To determine the safety of regadenoson for vasodilation in cardiac MRI stress tests to detect myocardial ischemia.
Material and methodsWe retrospectively analyzed cardiac MRI studies done in 120 patients (mean age, 67±11.6 years; 88 men) with suspected ischemic heart disease or known coronary disease who had clinical indications for cardiac MRI stress tests. All studies were done on a 1.5T scanner (MAGNETOM Aera, Siemens Healthineers) using regadenoson (5ml, 0.4mg) for vasodilation. We recorded cardiovascular risk factors, medications, and indications for the test as well as vital signs at rest and under stress and the symptoms and adverse effects induced by the drug.
ResultsNo symptoms developed in 52.6 % of patients. The most common symptoms were central chest pain (25 %) and dyspnea (12 %). At peak stress, the mean increase in heart rate was 23.9±11.4 beats per minute and the mean decreases in systolic and diastolic blood pressure were 7.1±18.8mmHg and 5.3±9.2mmHg, respectively (p< 0.001). The response to regadenoson was less pronounced in obese and diabetic patients. The increase in heart rate was greater in symptomatic patients (27.4 ± 11.2 bpm vs. 20.6 ± 10.7 bpm in asymptomatic patients, p= 0.001). No severe adverse effects were observed.
ConclusionRegadenoson is well tolerated and can be safely used for cardiac MRI stress tests.
Describir la seguridad del regadenosón como fármaco vasodilatador en estudios de resonancia magnética cardíaca (RMC) de estrés realizados para detectar isquemia miocárdica.
Material y métodosSe analizaron de manera retrospectiva los estudios de 120 pacientes (88 varones, edad media 67±11,6 años) con sospecha de cardiopatía isquémica o con enfermedad coronaria conocida e indicación clínica para RMC de estrés. Los estudios se realizaron en un equipo de 1,5Tesla (MAGNETOM Aera, Siemens Healthineers) empleando regadenosón (5ml, 0,4mg) como agente vasodilatador. En todos los pacientes se recogieron los factores de riesgo cardiovascular, fármacos que tomaban e indicación de la prueba, además de las constantes vitales en situación de reposo y bajo estrés y los síntomas y efectos adversos inducidos por el fármaco.
ResultadosEl 52.6% de los pacientes permanecieron asintomáticos. Los síntomas más frecuentes fueron la opresión centrotorácica (25%) y la disnea (12%). Durante el pico de estrés, el incremento medio de la frecuencia cardíaca fue de 23,9±11,4 lpm y el descenso medio de la presión arterial sistólica y diastólica de 7,1±18,8mmHg y 5,3±9,2mmHg, respectivamente (p< 0,001). En pacientes obesos y diabéticos se objetivó menor respuesta hemodinámica al regadenosón, mientras que los pacientes sintomáticos presentaron mayor incremento de la frecuencia cardíaca (27,4 ± 11,2 lpm frente a 20,6 ± 10,7 lpm, p= 0,001). No se evidenció ningún efecto adverso grave.
ConclusiónEl regadenosón es un fármaco bien tolerado que se puede utilizar con seguridad en RMC de estrés.
Cardiac imaging techniques are used to confirm the diagnosis of coronary heart disease, document ischaemia, stratify risk, help choose the most appropriate treatment and assess its effectiveness.1
In patients with stable symptoms suggestive of coronary heart disease, ischaemia screening tests are recommended to either identify the vascular territory involved prior to coronary angiography or to rule out ischaemia as a cause of symptoms.2 These tests include functional techniques, such as stress echocardiography, single photon emission computed tomography (SPECT), positron emission tomography (PET) and stress cardiac magnetic resonance imaging (stress CMR). In a recent meta-analysis, the authors concluded that stress CMR offers the highest diagnostic performance for ischaemia-causing coronary artery disease.3
Stress CMR is usually performed with vasodilators, such as dipyridamole or adenosine.4 These drugs bind to adenosine receptors (A1, A2A, A2B and A3) located in different organs and cause vasodilation of coronary vessels and peripheral vascularisation by activating A2A5 receptors. The adverse effects associated with these drugs are caused by A1, A2B and A3 receptor activation. Minor adverse reactions have been described in up to 80% of patients. The most common are facial flushing (35%-40%), chest pain (25-30%), dyspnoea (20%), dizziness (7%), nausea (5%) and symptomatic hypotension (5%), in addition to atrioventricular block (AV) (7%-8%) and ST segment depression (5%-7%).6
Regadenoson, a selective A2A receptor agonist with none of the side effects associated with A1, A2B and A3 receptor activation, has recently been introduced in stress tests.7 Regadenoson has been used in the field of nuclear medicine, and has been found to be safe in different clinical settings and in various patient groups in which conventional vasodilators have usually been relatively contraindicated, such as patients with chronic obstructive pulmonary disease (COPD) or mild to moderate asthma.8 The latest scientific literature also reports that regadenoson is safe in stress CMR, although its use in this context is not yet widespread.9
The aim of this study is to describe the safety profile of regadenoson used as a vasodilator in unselected patients clinically indicated for stress CMR studies.
