
Radiologists in the emergency department must be prepared to deal with any type of disease in any organ at any time. Many entities involving the chest can result in patients’ presenting at the emergency department. This chapter deals with entities that manifest with multifocal lung opacities and that can be mistaken for pneumonia. To facilitate their identification, this chapter approaches these entities by considering their most characteristic distribution on chest X-rays, the main diagnostic modality used for thoracic problems in the emergency department. Our schematic approach includes the key findings in patients’ personal histories, clinical examination, laboratory tests, and imaging studies that can be available during the initial workup.
El radiólogo de urgencias ha de prepararse para enfrentarse a enfermedad de todo tipo, de cualquier órgano y en cualquier momento. Son muchas las entidades con afectación torácica por las que un paciente puede acudir a urgencias. En este capítulo se exponen las enfermedades que se manifiestan con opacidades pulmonares multifocales y que clínicamente pueden simular y diagnosticarse erróneamente como una neumonía. Se han abordado considerando la distribución más característica, con el objetivo de que fuesen identificables empleando la principal herramienta diagnóstica torácica de que se dispone en urgencias: la radiografía de tórax. Se exponen de forma esquemática, aportando claves diagnósticas radiológicas, clínicas, de laboratorio y antecedentes personales, a las que se puede tener acceso en la primera valoración del paciente.
This chapter will schematically address the differential diagnosis in emergencies of pulmonary conditions whose radiological semiology consists of pulmonary opacities, either ground-glass or consolidated or both, multifocal or bilateral, known as multifocal pulmonary opacities (MPOs). Useful and accessible aspects from the emergency room will be pointed out, such as symptoms, the onset of symptoms (acute-subacute), laboratory test abnormalities and clinical and radiological history that help in a first differential diagnosis.
Except for acute pulmonary edema, all the diseases that present can present with fever or low-grade fever, leading to confusion with infections. Symptoms such as cough, dyspnea and chest pain may be present in all the diseases that appear, so, in practice, they will be of limited utility for discriminating between them.
Conditions whose main semiology is different from MPOs and those typical of pediatric, traumatized or cancer patients, or related to hematopoietic stem cell or solid organ transplantation, have been excluded.
Review of the subjectThe differential diagnosis will be established based on the distribution of the MPOs in the axial axis and the craniocaudal axis and, finally, taking into account other distributions.
Fig. 1 summarizes the key data of the conditions that will be discussed throughout the chapter so that it can serve as an index and final reference document for the differential diagnosis of MPOs.
Schematic of the key diagnostic data of the conditions that can present in the emergency room with multifocal pulmonary opacities (MPOs).
