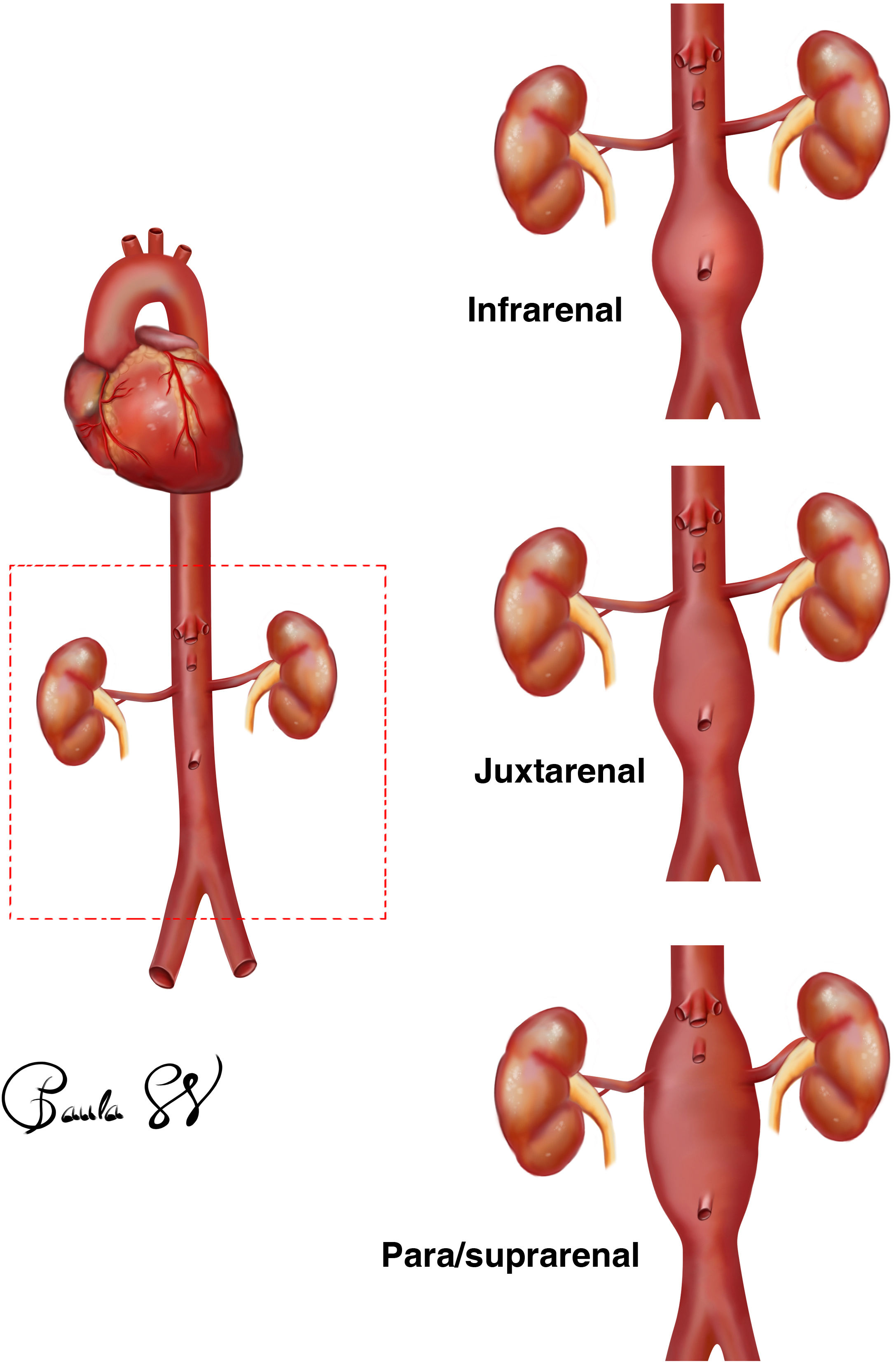
Abdominal aortic aneurysm is defined as a dilatation of the abdominal aorta greater than 3cm. Its prevalence is between 1 and 1.5 cases per 100 people, constituting an important cause of morbidity and mortality. Rare in women, its frequency increases with age and its most frequent location is between the renal arteries and the aorto-iliac bifurcation. Approximately 5% of cases will involve the visceral branches.