Material and methodsA retrospective analysis of the results of stress CMR tests performed in 120 consecutive patients between May and December 2017 was conducted. Patients with pacemakers, defibrillators or any device that could interfere with magnetic resonance imaging (MRI), intracranial aneurysm clips or intraocular metallic elements, body habitus that exceeds MRI limits, glomerular filtration rate of 30ml/min or less, claustrophobia not responding to mild intravenous anxiolytics and pregnant or breastfeeding women were excluded. Patients who were unable to remain supine for at least 30min or endure apnoea for 15s, those who were haemodynamically unstable or had a contraindication for regadenoson, such as hypersensitivity to the active substance or to any of the excipients, and patients with second- or third-degree AV block, sinus node dysfunction, unstable angina refractory to medical therapy, severe hypotension or decompensated heart failure were also excluded. Patients were asked not to drink coffee or other drinks or foods containing caffeine for 24h before the scan. Demographic data were collected from all patients, in addition to cardiovascular risk factors, usual medication and clinical indication for the study.
The use of regadenoson in CMR studies was approved by our hospital’s pharmacy and therapy committee. The study was classified as a “post-authorisation study-other design (abbreviated as EPA-OD)” by the Agencia Española de Medicamentos y Productos Sanitarios [Spanish Agency of Medicines and Medical Devices] (CUN-REG-2019-01) and approved by our hospital’s ethics committee. All patients gave their signed informed consent for the study.
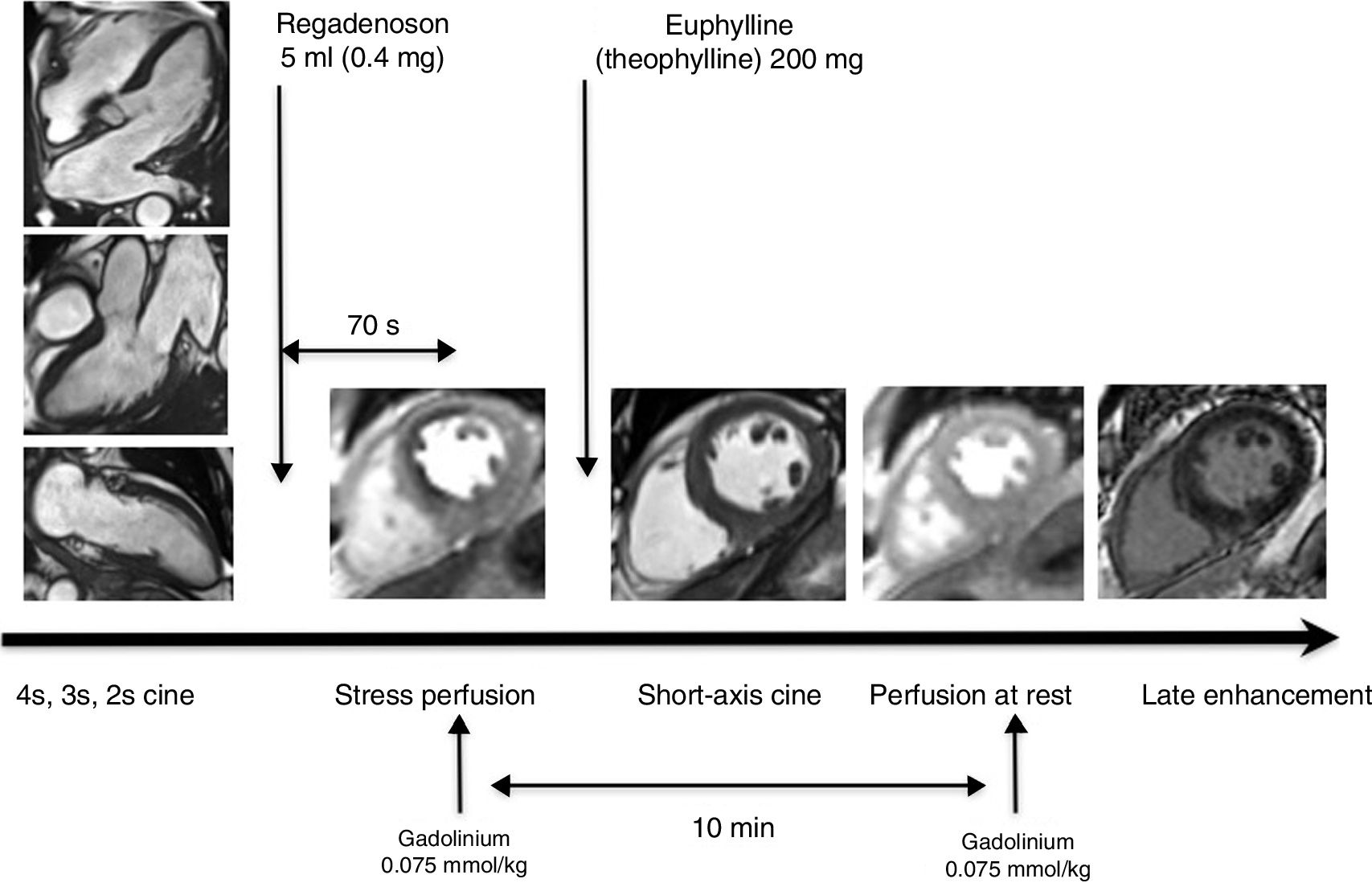
Stress cardiac magnetic resonance imaging protocolImaging studies were carried out using a 1.5Tesla device (Magnetom Aera, Siemens Healthcare, Erlangen, Germany) with a 6-channel surface coil. A conventional stress CMR protocol consisting of specific sequences was used to assess the anatomy and function of the heart, coronary perfusion, and to characterise the myocardium. The protocol included a long-axis functional study of the heart. Then regadenoson was administered and the stress perfusion study was performed 70s after its injection. Intravenous Euphyllin (theophylline) was administered within three minutes of regadenoson administration to reverse the vasodilator effect of the drug. Following this, the short-axis functional study was performed. Perfusion at rest was performed at least 10min after the stress perfusion study to leave sufficient time for gadolinium wash-out of cardiac cavities. The protocol concluded with the acquisition of late enhancement images (Fig. 1).