The predominant distribution of opacities has been considered as the central axis, together with the clinical course, with those of acute onset on the left of the image, and those of subacute onset on the right. The key data for each disease have been added and, in italics, the radiological findings, highlighting the type of MPO and other frequent or characteristic radiological findings. From top to bottom, the MPOs predominant in central and lower regions (a), in central and upper regions (b), in peripheral and lower regions (c), in peripheral and upper regions (d), in upper regions versus lower regions (e), and with diffuse distribution (f).
DAD: diffuse alveolar damage; DILD: diffuse interstitial lung disease; GM-CSF: granulocyte-macrophage colony-stimulating factor; DAH: diffuse alveolar hemorrhage; AEP: acute eosinophilic pneumonia; CEP: chronic eosinophilic pneumonia; HP: hypersensitivity pneumonitis; nfHP: non-fibrotic hypersensitivity pneumonitis; NSIP: non-specific interstitial pneumonia; OP: organizing pneumonia; UIP: usual interstitial pneumonia; QT: chemotherapy; HU: Hounsfield units; GG: ground glass.
*Conditions previously described in another more characteristic distribution.
The MPOs with central and peripheral predominance and with an anteroposterior gradient are distinguished.
MPOs with central predominanceAcute cardiogenic pulmonary edema. When the disease is advanced, the typical consolidations take place in "butterfly wings" with a typically central, perihilar distribution, corresponding to the last phase of cardiogenic pulmonary edema, when excess fluid accumulated in the lung due to failure of the cardiac pump already occupies the alveolar space. In pulmonary edema of cardiogenic origin, unlike edema due to increased permeability, the pulmonary architecture is preserved, thus allowing different compartments to be affected as the process evolves.1 This makes it possible to establish a series of phases that can be differentiated radiologically, the detection of which helps to determine the picture. The manifestations of each phase in a standing chest X-ray in cardiogenic edema are2:
–Retrograde increase in blood volume into the pulmonary veins and arteries due to failure of the cardiac pump. It manifests radiologically with dilation of the pulmonary veins of the upper lobes, which will be recognized above all by comparing the caliber of the vessels with that visible in previous X-rays. In the veins of the lower lobes, the finding is less recognizable because they are more engorged in the basal situation due to the gravitational effect, so that the excess volume seeks less congested areas, such as the upper lobes. As it progresses, the pulmonary arteries will also dilate (postcapillary pulmonary hypertension), which will show a larger diameter than the accompanying bronchus, assessable on a chest X-ray in the proximity of the pulmonary hilum (Fig. 2a and b)1
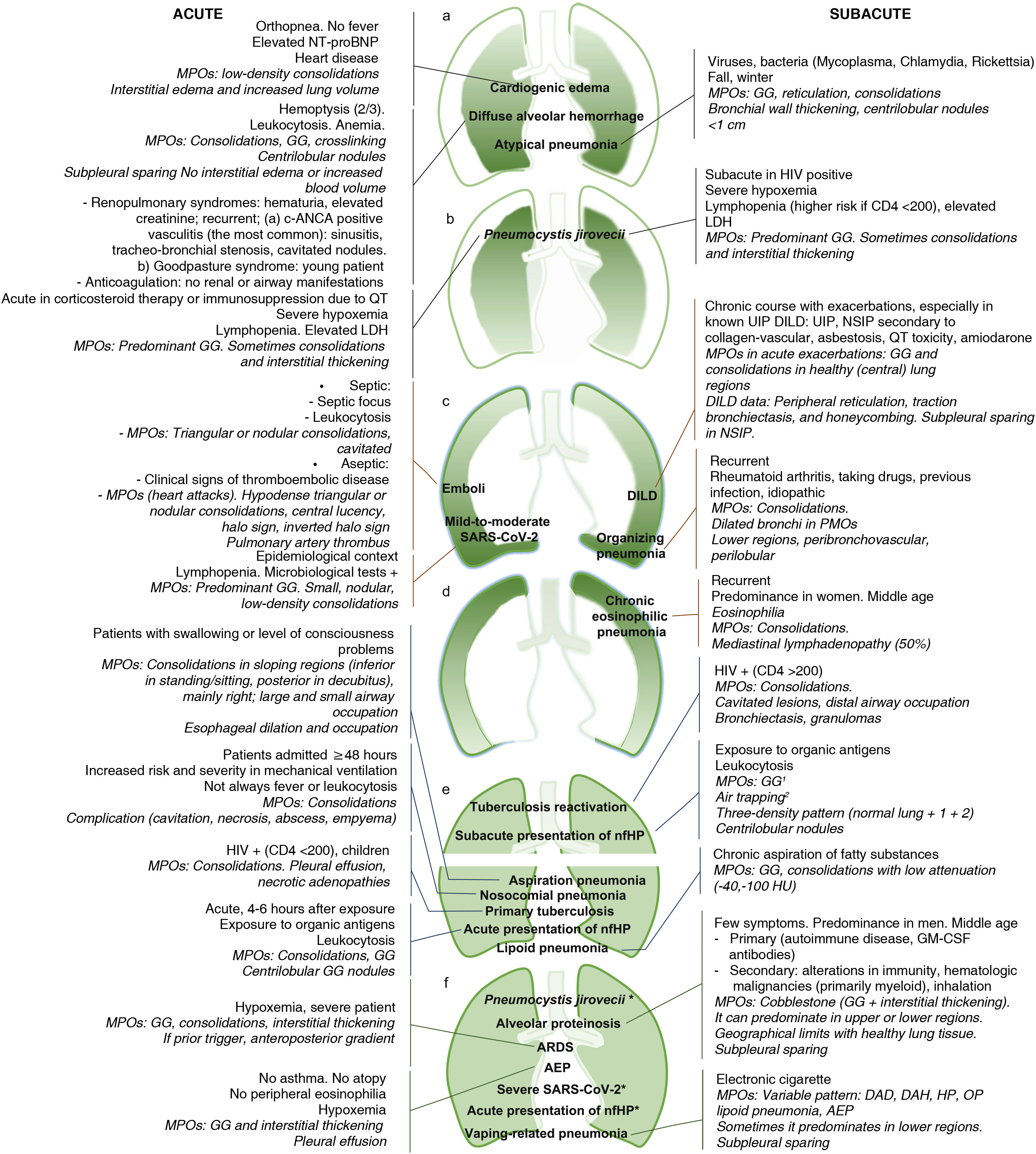
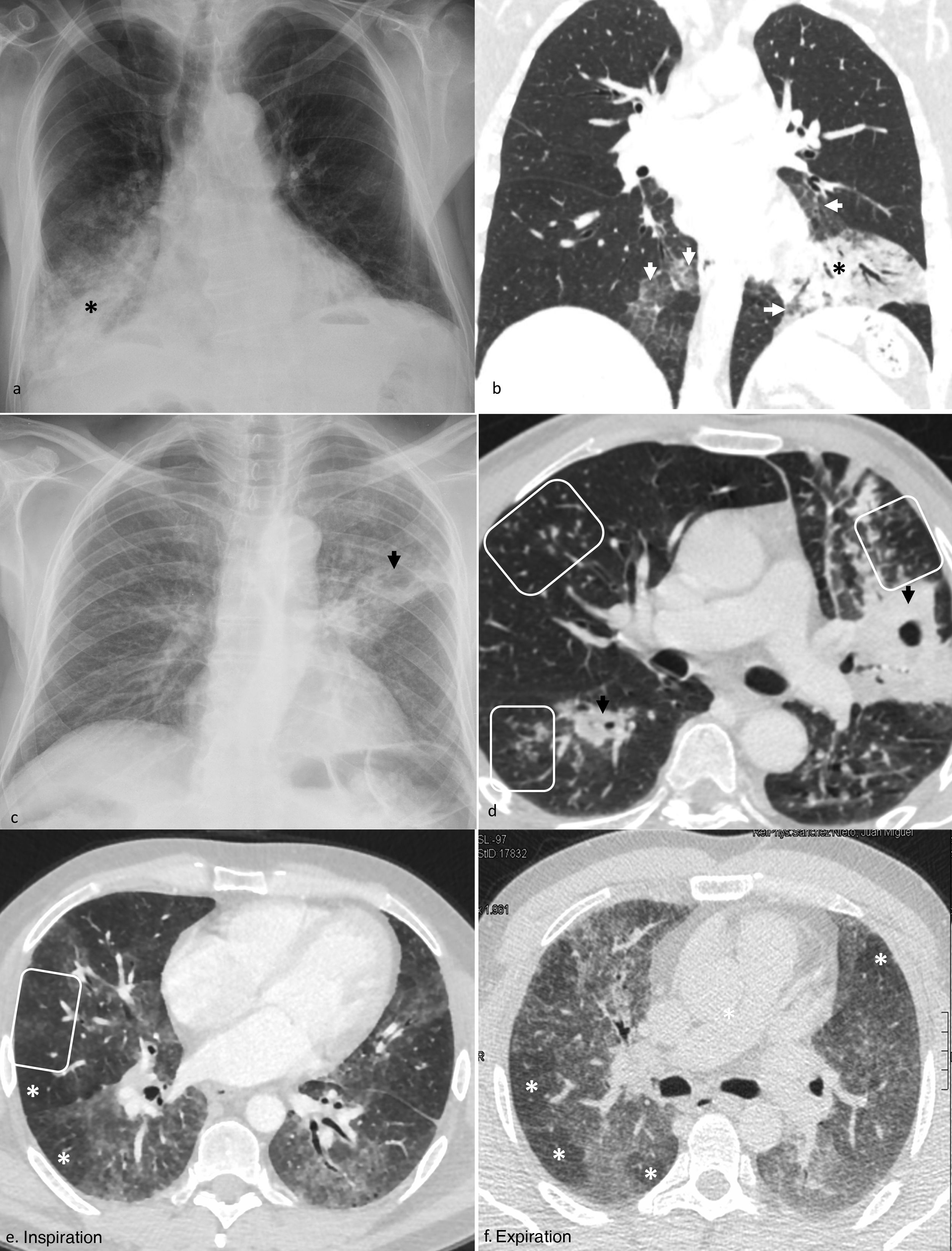
Figure 2.Multifocal pulmonary opacities with a predominantly central distribution.
