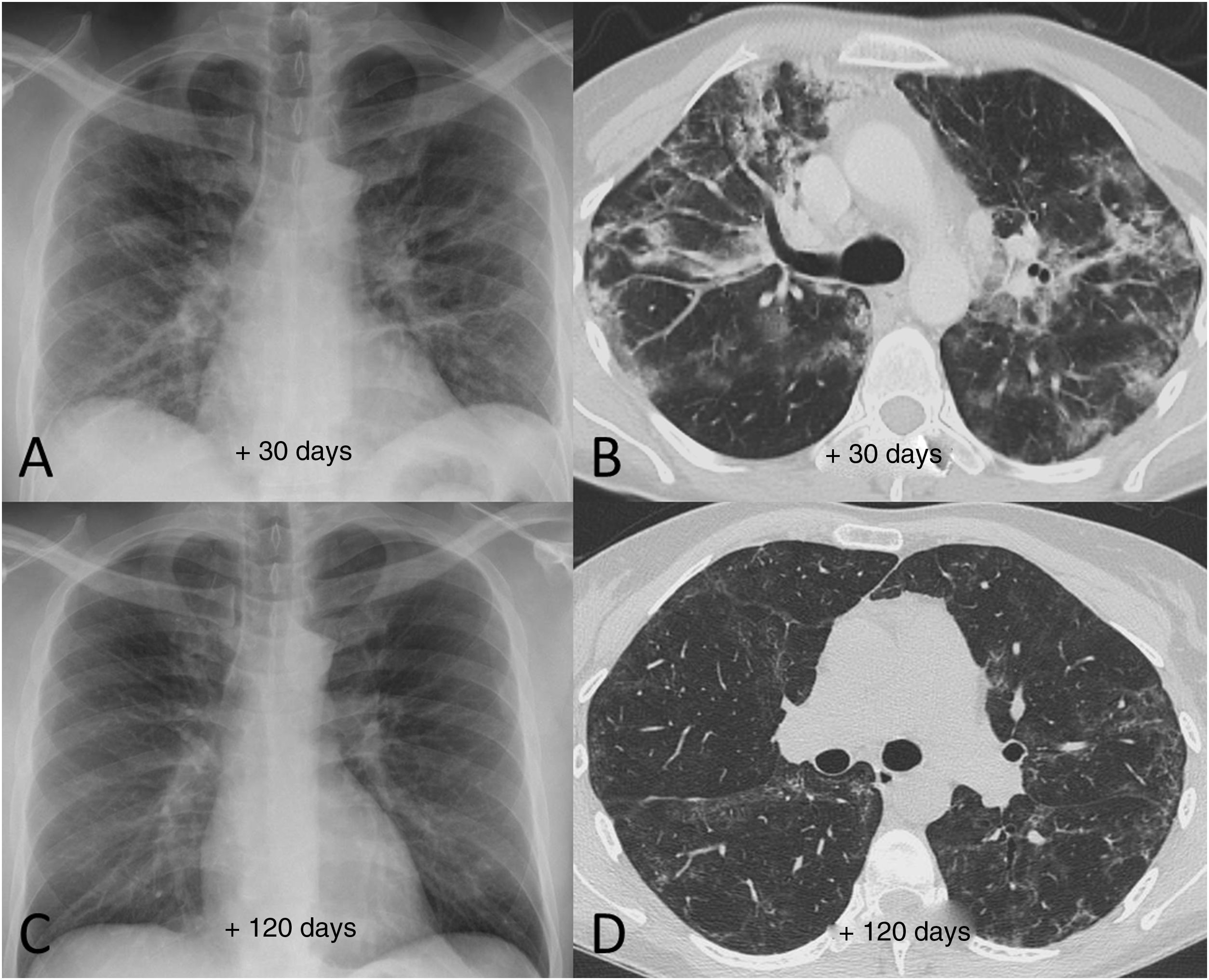
Example of good radiological recovery in terms of chest X-ray and computed tomography findings (lung parenchyma window) in a 45-year-old man with severe SARS-CoV-2 pneumonia after hospital discharge. A and B) Radiological tests 30 days after the onset of symptoms show patchy consolidation along with areas of ground-glass opacification and loss of volume. C and D) Radiological tests 120 days after onset of symptoms show radiological improvement with resolution of the consolidation and a reduction in the extension of the ground-glass opacification, with persistence of slight peripheral reticular interstitial involvement.
