For women with a high risk of breast cancer, early detection plays an important role. Due to the high incidence of breast cancer, and at a younger age than in the general population, screening begins earlier, and there is considerable evidence that magnetic resonance is the most sensitive diagnostic tool, and the principal American and European guidelines agree on the recommendation to perform annual magnetic resonance (with supplemental annual mammography) as an optimal mode of screening.
In addition to the absence of current consensus on which patients should be included in the recommendation for magnetic resonance screening (widely discussed in the introduction of part 1 of this work), there are other aspects that are different between guidelines, that are not specified, or that are susceptible to change based on the evidence of several years of experience, that we have called «controversies», such as the age to begin screening, the possible advisability of using a different strategy in different subgroups, performing alternate versus synchronous magnetic resonance and mammography, the age at which to terminate the two techniques, or how to follow up after risk reduction surgery. The aim of the second part of the paper is, by reviewing the literature, to provide an update in relation to some of the main «controversies» in high risk screening with magnetic resonance. And finally, based on all this, to propose a possible model of optimal and updated screening protocol.
En mujeres con alto riesgo de padecer cáncer de mama, la detección precoz tiene un importante papel. Debido a la alta incidencia de cáncer de mama en ellas, y a edades más tempranas que en la población general, el cribado comienza antes, y existe amplia evidencia de que la resonancia magnética es la herramienta diagnóstica más sensible. Las principales guías americanas y europeas coinciden en la recomendación de realizar resonancia magnética anual (con suplemento mamográfico anual) como modalidad óptima de cribado.
Además de la ausencia de consenso actual sobre qué pacientes deben incluirse en la recomendación de cribado con resonancia magnética (ampliamente tratada en la introducción de la parte 1 de esta actualización (Radiología. DOI: 10.1016/j.rx.2020.01.007)), existen otros aspectos no coincidentes en diferentes guías, o no concretados, o susceptibles de modificación según la evidencia de tantos años de experiencia, que hemos denominado «controversias», como son: la edad de comienzo del cribado, la posible conveniencia de emplear diferente estrategia en distintos subgrupos, la realización alterna vs. síncrona de la resonancia y la mamografía, la edad de finalización de las dos técnicas o la forma de realizar el seguimiento tras cirugía de reducción de riesgo. El objetivo de la segunda parte de nuestro trabajo es realizar, revisando la literatura científica, una actualización sobre esas principales «controversias» en el cribado con resonancia magnética en alto riesgo. Y finalmente, basándose en ello, proponer un posible modelo de protocolo de cribado óptimo y actualizado.
As set out in part 1 of this update, “Screening in patients with increased risk of breast cancer (part 1): pros and cons of MRI screening” (Radiología, DOI: 10.1016/j.rx.2020.01.007), and as seen since the first prospective magnetic resonance imaging (MRI) screening studies in high-risk (HR) cases,1–9 reaffirmed by subsequent studies,10–16 MRI sensitivity (90%)9,16 is double that of mammography (MG) (25%–59%17), and MRI specificity is also high (78%–97%,14 with a mean around 85%–90%, which increases following the first round and subsequently reaches 90%–95%13,15,16).
The use of MRI enables a higher rate of detection of breast cancer (BC) compared to screening with MG alone (16‰–30‰ with MRI18 versus 7.6‰–7.9‰ with MG19), and at a lower stage: cancers of a smaller size (mean size: 7–18mm; 50% ductal carcinomas in situ (DCIS) or T<1cm9) and cancers with less associated axillary lymph node involvement (11%–29%,9,11,12 a mean of 15%–16%, versus 30%–45% for screening with MG alone). In addition, there is a lower rate of interval cancers (with a mean around 5.4%, under 10%,9 versus a mean of practically 50% for screening with MG alone). Therefore, MRI has provided more effective screening in these patients which has justified its generalised use. The addition of ultrasound screening which coincides with annual MRI and MG screening does not significantly increase the rate of detection,5,6,12,13, although ultrasound plays a crucial role in investigating lesions detected on MRI.