Acute cardiogenic pulmonary edema on radiography. a) Incipient alveolar phase, with signs of increased blood volume (dilation of veins [black arrow] and pulmonary arteries [white arrow], and increased cardiothoracic ratio), interstitial edema (thickening of peribronchovascular cuffs [white arrowhead] and Kerley B lines [black arrowheads]) and alveolar edema, with small central consolidation in the right upper lobe (asterisk). b) More evolved alveolar phase in another patient, with perihilar consolidations with butterfly wings pattern.
Diffuse alveolar hemorrhage in a patient with Goodpasture syndrome on X-ray (c) and computed tomography (d), with predominantly mid-to-basal central consolidations, ground-glass opacities, and reticulation, with subpleural respect.
Pneumocystis jirovecii pneumonia in an HIV-positive patient diagnosed a posteriori, with central opacities predominantly in upper regions, mainly ground glass, on X-ray (e). In another patient, subjected to high doses of corticosteroids for a brain tumor, a more serious involvement was observed, with greater extension and density of the opacities, predominantly in ground glass and in upper regions, with subpleural sparing, in the computed tomography (f).
(0.73MB).–Interstitial edema. When the vascular space reaches its maximum capacity and the capillary hydrostatic pressure exceeds 18mmHg, the fluid continues to occupy the pulmonary interstitial space, both the axial or peribronchovascular (generating thickening of the peribronchovascular cuffs) and the interlobular space (generating a predominantly central and bibasal fine reticular pattern, and Kerley lines, with B lines being the most characteristic and easy to identify on X-ray, perpendicular to the pleural surface and at the lung bases) (Fig. 2a).3 During its clinical course, fissural thickening characteristically appears, followed by pleural effusion, with a bilateral distribution, although predominantly on the right, or only on the right if it is unilateral.
–Alveolar edema. From 25mmHg of capillary hydrostatic pressure, the interstitium does not support the accumulated fluid and it ends up occupying the alveolar space, producing central consolidations in "butterfly wings" (Fig. 2b), although this situation is only reached in 10% of cases.1,3 Atypical distributions of the edema can be found if it sits on a previously unstructured lung as in emphysema, and exceptionally in cases of severe mitral regurgitation (2%), in the form of consolidations in the right upper lobe.4,5 The diagnosis will be supported by finding heart disease data, such as an increased cardiothoracic index, pacemaker, stents or coronary by-pass, orthopnea symptoms and increased NT-proBNP in the analysis. Radiological findings appear and resolve quickly, with treatment.
Diffuse alveolar hemorrhage (DAH). Radiologically, it manifests with central PMOs that, although extensive, characteristically spare the subpleural area.6 The ground-glass component is usually extensive, and may also be associated with consolidations, occupation of the distal airway, with centrilobular or tree-in-bud nodules. During its clinical course, peripheral interstitial thickening appears. Its radiological appearance is usually rapid; its resolution also, although less so than that of the edema. Alveolar opacities and septal thickening caused by an acute bleeding episode resolve within two weeks.7 Hemoptysis is the cardinal symptom, but it is absent in a third of cases, in addition to not being exclusive to DAH. It presents with leukocytosis and anemia. The presence of hematuria and elevated creatinine indicates a renal-pulmonary syndrome, with these being responsible for the majority of alveolar hemorrhages.7 Granulomatosis with polyangiitis (formerly called Wegener's granulomatosis), one of the vasculitides with positive antineutrophil cytoplasmic antibodies, is the most common. It is associated with sinusitis in 75–90% and tracheo-bronchial stenosis in 20% of cases. It follows on from Goodpasture syndrome, with positivity for anti-glomerular basement membrane antibodies and with a disease peak in young males (Fig. 2c and d).7 In anticoagulated patients, anticoagulation should be indicated as the most probable cause; its association with acute pulmonary edema is not uncommon when dealing with older patients. Radiological differentiation between pulmonary edema and alveolar hemorrhage can be complex.8