Ventricular function was evaluated on four-chamber, two-chamber and three-chamber views and short axis images using steady-state free precession (SSFP) sequences with retrospective ECG synchronisation and the following acquisition parameters: TR: 43.26ms, TE: 1.3ms; matrix: 156×192; field of vision: 260−280×325−375mm; voxel size: 1.7×1.7mm×8mm; 8mm slice thickness with no overlap between slices, 14 segments, 25 phases.
The perfusion study was performed using a Turbo-FLASH sequence (TR: 2.96ms; TE: 1.1ms, matrix: 160×82; field of vision: 380×285mm; voxel size: 2.4×2.4×10mm; 10mm slice thickness, 59 segments, 50 acquisitions) under pharmacological stress and at rest. Three representative slices (basal, mid-ventricular and apical) of the left ventricle were acquired. Perfusion under stress and at rest was performed during administration of 0.075mmol/kg of gadobutrol (Gadovist, Bayer AG, Berlin, Germany) at a rate of 4ml/s (total dose of 0.15mmol/kg) using a dual-head injector (Medrad Inc., Warrendale, Pennsylvania, USA).
The late gadolinium enhancement was evaluated using SSFP-PSIR (phase-sensitive inversion recovery) (TR: 16.1ms; TE =1.74ms; FA=45°; matrix: 256×71; voxel size=1.3×1.3×8mm; 46 segments) 10−12min after administering the second dose of contrast.
Regadenoson administration protocolThe vasodilator regadenoson (Rapiscan, GE Healthcare AS, Oslo, Norway) was used to induce stress in all patients. It was administered as a single, fixed, intravenous dose of 0.4mg (5ml) in a manual injection lasting approximately 10s. Within three minutes of acquiring the stress study, 200mg of intravenous euphyllin was administered to reverse the effect of regadenoson in all patients, regardless of whether or not they manifested drug-related clinical symptoms.
The patients were monitored throughout the procedure by measuring their heart rate (HR) and blood pressure (BP) and the clinical signs and symptoms that arose during the scan. The imaging studies were supervised by at least one radiologist and one cardiologist, together with a radiology technician and a nurse. Patients were asked to describe their symptoms before receiving any of the drugs. Adverse effects that could be related to drug-induced stress were also recorded, such as bronchospasm, atrioventricular block, arrhythmias, ventricular tachycardia, ventricular fibrillation, need for hospitalisation, myocardial infarction or death.
The haemodynamic effect of regadenoson was evaluated on the basis of BP and HR measured at rest and under pharmacological stress, and the difference between these parameters was calculated. Peak HR was defined as the patient's highest heart rate during the perfusion study. Peak BP was defined as blood pressure measured before administering euphyllin. The haemodynamic response was defined as peak HR – baseline HR and peak BP – baseline BP.
Imaging study analysisImaging data were processed on a workstation equipped with specific software (cmr 42, Circle Cardiovascular Imaging Inc., Calgary, Canada). To analyse ventricular function, the endocardial and epicardial contours of the left ventricle were manually plotted on the end-diastolic and end-systolic short axis images obtained, in accordance with standard practice.10 Papillary muscles were excluded from the volumetric calculation and were included as myocardial mass. The ejection fraction (EF), end-diastolic volume (EDV), end-systolic volume (ESV) and stroke volume (SV) were recorded. The myocardial mass was obtained by adding the volumes of the tissue located between the endocardial and epicardial contours and multiplying the result by the specific density of the myocardium (1.05g/cm). Patients were considered to have normal left ventricle morphology if ventricular volumes and mass were within normal limits.11 Patients with normal myocardial mass and relative parietal thickness (RPT) greater than or equal to 0.42 were classified as concentric remodelling, and if they presented an increase in myocardial mass and RPT≥0.42, as concentric hypertrophy.12 Patients were classified as having ventricular dilation if their EDV adjusted for body surface area was higher than the mean ± 2 standard deviations, according to their age and gender.11
Myocardial perfusion was analysed qualitatively. The left ventricle basal and mid-cavity slices were divided into six equiangular segments, and the apical slice was divided into four segments, as recommended by the American Heart Association.13 Any myocardial segment that showed hypoperfusion during stress and normal perfusion at rest, and did not show infarction in late enhancement images was considered ischaemic.
Late enhancement was evaluated together with the function sequences to establish the correlation between segmental contractility alterations and the presence of infarction. Transmural infarction was defined as late enhancement greater than 50 % of myocardium thickness in the affected segment, while non-transmural infarction was defined as subendocardial enhancement that affected less than 50 % of myocardium thickness. Non-ischaemic late enhancement patterns were also recorded.
Statistical analysisData are presented according to the characteristics of the variables and their distribution. The Kolmogorov-Smirnov test was used to test the normality of the distribution of the data. Changes in stress-induced BP and HR in each individual were compared using the Student's t-test for paired samples, while the other continuous variables were compared using the Student's t-test for independent samples. Categorical data are presented as percentages and compared using the χ2 test. Statistical analysis was performed on SPSS for Mac (version 20/SPSS Inc., Chicago, IL) and WINPEPI (version 8.8, PEPI for Windows). Statistical significance was set at p<0.05.