It is a silent pathological process whose natural evolution is rupture, which often has a fatal outcome and whose diagnosis is part of the pathology that we will find in emergency radiology. The involvement of the radiologist and the preparation of an accurate diagnostic report, as soon as possible, is essential for decision-making by the team in charge of the patient's surgery.
Definimos el aneurisma de aorta abdominal como la dilatación de la aorta abdominal mayor de 3cm. Su prevalencia se sitúa entre 1 y 1,5 casos por cada 100 personas, constituyendo una importante causa de morbimortalidad. Rara en mujeres, su frecuencia aumenta con la edad y su localización más frecuente es la alojada entre las arterias renales y la bifurcación aorto-ilíaca. Aproximadamente un 5% de los casos va a comprometer las ramas viscerales.
Se trata de un proceso patológico silencioso cuya evolución natural es la rotura, que muchas veces tiene un desenlace fatal y cuyo diagnóstico forma parte de la enfermedad que nos encontraremos en la radiología de Urgencias. La implicación del radiólogo y la elaboración de un informe diagnóstico preciso, lo antes posible, es fundamental para la toma de decisiones por parte del equipo que se encargue de la cirugía del paciente.
The prevalence of abdominal aortic aneurysm (AAA) in the general population is 1–1.5 cases per 100 people. It continues to be a significant cause of morbidity and mortality; 1–2% of all deaths in the Western world are from a ruptured aneurysm and it is the tenth leading cause of death in the over-55s in the United States.1–5
The development of an AAA is a silent process that gradually progresses until it ruptures, often with a fatal outcome. With a diameter of 4−5cm, the risk of rupture is 14%, increasing to 10% when the aneurysm reaches a diameter of 5−6cm. According to the literature, this disease would seem to meet the criteria for a population screening programme with the aim of reducing death directly attributable to the aneurysm.6 A number of clinical studies have concluded that population screening would be effective in certain healthcare economies (United Kingdom, Sweden and Denmark), but it would take up to 10 years to gather the necessary scientific evidence to support its implementation.7 While AAA rupture has a mortality rate of 85–90%, the mortality rate for elective treatment of aneurysm is less than 3–5%.8
Up to 75% of AAAs are asymptomatic4 and in many cases the first evidence of aneurysm is when it ruptures and the patient ends up in hospital with acute symptoms. The management of AAA has fundamentally changed over the last thirty years, from open surgery as the only option, to the introduction of the endovascular aortic aneurysm repair (EVAR) technique. Parodi successfully treated the first EVAR case in history in Buenos Aires in September 1990.9
With the introduction of EVAR and improvements in anaesthetic and perioperative management, the survival of treated patients has increased. EVAR has made repair possible in older patients or patients whose degree of comorbidity contraindicated open surgery, and nowadays the perioperative mortality rate among octogenarians is comparable to that of younger patients.10
For decision-making in the management of these patients, the work of the radiologist forms an essential part and a precise and specific report is therefore of vital importance. The aim of this article is to provide guidance for writing the radiological report.