Pnemocystis jirovecii (P. jirovecii) pneumonia. Characteristically it causes MPOs of central or diffuse predominance, with respect to the subpleural area.6 The ground-glass component is clearly the main feature and may be associated with interstitial thickening, giving rise to a "cobblestone" pattern.9–11 During its clinical course, it is associated with consolidations that, according to some authors, tend to be distributed towards the upper lobes, unlike in DAH.11,12 The signs of increased blood volume and occupation by edema of the interstitial space, typical of edema, will be absent (Fig. 2e and f). In advanced stages, it may cause pulmonary cysts that can eventually be complicated by pneumothorax, but it is rare to find them at diagnosis. The diagnosis will be supported by a history of positive HIV status, although this is often the first manifestation of the disease, as well as a subacute onset (1–2 weeks), except if it occurs in the context of corticosteroid therapy or chemotherapy immunosuppression, circumstances in which the clinical picture and MPOs can develop more quickly.13 They usually present with severe hypoxemia refractory to treatment requiring admission to the Intensive Care Unit. Lymphopenia and elevated LDH will be other characteristic data.14
MPOs with peripheral predominanceSARS-CoV-2 pneumonia. The distribution of the MPOs is key. They predominate in the periphery and posterior regions of the middle and lower fields.11,15 If the MPOs predominate in the upper lobes or central region, other conditions should be considered.11 It usually begins, in the first week of symptoms, with patchy ground-glass opacities, sometimes associated with reticulation. Progressively, in more severe cases consolidations appear,16 sometimes pseudonodular, with a pattern of organizing pneumonia (Fig. 3a). The maximum peak of lung involvement is reached in three days for mild cases, and in two weeks in cases with moderate severity.15 Most of the symptoms are mild. However, some patients may develop dyspnea (typically 5–8 days after onset),15 lymphopenia and, in more severe cases, hypoxemia, similar to P. jirovecii pneumonia. In addition to the radiological semiological differences between the two, SARS-CoV-2 pneumonia generally develops faster, in hours or days from the onset of symptoms.11 On the other hand, vascular dilatations within the parenchymal involvement and the inverted halo sign with its variant, the target sign, are characteristic signs of SARS-CoV-2 pneumonia.17,18 When the disease is severe, they can develop between the first and third week from the onset of symptoms into diffuse opacities, whose main differential diagnosis and possible outcome is acute respiratory distress syndrome (ARDS).5,9 In the second or third week, reparative changes appear, with reticulation, curvilinear subpleural lines, parenchymal bands, retractile opacities with a "band" morphology, parallel to the pleural surface, bronchial dilatations and distortion, mostly reversible.15 This pattern of the disease corresponds to the first waves. Vaccination and new virus variants will probably modify this form of presentation towards milder radiological forms.19
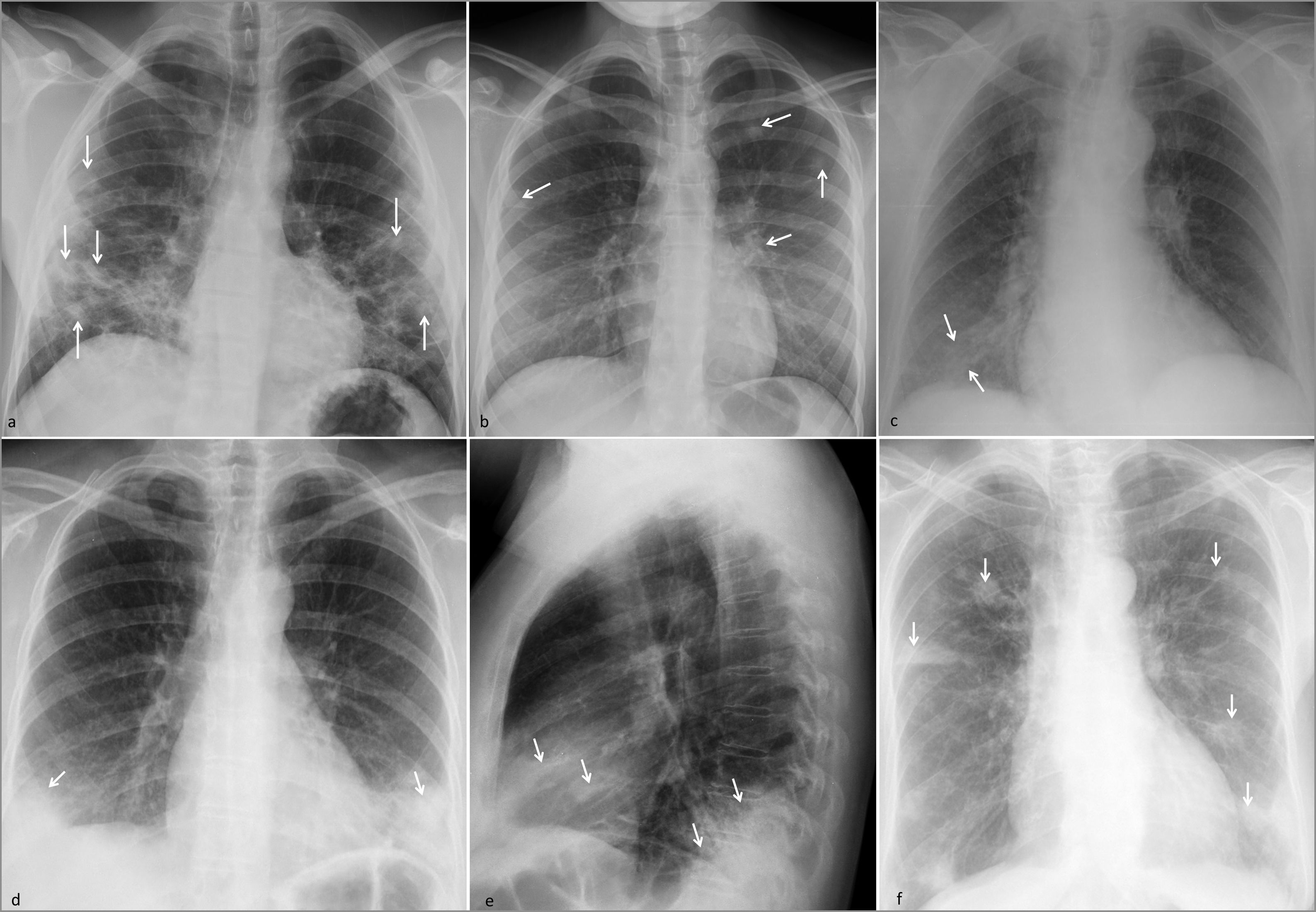
Multifocal pulmonary opacities of predominantly peripheral distribution.