ResultsStudy populationIn total, 120 consecutive subjects were included (88 men, 32 women; mean age 67±11.6 years). In most cases, the test was performed in patients who had previously undergone revascularisation and who presented atypical chest pain or possible ischaemic equivalent (46 of 120 subjects, 38.3 %), and in patients with suspected cardiomyopathy (27 of 120 subjects, 22.5 %). No patient was excluded due to claustrophobia or contraindications for CMR, or for administration of regadenoson or paramagnetic contrast. The study protocol was performed in full in all patients. Patient characteristics are shown in Table 1.
Clinical characteristics of study patients and indications for stress cardiac magnetic resonance imaging.
| Gender (male/female) | 88/32 (73.3 %/26.7 %) |
| Age (years) | 67±11.6 |
| Weight (kg) | 78.2±15.1 |
| Height (cm) | 169.4±7.7 |
| BMI (kg/m2) | 27.2±4.5 |
| BSA (m2) | 1.9±0.2 |
| HR (bpm) | 65.5±13.2 |
| Systolic BP (mmHg) | 151.3±21.6 |
| Diastolic BP (mmHg) | 76±10.6 |
| Heart rhythm | |
| Sinus | 81 (67.5 %) |
| Sinus with conduction defect | 31 (25.8 %) |
| Atrial fibrillation | 8 (6.7 %) |
| Cardiovascular risk factors | |
| Smoker (yes/no/former) | 48/13/59 (40 %/10.8 %/49.2 %) |
| HTN | 77 (64.2 %) |
| Dyslipidaemia | 75 (62.5 %) |
| Diabetes mellitus | 38 (31.7 %) |
| COPD | 21 (17.5 %) |
| Obesity (BMI≥30kg/m2) | 31 (25.8 %) |
| Previous revascularisation (bypass/stent) | 46 (9/37) (38.3 %) |
| Family history of ischaemic heart disease | 40 (33.3 %) |
| Pharmacological therapy | |
| Angiotensin II receptor antagonists | 60 (50 %) |
| Calcium channel blockers | 23 (19.2 %) |
| Acetylsalicylic acid | 60 (50 %) |
| Clopidogrel | 23 (19.2 %) |
| Diuretics | 37 (30.8 %) |
| Oral anticoagulants | 18 (15 %) |
| Beta blockers | 55 (45.8 %) |
| Statins | 76 (63.3 %) |
| Antidiabetics | 38 (31.7 %) |
| Antianginals | 14 (11.7 %) |
| Clinical indication for stress CMR | |
| History of coronary revascularisation and atypical chest pain or possible ischaemic equivalent | 48 (40 %) |
| Suspicion of cardiomyopathy or structural cardiomyopathy | 27 (22.5 %) |
| Atypical chest pain | 20 (16.7 %) |
| CV risk factors | 11 (9.2 %) |
| Ventricular tachycardia | 7 (5.8 %) |