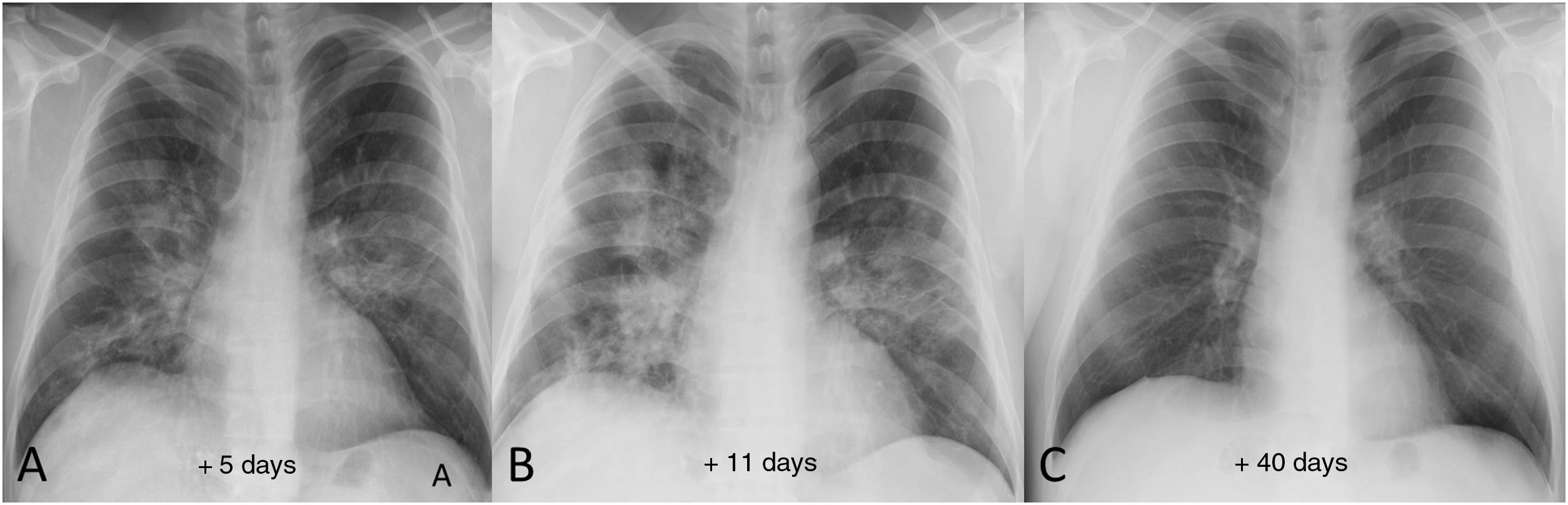
Example of good radiological recovery on chest X-ray in a 39-year-old male patient. A) Five days after the onset of symptoms, bilateral opacities can be seen, predominantly perihilar. B) 11 days after the onset of symptoms, radiological worsening is identified (greater density and extension of the opacities). C) Once discharged from hospital, follow-up chest X-ray 40 days after onset of symptoms shows complete radiographic resolution of the lung opacities.