SARS-CoV-2 pneumonia. (a) Ground-glass opacities with small consolidations in the middle and lower fields (arrows) in a young patient with a clinical picture weeks after onset.
Emboli. (b) Septic emboli in a patient with a cutaneous septic focus and Staphyloccocus aureus bacteraemia, with faint predominantly peripheral consolidations (arrows). (c) Peripheral consolidation due to pulmonary infarction secondary to acute pulmonary thromboembolism (arrows).
Organizing pneumonia. (d and e) Dense peripheral consolidations in the lower lobes and middle lobe (arrows). The patient had a history of rheumatoid arthritis and previous episodes of organizing pneumonia.
Chronic eosinophilic pneumonia. f) Dense peripheral consolidations with a certain predominance in upper regions (arrows). The patient, a middle-aged woman, had a history of previous episodes.
Emboli They cause MPOs that are characteristically peripheral (subpleural) consolidations, predominantly in the middle and lower fields, with a nodular or triangular shape, corresponding to infarcts. In a septic context, the clinical picture can indicate septic emboli, especially if the MPOs are cavitated and a septic focus is found, some of which are detectable in the emergency setting (skin, dentigenic, etc.) (Fig. 3b). On CT, the ground-glass halo can be seen, corresponding to hemorrhage, which reflects its condition as a hemorrhagic infarct, as well as the inverted halo sign, characteristic in an appropriate context20 and the nutrient vessel sign, corresponding to a vessel that reaches the center of the consolidation. In aseptic infarcts, mainly due to acute pulmonary thromboembolism, cavitation is less common, but central radiolucent areas may be found. Detecting filling defects in the pulmonary arteries will be key (Fig. 3c).
Organizing pneumonia.21 It presents with peripheral MPOs, predominantly in the lung bases, but also with peribronchovascular distribution and perilobular pattern (Fig. 3d and e).22 Bronchial dilatations occur within consolidations and the inverted halo sign is characteristic.22 It has a subacute, migratory and recurrent clinical course. It is frequently related to other conditions, such as rheumatoid arthritis, drugs or previous infections, although it can be cryptogenic. It is often initially approached as a pneumonic process.
Chronic eosinophilic pneumonia. MPOs in the form of characteristically peripheral consolidations, and predominantly in the upper and middle fields (Fig. 3f).23 When they are very extensive, they can manifest as the “negative of acute pulmonary edema”, although it only occurs in a third of cases.23 They are associated with mediastinal adenopathies in 50% of cases. Migratory and recurrent MPOs are a characteristic feature, although not pathognomonic, also visible in organizing pneumonia and vasculitis, but useful to dissuade us from the possibility of infection.23 It occurs frequently in middle-aged women, with a subacute course and peripheral eosinophilia.
MPOs with anteroposterior gradientEdema due to increased permeability or ARDS.2 Parenchymal damage occurs, producing alveolar damage and edema due to increased permeability. Unlike cardiogenic edema, there is no interstitial thickening that precedes alveolar involvement or engorgement of pulmonary veins or arteries, since there is no cardiac pump failure.1 In ARDS, MPOs are diffusely or patchily distributed, reaching the lung periphery. It usually has a trigger, which can be immediate, such as gastric acid aspiration or blood product transfusion, or produced hours or days before the edema, such as sepsis, trauma, surgery, inhalation of toxins, or "near drowning".24 When the trigger is extrapulmonary, a gradient of anteroposterior involvement may develop, with the anterior or non-sloping lung portion normal or even hyperinflated, the middle portion affected by ground-glass opacities, and the sloping portion due to consolidations with dilated bronchi and atelectasis. The loss of volume in sloping regions can be compensated by the mechanical ventilation that these patients usually undergo.25 Pleural effusion is variable in this type of edema. The onset of symptoms of ARDS usually precedes the radiological signs, with hypoxia refractory to treatment and early requirements for mechanical ventilation, and it may be associated with fever.25,26 If the clinical and radiological pictures are compatible but there is no trigger, we speak of acute interstitial pneumonia.25 Transfusion-related acute lung injury (TRALI) is an underdiagnosed cause of ARDS but is the leading cause of transfusion-associated mortality. It can appear early, even just one hour post-infusion.27 In the presence of radiological signs of non-cardiogenic edema, detecting anemia or thrombocytopenia will be key data. It must be differentiated from hydrostatic edema due to the fluid overload caused by fluid infusion, with radiological characteristics of cardiogenic edema, but without data of vascular engorgement in veins or pulmonary arteries, since there is no cardiac pump problem.28 Another condition that causes ARDS is fat embolism syndrome, which manifests as bilateral MPOs with an anteroposterior gradient from lower to higher density; it can be associated with reticulation and, unlike other causes of ARDS, poorly defined nodules <1cm of random distribution.2,29 The respiratory picture characteristically develops within three days of trauma with a long bone fracture. It is produced by migration of fat from the bone marrow of the fractured bone towards the intramedullary veins. It is prevented by early immobilization of the fracture. Finding evidence of fat embolism in other organs, typically the skin, retina, brain, sputum and urine, will support this diagnosis.