| Inconclusive results from previous stress test or heart CT scan | 7 (5.8 %) |
BMI: body mass index; BP: blood pressure; bpm: beats per minute; BSA: body surface area; cm: centimetres COPD: chronic obstructive pulmonary disease; CV: cardiovascular; HR: heart rate; HTN: hypertension; kg: kilograms; m: metres; mmHg: millimetres of mercury.
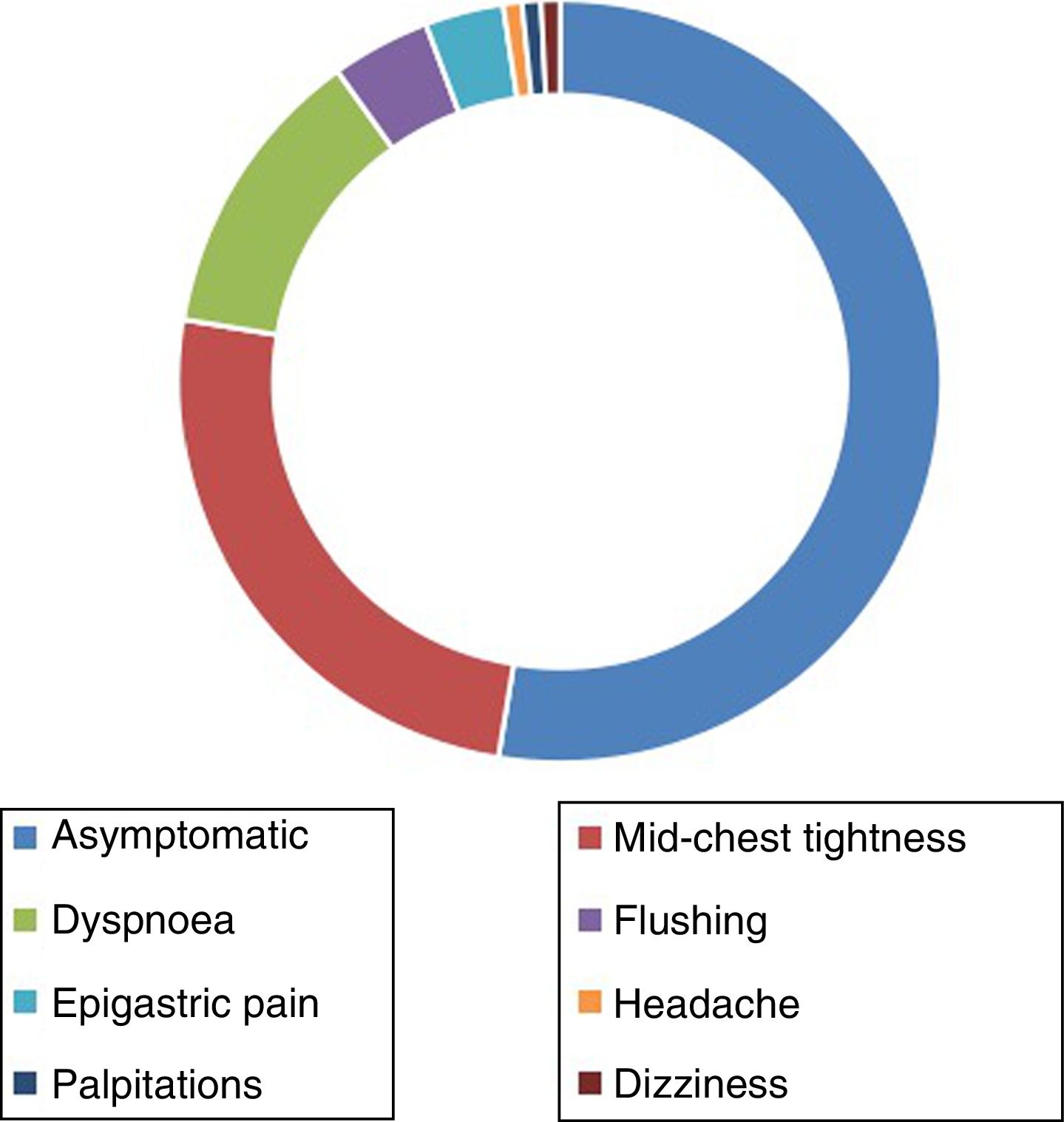
More than half of the study patients (52.6 %) did not report any symptoms during regadenoson administration. The clinical symptoms most frequently described by patients were mid-chest pain (25 %) and dyspnoea (12.5 %). Other less common symptoms were facial flushing (4.2 %), epigastric pain (3.3 %), headache (0.8 %), palpitations (0.8 %) and dizziness (0.8 %) (Fig. 2).
None of the complications observed required medical attention. Specifically, no episodes of infarction, decompensated heart failure, ventricular tachycardia or high-grade atrioventricular block occurred, and there were no study-related fatalities. No patients presented bronchospasms that required medical treatment. None of the patients reporting chest pain required pharmacological therapy to alleviate symptoms. Contrast extravasation occurred in one patient, which was treated conservatively. No patient required hospital admission.
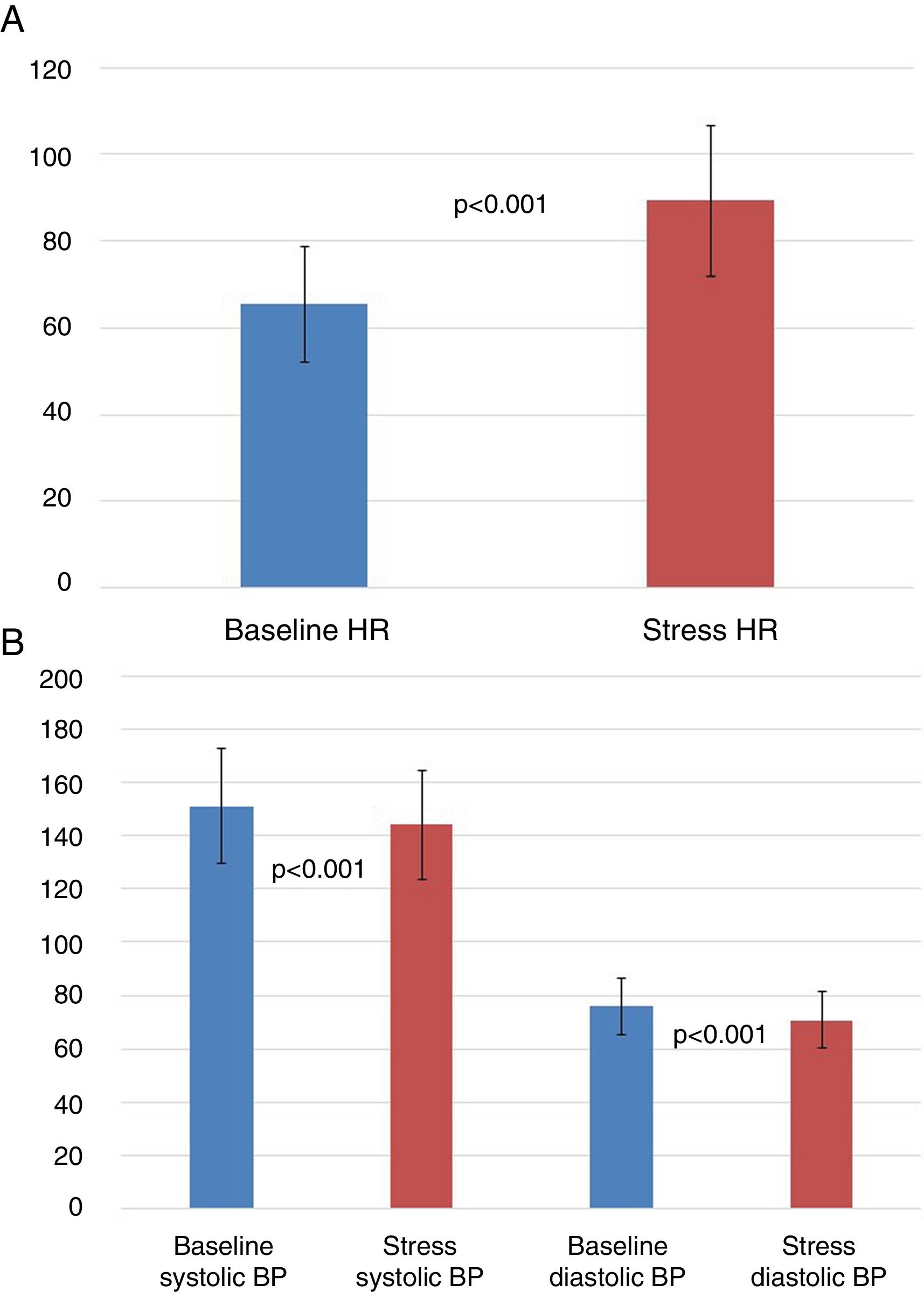
Haemodynamic response to regadenosonVasodilation caused an average increase in HR of 23.9±11.4 bpm (p< 0.001) and a mean decrease in systolic and diastolic BP of 7.1 ± 18.8 mmHg and 5.3 ± 9.2 mmHg, respectively (p<0.001) (Fig. 3). An analysis of differences in HR variation based on the patient’s cardiovascular risk factors and clinical characteristics showed no statistically significant differences, except in obese patients (BMI≥30kg/m2) (increase in mean HR of 19.7±7.8 bpm vs. 25.5±12.1 bpm in non-obese patients, p< 0.001) and in diabetics (increase in mean HR of 18.4 ± 9.2 bpm vs. 26.5 ± 11.4 bpm in non-diabetics, p< 0.001), who exhibited less haemodynamic response to regadenoson. A comparison of the haemodynamic response according to pharmacological treatment received revealed no statistically significant differences between patients.