Open surgery or EVARThe clinical presentation of a ruptured AAA (rAAA) is often subtle and confusing, with signs ranging from syncope, loss of consciousness or transient hypotension to simple backache. The classic triad is abdominal or low back pain, hypotension and a pulsatile abdominal mass, although these signs are only present in 25–50% of patients.11–14
The gold standard as imaging test for suspected rAAA is currently computed tomography (CT). An abdominal and pelvic CT angiogram with 1-mm slice thickness will provide sufficient information to determine whether or not the patient is a candidate for EVAR and, if so, obtain the necessary measurements for planning the procedure. This scan should be performed in stable patients (class I recommendation, level of evidence B) and even considered in haemodynamically unstable patients (class II recommendation, level of evidence B).14 In emergency cases, the patient should be taken to the operating theatre immediately for open surgery or intraoperative aortography to determine the feasibility of performing an EVAR.14
The IMPROVE trial,15 which compared the two techniques, found no differences in the mortality rate at 30 days (35% EVAR, 37% open surgery) or at one year. It did demonstrate better quality of life, decreased hospital stay and cost, and better three-year survival with endovascular treatment, and similar levels of re-operation in both techniques. Currently, the European and American guidelines16,17 recommend endovascular treatment whenever anatomically feasible, with level of evidence B (moderate, European guidelines) and C (low, American guidelines). The NICE guidelines18 (United Kingdom) determine that EVAR provides a greater benefit for most patients, especially men over the age of 70 and women in any age range, while open surgery boasts a greater benefit-risk balance in under-70s. EVAR is associated with a decrease in early death, but this benefit is not maintained in the medium and long term due to the development of complications.
For all these reasons, the decision to perform EVAR routinely is not recommended, even if it is feasible. Rather, this decision will depend on the patient's age, comorbidities, previous abdominal surgery, anatomical considerations and the experience of the working team.
Types of EVARStandard EVAR: aortic prosthetic endografts, typically consisting of two components: a bifurcated body and extensions. Polytetrafluoroethylene (PTFE, registered as TeflonTM) prosthetic fabric is sewn to a metal support structure using non-absorbable sutures of braided polyester, monofilament polypropylene or laminated fluoroethylene-propylene/expanded PTFE. The support stents are made of nitinol or stainless steel wire. They usually have radiopaque markings to enable their position to be checked once placed.
Chimney EVAR (CHEVAR): emerged as a rescue technique making it possible to maintain visceral branches that would be occluded with the placement of the endograft body.
A conventional endograft is overlapped over coated stents, which are placed in the visceral branches or renal arteries, in order to maintain their patency while achieving an optimal seal zone.
Fenestrated endografts (FEVAR): there are certain limitations with conventional stent grafts in the case of a juxtarenal or adrenal aneurysm, where there is no healthy area of the aortic wall to support the device stents. Fenestrated endografts have been developed for that purpose. These grafts have holes in the body, corresponding to the ostia of the visceral arteries and renal arteries involved in the aneurysm, in order to prevent them from becoming occluded. After placement of the fenestrated body, the covered stent is placed in the corresponding artery. These grafts are custom-made, adjusting to the individual anatomical requirements of each patient, so they would not be available for an urgent case.
Branched endografts (BEVAR): this is a main graft body to which secondary grafts (coated stents) are added through which the different arteries involved in the aneurysm are channelled. Within this group, there are grafts with branches external to the main body and, more recently, grafts with internal branches have been developed. There is currently a non-customised endograft with internal branches on the market that could be an effective alternative for a wide range of patients with rAAA who are not eligible for standard EVAR. For the moment, however, prospective studies are needed to determine its suitability (Fig. 1).
Radiology reportWhether the rAAA is to be treated by open surgery or endovascular repair, there are some essential elements that have to be systematically reflected in the diagnostic report to describe the findings. However, this article focuses on endovascular repair, as this is where the radiology report has to be more specific.
The first important aspect is to identify the ruptured aortic aneurysm itself or, if it is not ruptured, signs of imminent rupture. Clinical suspicion can be helpful in making the diagnosis and, in this case, as pain is a sign associated with aneurysm expansion and rupture, the presence of pain per se in association with an abdominal aneurysm increases the patient's morbidity and mortality risk.6
There are various radiological signs that are helpful in diagnosing an imminent rupture, an already ruptured and bleeding aneurysm, or a chronic or contained rupture (Table 1).19
Radiological signs in an abdominal aortic aneurysm.