According to distribution in the craniocaudal axisMPOs predominant in lower regionsAspiration pneumonia. It settles in sloping regions, which correspond to the lower lobes, especially the right one, and the middle lobe in patients in standing or sitting position, and in the posterior segments of the upper and apical lobes of the lower lobes in patients in supine decubitus position. They can be unilateral in the sloping region in patients in lateral decubitus poisition.30 They are characterized by dense consolidations with occupation of the airway both distally and in the trachea and bronchi. It may be associated with a loss of lung volume due to the occupation of the large airway by secretions (Fig. 4a). It is the most common cause of lung abscesses. Radiological findings often do not appear on the first X-ray taken in the emergency setting. It occurs characteristically in patients with swallowing difficulties, whether of digestive or neurological origin. Radiologically visible esophageal dilation and occupation may be key data.31 If the aspirated content is gastric acid, a chemical pneumonitis will occur, with development of symptoms in minutes, with bronchospasm and hypotension, which radiologically behaves like a non-cardiogenic edema.32
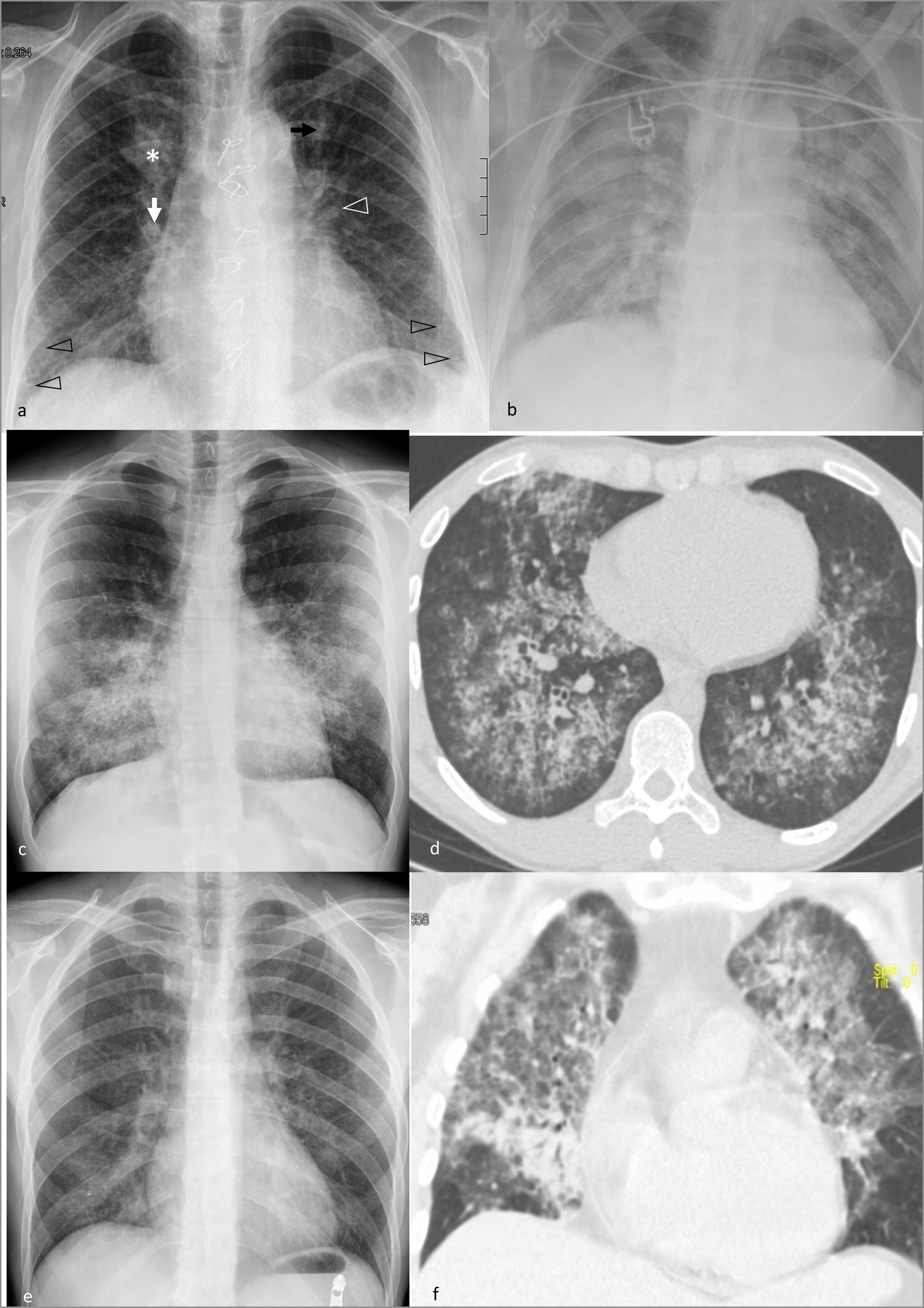
Multifocal pulmonary opacities according to their distribution in the craniocaudal axis.
Predominance in lower regions: aspiration pneumonia (a), X-ray with consolidations in both lower lobes, especially the right one (asterisk); Lipoid pneumonia (b), CT scan with ground-glass opacities in lower lobes (arrows), with associated reticulation in the right lobe and low-attenuation consolidation (asterisk) in the mediastinal window (not shown) in the left lobe.
Predominance in upper regions: tuberculous reactivation, X-ray (c), and computed tomography (d), with cavitated consolidations (black arrows) and tree-in-bud nodules (insets) in upper regions (upper lobes and apical segments of lower lobes); Non-fibrotic hypersensitivity pneumonitis in the subacute phase on computed tomography in forced inspiration (e) and expiration (f), with a three-density pattern: diffuse ground-glass opacities, air trapping (white asterisks), and normal lung tissue (not shown), as well as ground-glass centrilobular nodules (inset in e).