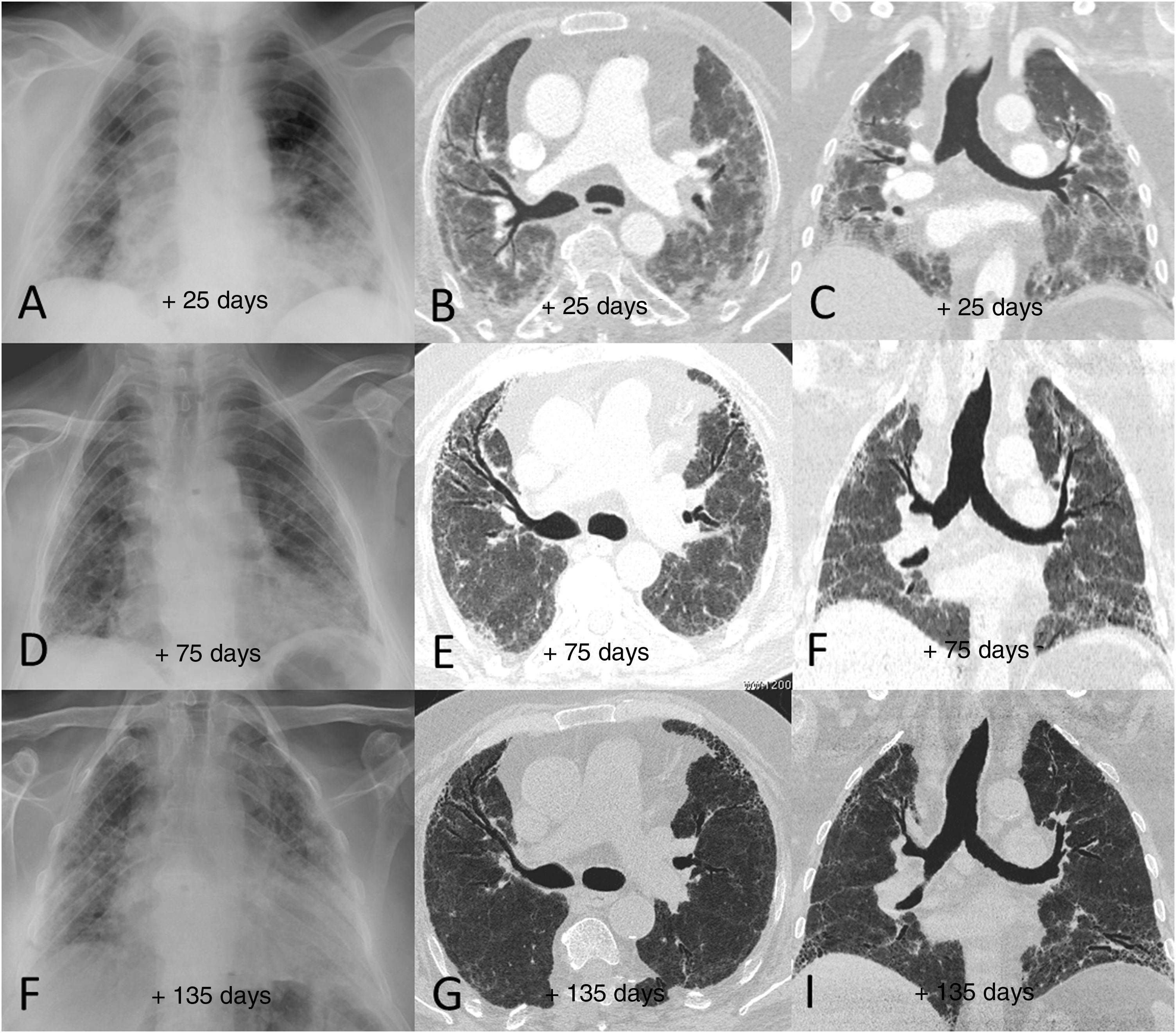
Example of poor radiological outcome in a 65-year-old male patient who required hospital admission due to SARS-CoV-2 infection and invasive mechanical ventilation for more than 15 days. The patient was finally discharged after 90 days in hospital, requiring home respiratory support and having significant limitations performing basic daily activities. A–C) Radiological tests performed 25 days after the onset of symptoms (7 days after extubation) show peripheral patchy parenchymal opacities predominantly in both middle and lower lung fields. D–F) The tests 75 days after the onset of symptoms showed slight radiological improvement of the opacities, with an architectural distortion detected in the computed tomography consisting of incipient areas of subpleural reticulation and bronchial dilation, mainly in the anterior segments of both upper lobes and in the left lower lobe. G–I) In the radiological follow-up 135 days after the onset of symptoms, the findings have barely changed with respect to the previous study (persistence of areas of subpleural reticulation and bronchial dilation).