Patients who reported clinical symptoms with regadenoson had a greater increase in HR than subjects who remained asymptomatic (increase in mean HR of 27.4±11.2 bpm vs. 20.6±10.7 bpm in asymptomatic patients, respectively, p= 0.001). The haemodynamic response in patients with prior revascularisation was similar to non-revascularised patients. The presence of myocardial ischaemia during the stress test did not influence the haemodynamic response to regadenoson.
Results of the cardiac magnetic resonance imaging studyThe results of the stress CMR studies are shown in Table 2. In the left ventricle, the mean ejection fraction (EF) was 62.7±13.8 %, mean end-diastolic volume index (EDVi) was 77.9±28.5ml/m2 and mean end-systolic volume index (ESVi) was 31.9±25.65ml/m2. The morphology of the left ventricle was normal in more than half of all patients (53.3 %), concentric remodelling was observed in 15.8 % and ventricular dilation in 13.3 %.
Results of the stress cardiac magnetic resonance imaging study.
| Ventricular parameters | |
|---|---|
| LV-EF (%) | 62.7±13.8 |
| EDVi-LV (ml/m2) | 77.9±28.5 |
| ESVi-LV (ml/m2) | 31.9±25.6 |
| LV-Mi (g/m2) | 63.8±15.3 |
| RV-EF (%) | 63.1±10.4 |
| EDVi-RV (ml/m2) | 70.1±23.3 |
| ESVi-RV (ml/m2) | 31.6±25.3 |
| Left ventricle morphology | |
| Normal | 64 (53.3 %) |
| Concentric remodelling | 19 (15.8 %) |
| Asymmetric hypertrophy | 8 (6.7 %) |
| Concentric hypertrophy | 8 (6.7 %) |
| Eccentric hypertrophy | 5 (4.2 %) |
| Dilated | 16 (13.3 %) |
| Stress test | |
| Positive | 36 (30 %) |
| Negative | 84 (70 %) |
| Late enhancement | |
| No | 47 (39.2 %) |
| Ischaemic pattern | 41 (34.2 %) |
| Non-ischaemic pattern | 32 (26.6 %) |
Note. Ventricular parameters are shown adjusted for body surface area.
EDVi: end-diastolic volume index; EF: ejection fraction; ESVi: end-systolic volume index; LV: left ventricle; Mi: indexed mass; RV: right ventricle.
The mean EF of the right ventricle was 62.7±13.8 %; the mean EDVi was 70.1±23.3ml/m2 and the mean ESVi was 31.6±25.3ml/m2.
The stress test was positive in 36 patients (30 %) and negative in 84 (70 %) (Fig. 4). Over half (60.8 %) of all patients presented late gadolinium enhancement, with a pattern of ischaemic enhancement in 34.2 % and a pattern of non-ischaemic enhancement in 26.6 %.