Nosocomial pneumonia It is pneumonia that appears 48h after admission. Fever and leukocytosis may be absent, making clinical suspicion difficult. They are usually due to gram-negative germs, with a higher risk of complications than community-acquired pneumonia. Mechanical ventilation increases the risk, with prevalence around 40%, and worsens its prognosis, with mortality around 80%. They usually manifest as bronchopneumonia predominantly in the lower lobes, although not always.33 It can complicate underlying acute pulmonary edema, a superinfection that should be suspected in the event of new progressive unifocal or multifocal pulmonary opacities, with a different distribution from the edema, that is, peripheral, asymmetric and with a slower development.
Other less common diseases: a) acute non-fibrotic hypersensitivity pneumonitis (nfHP), b) primary tuberculosis, and c) lipoid pneumonia (Fig. 4b).30,34,35
MPOs predominant in upper regionsTuberculosis reactivation. As they are the most oxygenated regions of the lung and with the worst lymphatic drainage, the upper lobes and apical segments of the lower lobes are the typical site, in immunocompetent or mildly immunosuppressed patients (Fig. 4c and d).35
Subacute presentation of nfHP. The “three-density pattern” is characteristic, which refers to the combination of normal lung volume with ground-glass opacities and air trapping, together with ill-defined centrilobular nodules (Fig. 4e and f).34
Other distributionsMPOs with diffuse distribution. It is common in previously explained acute conditions, such as pneumonia due to P. jirovecii, severe pneumonia due to SARS-CoV-2, ARDS (Fig. 5a–c) and acute presentation of nfHP. It is also the most common pattern in acute eosinophilic pneumonia, showing non-peripheral patchy diffuse consolidations, with marked thickening of the interlobular septa on CT and pleural effusion. It is not associated with adenopathies. It presents with acute febrile symptoms lasting less than five days and acute respiratory failure, simulating pneumonia.23,36 Unlike other eosinophilic processes, it is not usually associated with peripheral eosinophilia, but it is associated with bronchoalveolar lavage, or a history of atopy or asthma.23 It can rapidly progress to acute respiratory failure without treatment. Subacute processes such as alveolar proteinosis or vaping-related pneumonia also follow this distribution more frequently, both with subpleural involvement.6,11
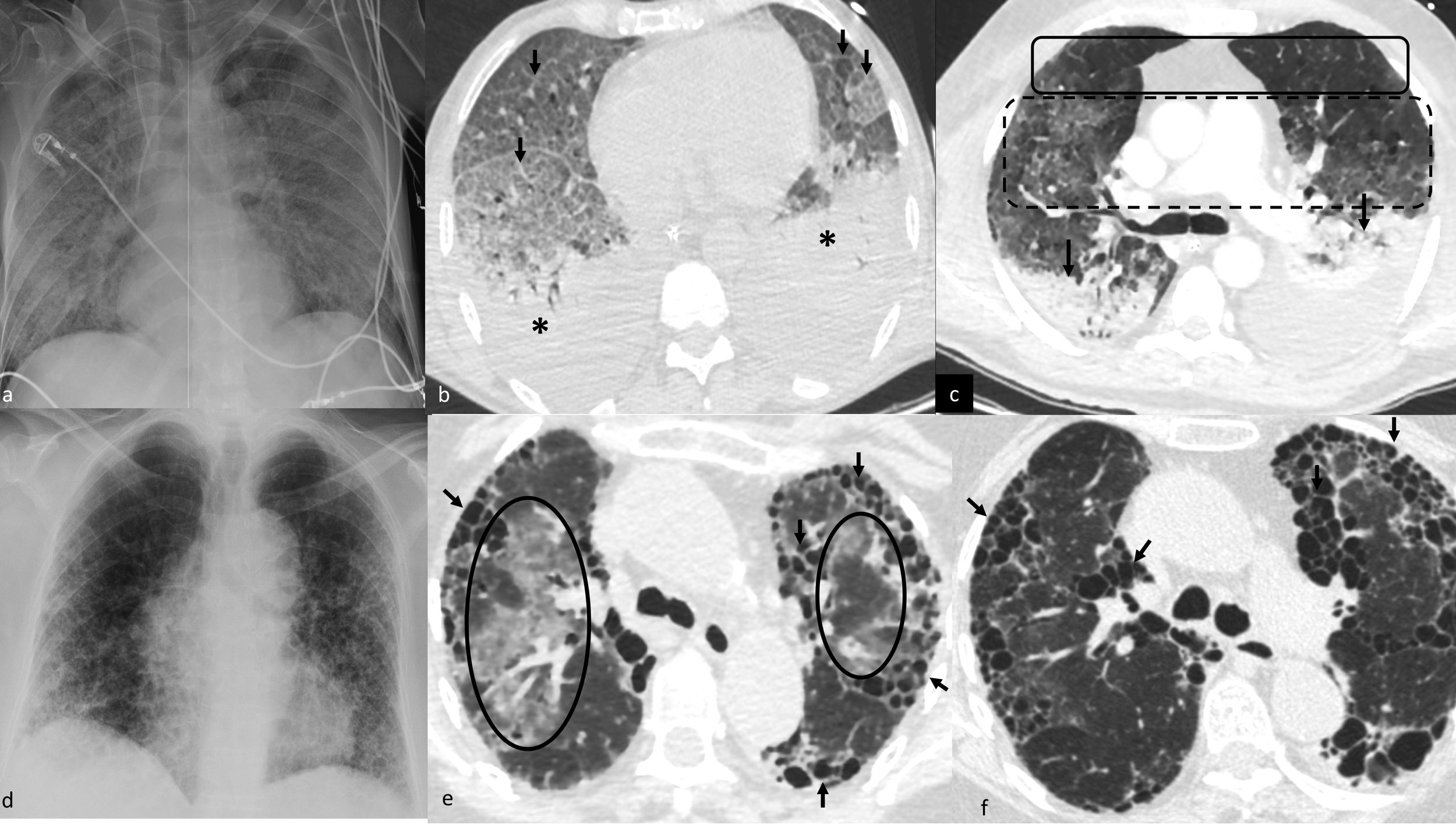
Multifocal pulmonary opacities (MPOs) with other distributions.