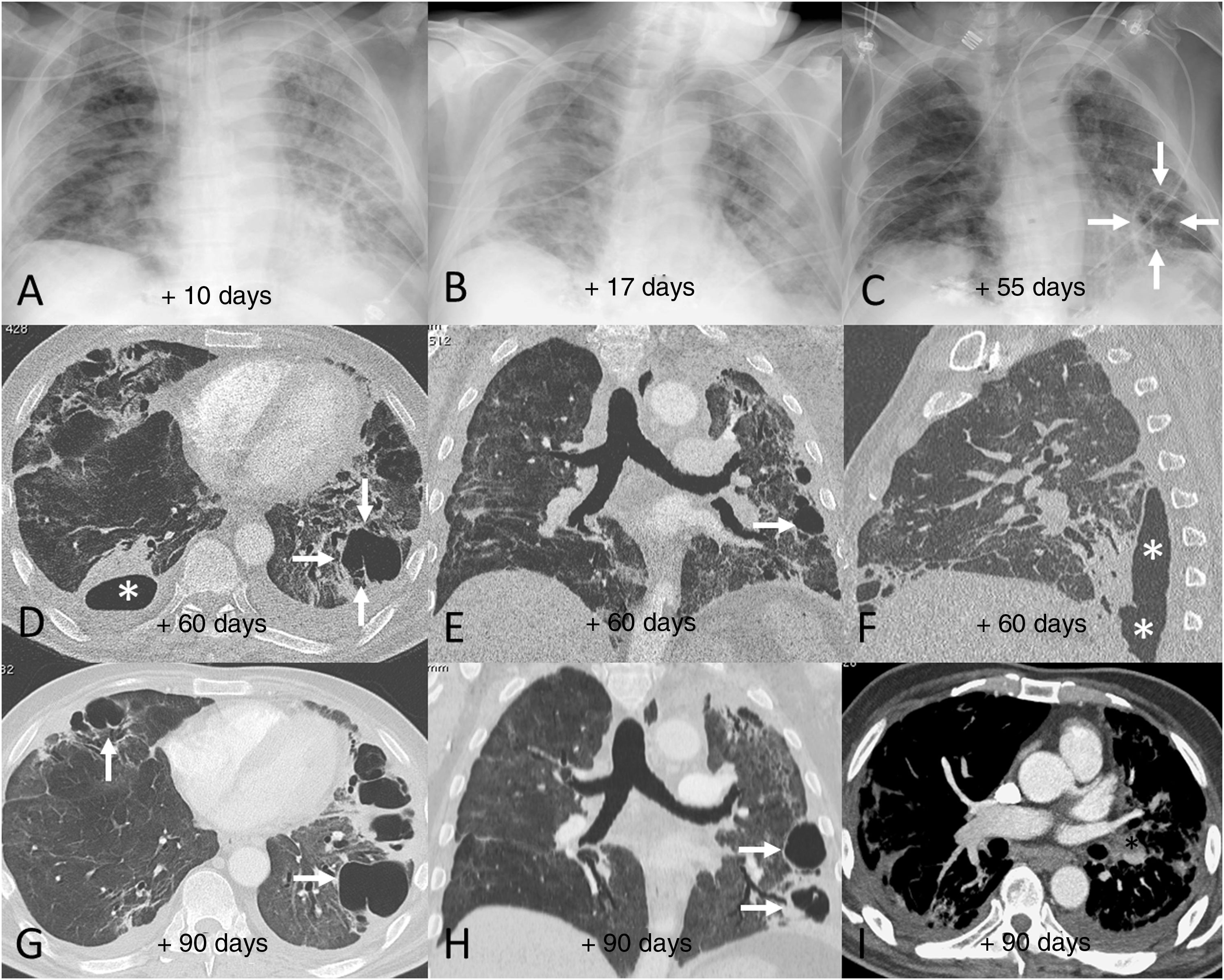
Example of poor radiological outcome in a 62-year-old male patient who required hospital admission due to SARS-CoV-2 infection and invasive mechanical ventilation for 12 days. After 90 days in hospital, the patient was discharged requiring long-term home oxygen therapy and needing rehabilitation. A–C) Chest X-rays corresponding to days 10 (A), 17 (B) and 55 (C) after the onset of symptoms showing extensive persistent bilateral opacities, which are beginning to resolve by the end of the hospital stay. Note the appearance of areas of lower density in the lung bases in relation to pneumatoceles (C, arrows). D–F) Chest computed tomography (CT) images 60 days after the onset of symptoms, in which extensive bilateral fibrotic lesions in the form of subpleural bands and bronchial dilation can be seen, in addition to peripheral air cysts compatible with pneumatoceles (arrows) in the right middle lobe and both lower lobes. One of the lesions in the right lower lobe is in contact with the pleural space, causing a hydropneumothorax (D and F, asterisks). G–I) Chest CT images 90 days after the onset of symptoms, showing a slight improvement in the fibrotic lesions but slightly more growth of the pneumatoceles in the right middle lobe and left lower lobe (arrows). Note the incidental detection of an eccentric filling defect in the left lower lobe artery (I, asterisk) consistent with a subacute pulmonary embolism.
COVID-19 is a new disease with very diverse clinical and radiological manifestations (pulmonary effects predominating due to their frequency and severity), and we are now starting to discover its medium-to-long term complications and sequelae. It is vitally important that centres and physicians dedicated to respiratory disease are prepared for optimal detection and