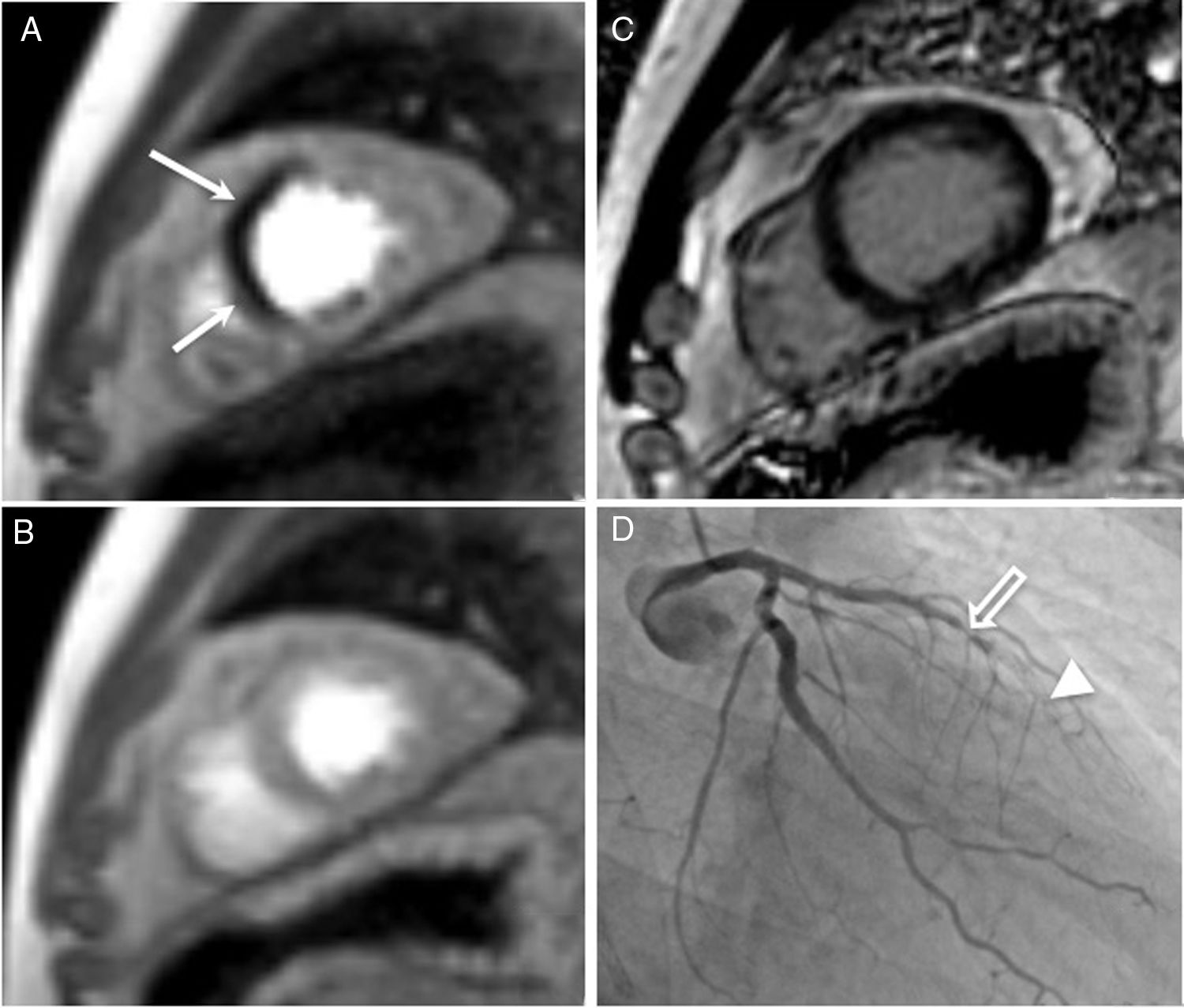
Stress cardiac magnetic resonance imaging with regadenoson in a 61-year-old patient with a history of ischaemic heart disease percutaneously revascularised by placing stents in the proximal and middle segments of the anterior descending artery, who was seen for chest tightness. A) Stress perfusion. B) Perfusion at rest. C) Late enhancement. D) Conventional coronary angiography. The stress study showed a perfusion defect in the apical septal segment (arrow in A), with normal perfusion in this segment at rest (B) and no late gadolinium enhancement suggestive of infarction (C). The patient was diagnosed with ischaemic heart disease in the territory of the anterior descending coronary artery and referred for percutaneous coronary intervention. Conventional coronography showed severe restenosis (95 %) in the segment joining both stents (open arrow) and occlusion of the more distal stent (arrow head), thus confirming the diagnosis. The patient underwent percutaneous coronary intervention with implantation of a new drug-eluting stent.
This study shows that regadenoson is a safe and well-tolerated vasodilator for stress CMR imaging studies.