Noncardiogenic acute pulmonary edema of secondary origin: ground-glass MPOs of diffuse distribution on X-ray (a) and computed tomography (b), with associated interstitial thickening (cobblestone pattern, arrows in b) and consolidations in sloping regions (asterisks in b). MPOs in another patient (c) with a more evident anteroposterior gradient, observing normal lung tissue (solid-line box), ground-glass opacities (broken-line box), and consolidations/atelectasis with dilated bronchi (arrows) in the most sloping region.
Acute exacerbation of usual interstitial pneumonia. Predominantly peripheral coarse reticular pattern on X-ray (d) corresponding with areas of honeycombing on computed tomography (arrows in e) and faint ground-glass opacities in central lung regions (circles in e). The progressive computed tomography shows the progression of honeycombing (arrows in f).
MPOs in diffuse interstitial lung disease (DILD). It is produced by acute exacerbation of DILD, mainly usual interstitial pneumonia (UIP).37 It is underdiagnosed. Acute exacerbation is defined as “acute respiratory impairment, radiologically characterized by bilateral MPOs with ground-glass density or consolidations, not clearly explained by acute cardiopulmonary decompensation, fluid overload, or infection.”38 They appear in healthy lung tissue, which usually corresponds to the central regions in these patients (Fig. 5d–f). It can be triggered by a direct attack on the lung, such as a biopsy, surgery, radiotherapy, or chemotherapy.39 It leads to a subsequent development of fibrosis (Fig. 5d–f). Recognizing the characteristic chronic peripheral gross reticular pattern of DILDs will be very helpful, although the acute form may be its first manifestation.
Conclusions- 1
Fig. 1 summarizes the characteristics of the diseases that can present in the emergency room with MPOs.
- 2
In a patient with MPOs, radiologists must dwell on the subtleties of the radiological findings, the clinical history and the laboratory data, before talking only about edema and bronchopneumonia.
- 3
In urgent chest radiology:
–Fever and low-grade fever are not synonymous with infection, and can appear in all the aforementioned conditions, except acute pulmonary edema.
–Comparing with previous studies will make it possible to detect the speed of onset of the findings, if they precede the clinical manifestations, underlying disease (cardiopathy, oncological disease, DILD, recurrent process) and subtle findings, such as increased pulmonary volume and interstitial edema.
- 1
Responsible for the integrity of the study: JMPM
- 2
Study conception: JMPM
- 3
Study design: JMPM
- 4
Data collection. Not applicable
- 5
Data analysis and interpretation. Not applicable
- 6
Statistical treatment. Not applicable
- 7
Literature search: JMPM
- 8
Drafting of the manuscript: JMPM
- 9
Critical review of the manuscript with intellectually relevant contributions: JMPM
- 10
Approval of the final version: JMPM
The author declares that she has no conflicts of interest.





![Multifocal pulmonary opacities with a predominantly central distribution. Acute cardiogenic pulmonary edema on radiography. a) Incipient alveolar phase, with signs of increased blood volume (dilation of veins [black arrow] and pulmonary arteries [white arrow], and increased cardiothoracic ratio), interstitial edema (thickening of peribronchovascular cuffs [white arrowhead] and Kerley B lines [black arrowheads]) and alveolar edema, with small central consolidation in the right upper lobe (asterisk). b) More evolved alveolar phase in another patient, with perihilar consolidations with butterfly wings pattern. Diffuse alveolar hemorrhage in a patient with Goodpasture syndrome on X-ray (c) and computed tomography (d), with predominantly mid-to-basal central consolidations, ground-glass opacities, and reticulation, with subpleural respect. Pneumocystis jirovecii pneumonia in an HIV-positive patient diagnosed a posteriori, with central opacities predominantly in upper regions, mainly ground glass, on X-ray (e). In another patient, subjected to high doses of corticosteroids for a brain tumor, a more serious involvement was observed, with greater extension and density of the opacities, predominantly in ground glass and in upper regions, with subpleural sparing, in the computed tomography (f). Multifocal pulmonary opacities with a predominantly central distribution. Acute cardiogenic pulmonary edema on radiography. a) Incipient alveolar phase, with signs of increased blood volume (dilation of veins [black arrow] and pulmonary arteries [white arrow], and increased cardiothoracic ratio), interstitial edema (thickening of peribronchovascular cuffs [white arrowhead] and Kerley B lines [black arrowheads]) and alveolar edema, with small central consolidation in the right upper lobe (asterisk). b) More evolved alveolar phase in another patient, with perihilar consolidations with butterfly wings pattern. Diffuse alveolar hemorrhage in a patient with Goodpasture syndrome on X-ray (c) and computed tomography (d), with predominantly mid-to-basal central consolidations, ground-glass opacities, and reticulation, with subpleural respect. Pneumocystis jirovecii pneumonia in an HIV-positive patient diagnosed a posteriori, with central opacities predominantly in upper regions, mainly ground glass, on X-ray (e). In another patient, subjected to high doses of corticosteroids for a brain tumor, a more serious involvement was observed, with greater extension and density of the opacities, predominantly in ground glass and in upper regions, with subpleural sparing, in the computed tomography (f).](https://static.elsevier.es/multimedia/21735107/00000065000000S1/v2_202304071829/S2173510723000356/v2_202304071829/en/main.assets/thumbnail/gr2.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)