Our experience has yielded some results corresponding closely to those reported in the scientific literature. At present, our BC detection rate is 19.7‰ with MRI. We have detected 13 cancers: 11 invasive cancers (ICs) and 2 DCISs (84.62% ICs and 15.38% DCISs). A total of 84.6% of BCs were “minimal BCs” (PT1a/b or DCIS); there was one PT1c IC and one T2 IC (the T2 was an interval cancer in a BRCA1 patient with a complete radiological and pathological response to neoadjuvance). We had just 1 out of 13 cases (7.69%) with lymph node macrometastases and 1 out of 13 cases (7.69%) of interval cancers.
In patients with a high risk of BC, an absolute lifetime risk greater than or equal to 20%–25%, there has been widespread agreement in recent years among the main European and American radiology associations (the European Society of Breast Cancer Specialists (EUSOMA), European Society of Breast Imaging (EUSOBI), Society of Breast Imaging (SBI), American College of Radiology (ACR) and American Cancer Society (ACS)) on recommending annual screening with MRI added to MG.20 These associations’ guidelines are consistent with regard to patients to be included21–28: patients carrying or with first-degree relatives carrying a genetic mutation associated with a high risk of developing BC, patients with a risk due to family history greater than or equal to 20%–25% and patients having experienced exposure to (mantle-field) chest radiation at an age under 30 years.
In 2018, the ACR included in its update17 a recommendation for screening with MRI for some subgroups of women who until then had belonged to the “intermediate risk” group – specifically, “women with a personal history of BC and dense tissue” and “women with a personal history of BC, if the BC was diagnosed before the age of 50”. They also indicated that the additional MRI should be considered in all other women with a personal history of BC or a personal history of biopsy yielding a result of atypical ductal hyperplasia (ADH) or lobular neoplasia (lobular carcinoma in situ (LCIS) or atypical lobular hyperplasia (ALH)), especially if accompanied by other risk factors.
Another American guideline (from the National Comprehensive Cancer Network (NCCN))29 recommends considering screening with MRI when a patient has been diagnosed with LCIS, AHL or ADH, based on emerging evidence if the risk is 20% or higher.
In its latest update in 2019,30 the ACS reported that it is reviewing recommendations in other subgroups of patients so far not included in the recommendation for screening with MRI due to a lack of evidence since what was established in 200721: patients with a personal history of BC, patients with a prior diagnosis of a high-risk histological lesion, patients with significantly dense breasts, patients using hormone replacement therapy and black patients.
Table 1 shows the genes with mutations considered to be associated with a high risk of BC. We have added a new one to those originally included.17,29,31
Genes associated with a high risk of breast cancer (carriers of mutations in those genes, included in the high-risk screening protocol).36
| • BRCA1 |
| • BRCA2 |
| • TP53 (Li-Fraumeni) |
| • PTEN (Cowden, Bannayan-Riley-Ruvalcaba) |
| • PALB2a |
Carriers of a PALB2 mutation are considered to have the same probability of breast cancer as BRCA2 carriers (hence, they are also included in the recommendation for screening with MRI at present). (Other genes associated with a lower (though also high) risk than those cited, mentioned in some guidelines, and included in the recommendation for screening with MRI are: SKT11, CDH1, CHEK 2, ATM, NBN, NF1, etc.)17,29,31.
In addition to the absence of a current consensus among the main associations as to which patients should be included in the recommendation for MRI screening, especially since the 2018 ACR update17 (a topic extensively addressed in the introduction to part 1 of this update), certain other aspects of different guidelines are inconsistent, not always specified or subject to amendment based on evidence derived from so many years of experience. These we have designated as “controversies”, and they include: the age at which to begin screening, the possible advisability of using a different strategy in different subgroups, performing alternate versus synchronous MRI and MG, the age at which to stop using the two techniques, and how to follow up after risk-reduction surgery. The objective of the second part of our study is to review the specialised literature and prepare an update on those “controversial” principles in screening with MRI in HR cases. Finally, we aim to propose, based on that review, a possible optimised and updated screening protocol model.