| Signs of high risk of rupture/imminent rupture of the AAA | Signs of AAA rupture | Chronic contained rupture |
|---|---|---|
| Accelerated growth of the sac: 6−12mm/year | Retroperitoneal haematoma (40−70 HU) | Draped aorta signb: associated with chronic contained rupture, which should meet the following conditions: |
| – Known abdominal aortic aneurysm | ||
| – Previous acute symptoms resolved | ||
| – Haemodynamically stable patient with normal haematocrit | ||
| – Computed tomography showing retroperitoneal haemorrhage or draped aorta with an organised thrombus | ||
| Saccular or very eccentric morphology | Haematoma at the root of the mesentery | |
| Absence of thrombus or thinning of the aneurysmal sac wall | Blood collection in the psoas muscle without a cleavage plane with the aneurysmal sac | |
| Ulceration of the aortic wall or de novo ulceration of the mural thrombus. Fragmentation of existing mural thrombus | Active extravasation of contrast outside the aneurysmal sac | |
| High-attenuation crescenta | ||
| Draped aorta signb | ||
| Discontinuity of parietal calcium lodged in the intima layer of the artery | ||
| Mycotic/inflammatory aneurysms |
HU: Hounsfield units.
The location of the aneurysm is fundamental as it will determine the choice of graft. Aneurysms are most often located between the renal arteries and the aortoiliac bifurcation. Approximately 5% of cases will compromise the visceral branches.20
It is necessary to assess the number and location of visceral arteries involved in the abdominal aneurysmal sac. Essentially, we will see that the inferior mesenteric artery originates from the sac wall and will be excluded both in open surgery and with the implantation of the graft body. Thanks to the collateral circulation coming from the arc of Riolan, in most cases this will not have any clinical implications (Fig. 2).
Infrarenal: the AAA is located below the origin of both renal arteries. In this case, it is necessary to know the distance from the lowest renal artery to the beginning of the aneurysmal sac and also to know whether or not this space, called the neck, has a healthy aortic wall (free of thrombus and atheromatous plaques), as this is where the stent for fixation of the endograft body will be anchored. It is important to know the shape of the neck of the aneurysm (for example, whether it is conical or inverted conical, its angle with respect to the axis of the aorta) and the diameter. Based on the specifications for use of the different devices, the treatment range is from 18 to 32mm. However, a diameter greater than 28mm increases the risk of associated complications.
At this point we have to be aware of the concept of hostile neck.21 This term refers to aneurysm necks that meet some or all of the following criteria:
- -
Length of 15 to 10mm.
- -
Diameter greater than 28mm.
- -
Angulation greater than 60°.
- -
Calcification of the aortic wall greater than 50%.
- -
Circumferential thrombus.
- -
Conical shape.
When measuring the neck of the aneurysm, there are two important angles:
- -
The alpha/α angle or suprarenal angle: this is the angle formed between the central axis of the suprarenal aorta and the proximal neck of the aneurysm.
- -
The beta/β angle or infrarenal angle: formed between the axis of the proximal neck and the axis of the lumen of the aneurysmal sac. We are always referring to this β angle when we state that the angulation of the aneurysmal sac with respect to the axis of the abdominal aorta should be <60°.
Juxtarenal: if the AAA begins infrarenally but the distance from the lowest renal artery to the sac is less than 15mm in length, the length may not be adequate for fixation of the body of the stent. There are occasions when the instructions for use of the devices are not strictly adhered to, with standard EVAR being performed in necks of up to 10mm. If this is not possible, endovascular repair using the CHEVAR technique or open surgery would have to be considered.
Para/suprarenal: the aneurysmal sac includes both renal arteries, and may also include the outlet of the coeliac trunk and the superior mesenteric artery (SMA). In this case, the options are the placement of a chimney graft or open surgery (aneurysm resection and graft interposition and re-implantation or bypass of all the arterial branches involved).
SacWe have to indicate the size of the sac, whether there is a mural thrombus and, if so, whether or not the distribution is concentric and how much lumen is left for blood to flow through. It is essential to assess the angulation of the aneurysmal sac with respect to the axis of the abdominal aorta, with the recommendation being for angulations of less than 60° in EVAR.