management of the long-term sequelae of SARS-COV-2 infection and that they have the technical and human resources to be able to properly diagnose and treat these sequelae. Although the lack of a solid, standardised basis for the management of post-COVID-19 patients means that each centre is having to adapt its resources according to its own situation and needs, we believe that the recommendations and suggestions we have made here (based on the experience of two public hospitals in Madrid with a high incidence of patients with COVID-19 and on several international guidelines, which are beginning to be published on the management of sequelae in these patients) can help in the development and implementation of clinical-radiological follow-up protocols for post-COVID-19 patients with respiratory involvement, as it is clear that the health consequences of COVID-19 are going to persist once the pandemic has passed.
Authorship- 1
Responsible for the study integrity: LGS and JAR.
- 2
Study conception: JAR, LGS, JJMP, AUV, MFV and JARB
- 3
Study design: JAR.
- 4
Data acquisition: LGS, JAR and MFV.
- 5
Data analysis and interpretation: LGS.
- 6
Statistical processing: LGS.
- 7
Literature search: JAR, LGS, JJMP, AUV, MFV, JARB and AJO.
- 8
Drafting of the manuscript: JAR, LGS, JJMP, AUV, MFV, JARB and AJO.
- 9
Critical review of the manuscript with intellectually relevant contributions: JAR, LGS, JJMP, AUV, MFV, JARB and AJO.
- 10
Approval of the final version: JAR, LGS, JJMP, AUV, MFV, JARB and AJO.
The authors declare that they have no conflicts of interest.
Please cite this article as: Alarcón-Rodríguez J, Fernández-Velilla M, Ureña-Vacas A, Martín-Pinacho JJ, Rigual-Bobillo JA, Jaureguízar-Oriol A, et al. Manejo y seguimiento radiológico del paciente post-COVID-19. Radiología. Radiología. 2021;63:258–269.