Due to its excellent safety profile, tolerability and mechanism of action, regadenoson has been used as a stress agent in ischaemia screening tests for more than a decade and is now the drug of choice for drug-induced stress tests in many nuclear medicine laboratories.14,15 In Europe, regadenoson is approved for nuclear medicine myocardial perfusion studies but is still used "off label" in CMR studies.16
The vasodilatory efficacy of regadenoson has been described as similar to that of adenosine and superior to that of dipyridamole, most likely due to its direct action on adenosine receptor agonists. Dipyridamole causes vasodilation by indirectly increasing adenosine levels.17
Furthermore, unlike adenosine and dipyridamole, regadenoson is a fast-acting drug that can be administered simply in a single fixed dose that is not weight-dependent. Regadenoson has additional advantages in CMR. For example, it is more convenient and inexpensive to administer than adenosine or dipyridamole because it does not required infusion pumps. In addition, due to its selective action on adenosine A2A receptors, regadenoson has an excellent safety profile and causes fewer symptoms than other vasodilators.9 In the Adenoscan multicentre study, performed to confirm the safety of intravenous adenosine infusion in myocardial perfusion tests, 81 % of patients experienced side effects, the most common being facial flushing (36.5 %), dyspnoea (35.2 %), chest pain (34.6 %), gastrointestinal discomfort (14 %) and headache (11 %).18 With regard to dipyridamole, a registry of 3911 patients published by Ranhosky et al.19 reported minor side effects in almost half of all patients (46.5 %), including chest pain (19.7 %), headache(12.2 %), dizziness (11.8 %), nausea (4.6 %) and facial flushing (3.4 %), and serious adverse effects in 0.26 % (four patients with myocardial infarction and four with bronchospasm).19 More than half of our cohort (52.6 %) remained asymptomatic during drug administration, and patients who reported symptoms did not require any specific treatment. This is consistent with the findings of Nguyen et al.9 after evaluating 728 patients receiving regadenoson for stress CMR. These authors observed stress-induced atrial or ventricular ectopic beats in 46 subjects (6 %), chest pain that required treatment with nitroglycerin in nine (1 %) and with intravenous metoprolol in six (<1 %), symptomatic hypotension in two patients (< 1 %) and bronchospasm in one patient. In addition, two patients in this cohort presented contrast extravasation, one patient suffered a mild allergic reaction to gadolinium and one required hospitalisation.9
When using vasodilators, it is important to determine if the drug used has achieved the desired haemodynamic effect. A false negative rate of 5 %–10 % with respect to conventional coronary angiography has been reported in myocardial perfusion scans performed with adenosine and dipyridamole.20 Over half of these false negatives are believed to be due to ineffective stress induction, either due to insufficient dosage or interaction with other medications or substances, such as caffeine. One way of determining the haemodynamic effect of the drug is to achieve a 10 bpm increase over baseline HR and a 10mmHg reduction in baseline systolic BP. However, these parameters do not predict successful coronary vasodilation.21 In fact, certain groups of patients, such as diabetics or patients with chronic kidney failure, have a decreased haemodynamic response.22 Patient-reported symptoms during administration are also taken into account when evaluating response, leading to subjective assessment errors. These limitations have not been reported with regadenoson. In our cohort, we observed a mean increase in HR of 23.9±11.4 bpm and a mean decrease in systolic and diastolic BP of 7.1±18.8mmHg and 5.3±9.2mmHg, respectively. The only subgroups of patients with less variation in HR were diabetics and obese patients, an observation also reported by Nguyen et al.9 and in perfusion studies using SPECT.23,24 In the case of diabetic patients, lower tachycardic response to the vasodilator is known be a predictor of poor prognosis.25 In some studies, a single 0.4mg dose was sufficient to cause vasodilation in obese patients26, suggesting, in line with Nguyen et al.9, that the use of the same dose in all patients does not explain the lower tachycardic response in obese patients. Targeted studies are needed to explain this particular mechanism. Patients who reported clinical symptoms with regadenoson experienced a greater increase in HR, probably due to the subjective sensation of drug-induced tachycardia. In terms of drug interactions, no significant differences in the haemodynamic effect of regadenoson were observed in patients treated with beta-blockers (23.8±10.5 bpm vs. 24±12.5, p=0.93).
The diagnostic efficacy of stress CMR in the detection of coronary heart disease has been confirmed in several studies.27 The findings of a recent cost-effectiveness study suggest that the faster administration of regadenoson compared to adenosine could reduce the cost of CMR studies.28 Although a similar protocol is used in both drugs, the single dose of regadenoson needed to achieve vasodilation reduces the duration of the procedure, whereas other drugs, such as adenosine, may require dose escalation. From a strictly economic perspective, regadenoson is more expensive than adenosine, although the cost of a stress test with adenosine may be more expensive if MRI-compatible infusion pumps are not available, because more vials will be needed to complete the circuit or to obtain the desired haemodynamic effect.
This study has several limitations. On the one hand, the sample size is small compared to other studies.9 However, our study reports the first experience in Spain of using regadenoson in stress CMR imaging studies in a clinical setting with non-selected patients. On the other hand, the haemodynamic effect of the drug has been assessed on the basis of variations in HR, changes in BP levels and drug-related symptoms, but not on myocardial hyperaemia, which would have provided a more objective assessment.17 Finally, although it was not the aim of the study, we did not correlate our CMR findings with the results of conventional coronary angiography. This correlation must be performed to establish the diagnostic efficacy of the technique in our setting.
In conclusion, regadenoson is a safe and well-tolerated vasodilator for stress CMR imaging. The few adverse reactions caused were mostly mild and transient and a hyperaemic response was obtained in all patients. Further studies with a larger sample size and more specific risk profiles are needed to evaluate the safety of the drug in specific patient subgroups.
AuthorshipResponsible for the integrity of the study: GB, AE, AGB
Study conception: GB, AE, JCP, JJG
Study design: GB, AE
Data acquisition: GB, AE, MC, MC, JCP
Data analysis and interpretation: GB, AE, AGB
Statistical processing: GB
Literature search: GB, AE, AGB, MC
Drafting of the article: GB
Critical review of the manuscript with intellectually relevant contributions: GB, AE, MC, AGB, MC, JCP, JJG
Approval of the final version: GB, AE, AGB, MC, MC, JCP, JJG
Conflict of interestThe authors have no conflicts of interest to declare.
Please cite this article as: Bastarrika G, Ezponda A, García Baizan A, Calvo M, Pueyo JC, Gavira JJ, et al. Seguridad del empleo de regadenosón como fármaco vasodilatador en resonancia magnética cardíaca de estrés. Radiología. 2020;62:213–221.