The length of the sac has to be determined, indicating where it begins (para/supra/juxta or infrarenal) and where it ends, and if it includes the iliac bifurcation caudally.
The aneurysmal sac may be fusiform, the most common, or have a saccular or “hourglass” shape. Mycotic or inflammatory aneurysms can appear as a thickening of the aortic wall; the fibrotic appearance, a peri-aortic striation or an atypically located focal saccular aneurysm should raise the suspicion of a mycotic aneurysm or aneurysm secondary to an inflammatory process, which have a greater risk of rupture (53–75%).22 The treatment must be carefully assessed as, if performing an EVAR (graft material which is a foreign body), any underlying infectious/inflammatory process has to be taken into account.
Aortic conusDiameter of distal neck. In cases with a distal aortic neck of less than 18mm, placement of an aorto-uni-iliac stent graft should be considered.
Arterial accessRepair using the EVAR technique will be performed using femoral arterial approaches, so knowing the status of the femoral and iliac arteries is vital. We have to report the morphology and maximum and mean diameter of the arteries on both sides; whether there is occlusion or stenosis of the arterial lumen and, if so, what the smallest diameter is.
We also have to report whether or not both hypogastric arteries are patent.
The optimal conditions are that both common iliac arteries have a length of at least 15mm prior to their bifurcation to obtain a good seal length, and that they have a diameter between 8 and 22mm.
Also important is the concept of hostile iliacs:
- -
Excessive angulation.
- -
Severe diffuse atheromatous disease causing stenosis.
- -
Aneurysmal common iliac arteries: in such cases, embolisation of the ipsilateral hypogastric artery is usually required during the procedure to achieve distal sealing in the external iliac artery.
- -
Anatomical variants: retroaortic or circumaortic renal veins.
- -
Existence of renal polar arteries, stating the location of their origin in the report.
- -
Arterial stenosis: can risk organ viability and even be life-threatening for the patient. The pre-existence of stenosis in the coeliac trunk, the SMA or the renal arteries is particularly important if the repair is with the CHEVAR technique, where these arteries are going to be manipulated endovascularly.
- -
Existence of visceral aneurysms.
- -
Existence of hypertrophic lumbar arteries, which can lead to type II endoleaks.
- -
Unknown concomitant processes: we must highlight space-occupying lesions or complications deriving from an abdominal oncological or infectious process.
- -
In the case of open surgery, the report should also include evidence of previous abdominal surgery, a factor that increases the morbidity risk attached to the procedure.
An AAA is considered when the diameter of the aorta exceeds 3cm. A 7-cm aneurysm carries a 25% risk per year of rupture.22 Although not necessary for us to determine this, if the point of rupture of the aneurysm is sought, the majority occur on the posterior-lateral aspect.
If repair using the EVAR technique is to be performed, there is a series of measurements that must be assessed, including what type of graft to use and with what dimensions.
It is essential when performing an aortic measurement, particularly when we need to assess an aneurysm's growth or establish the dimensions in an EVAR, that each measurement is made orthogonal to the variable axis of the aorta in each plane. This means that for each measurement, we have to angulate the aortic axis in the three spatial planes, as this provides real measurements of the dimensions (Fig. 3).
Ruptured abdominal aortic aneurysm with measurements in orthogonal planes of the infrarenal neck (upper image) and aneurysmal sac (central image). Ruptured abdominal aortic aneurysm with measurements in orthogonal planes and diameter of the sac prior to iliac bifurcation. The images are distributed in sagittal (A), coronal (B) and axial (C) planes.
We should also highlight the concepts of multiplanar reconstruction (MPR), which involves two-dimensional images in multiple planes with maximum intensity projection, enabling a more precise analysis of the vessel wall and calcium distribution (Fig. 3).
There is a tool in the reconstruction of the images that is part of the curved MPR analysis called the central lumen line. The aim is to virtually recreate the aneurysm stretched along the central axis of the lumen of the vessel. This is a useful tool, as it measures diameters, but it remains an automatic software measurement, which means there is the potential for error. The estimated lengths and diameters may not coincide with the final lengths of the artery, so it is always recommended to verify them manually to ensure precision when choosing the graft material.
The measurements required for planning an EVAR are shown in the diagram in Fig. 4; they will be made by the doctor in charge of the surgery, for choosing the type and size of each graft (Fig. 4).
From among these, there are some basic data that should be provided in the report:
- -
D2: infrarenal neck diameter.
- -
D7: diameter of the aorta prior to the bifurcation.
- -
D8: diameter of both common iliac arteries.
- -
D9: diameter of both external iliac arteries.
- -
Diameter of both common femoral arteries.
Decision-making in cases of rAAA must be systematic and as rapid as possible. We therefore have to be clear about the morphology of the aneurysm, as this will determine the type of intervention and the type of graft. For the purposes of facilitating and describing in clear, simple terms the findings of the abdominal CT, we have created the framework for a predefined report,21,23 which collates the necessary data in structured form (Table 2).
Proposal for structured report.
| PATIENT'S NAME: | |
| Med. Record No. | |
| DATE: | |
| DIAGNOSIS: | |
| Known ruptured/contained abdominal aortic aneurysm | |
| LOCATION: | |
| - Infrarenal: | |
| - Lowest renal artery: right/left | |
| - Distance from the lowest renal artery: () mm | |
| - Juxtarenal: | |
| - Length of the neck: () mm | |
| - Para/suprarenal: | |
| - Arteries involved: | |
| - Visceral stenosis: yes/no | |
| NECK: | |
| - Length: () mm | |
| - Condition of the neck: | |
| - Hostile neck: yes/no | |
| - Thrombus/Calcium: yes/no | |
| - Angle: > or <60 degrees | |
| SAC MORPHOLOGY: | |
| - Fusiform/Saccular | |
| - Mural thrombus: yes/no | |
| Circumferential: yes/no | |
| Eccentric: yes/no | |
| - Aortic lumen diameter: () mm | |
| - Angulation to the aortic axis: () mm | |
| - Length: () mm | |
| Includes iliacs: yes/no | |
| - Renal polar arteries involved: yes/no | |
| ARTERIAL ACCESS: | |
| - Diameter of external iliac arteries and common femoral arteries: () mm | |
| Calcium: yes/no | |
| Tortuosity: yes/no | |
| OTHER SIGNIFICANT FINDINGS: | |
| - Anatomical variants: yes/no. Location | |
| - Visceral aneurysms: yes/no. Location | |
| - Hypertrophic lumbar arteries: yes/no | |
| - Concomitant processes (cancer patient, concurrent abdominal disorders): yes/no | |
| - Previous abdominal surgery: yes/no | |
| - Suspected mycotic abdominal aortic aneurysm: yes/no | |
| - Lumen of the inferior mesenteric artery | |
| COMMENTS: |
rAAA is a medical emergency with high morbidity and mortality rates and is a challenge for the general radiologist. The radiologist's commitment to rigorously analysing the morphology and characteristics of the aneurysm is essential for preparing an accurate report, and a vital part of the decision-making process to determine the best procedure to offer our patients.
Authorship- 1.
Responsible for the integrity of the manuscript: SLL, PCÁ and PSV.
- 2.
Conception of the manuscript: SLL.
- 3.
Design of the manuscript: SLL, PCÁ and PSV.
- 4.
Data collection: not applicable.
- 5.
Data analysis and interpretation: not applicable.
- 6.
Statistical processing: not applicable.
- 7.
Literature search: SLL, PCÁ and PSV.
- 8.
Drafting of the article: SLL, PCÁ and PSV.
- 9.
Critical review of the manuscript with intellectually relevant contributions: SLL, PCÁ and PSV.
- 10.
Approval of the final version: SLL, PCÁ and PSV.
The authors declare that they have no conflicts of interest.