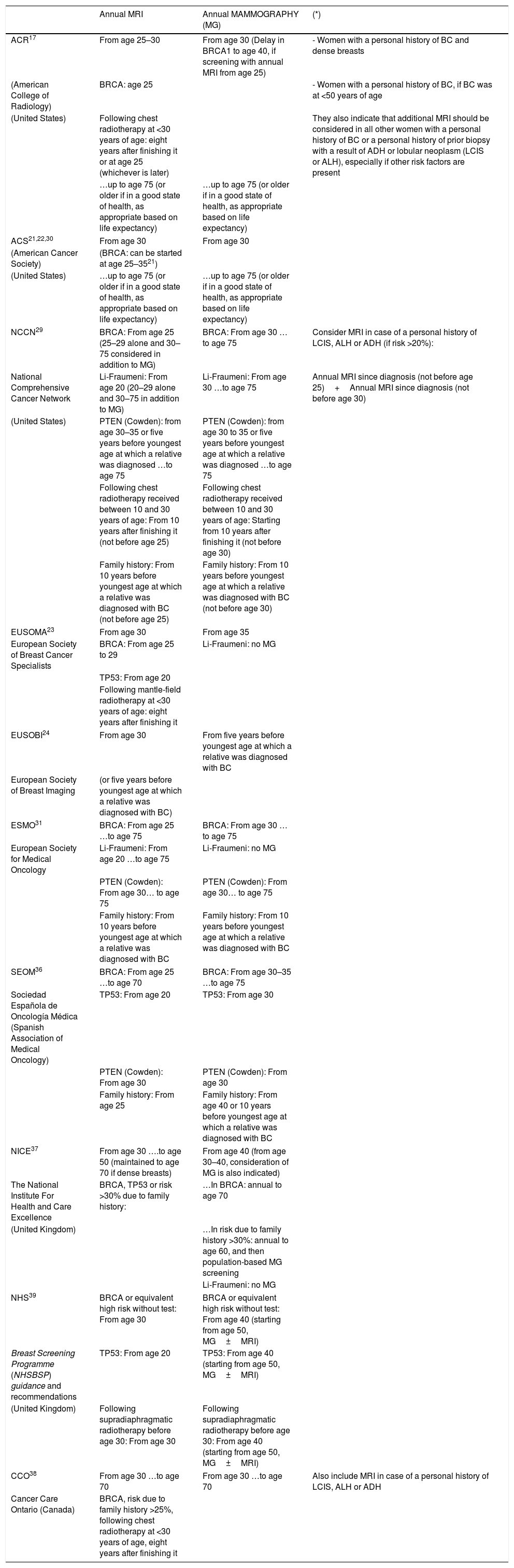
ControversiesAge to begin screening (age 25 or 30?)Guidelines are inconsistent as to the age to begin screening (age 25–30), and also vary depending on whether or not genetic mutations are present (Table 2).
Summary table with recommendations for screening in high-risk cases from the major American and European associations (we have added other specific representative recommendations from countries such as Spain, the United Kingdom and Canada).
| Annual MRI | Annual MAMMOGRAPHY (MG) | (*) | |
|---|---|---|---|
| ACR17 | From age 25–30 | From age 30 (Delay in BRCA1 to age 40, if screening with annual MRI from age 25) | - Women with a personal history of BC and dense breasts |
| (American College of Radiology) | BRCA: age 25 | - Women with a personal history of BC, if BC was at <50 years of age | |
| (United States) | Following chest radiotherapy at <30 years of age: eight years after finishing it or at age 25 (whichever is later) | They also indicate that additional MRI should be considered in all other women with a personal history of BC or a personal history of prior biopsy with a result of ADH or lobular neoplasm (LCIS or ALH), especially if other risk factors are present | |
| …up to age 75 (or older if in a good state of health, as appropriate based on life expectancy) | …up to age 75 (or older if in a good state of health, as appropriate based on life expectancy) | ||
| ACS21,22,30 | From age 30 | From age 30 | |
| (American Cancer Society) | (BRCA: can be started at age 25–3521) | ||
| (United States) | …up to age 75 (or older if in a good state of health, as appropriate based on life expectancy) | …up to age 75 (or older if in a good state of health, as appropriate based on life expectancy) | |
| NCCN29 | BRCA: From age 25 (25–29 alone and 30–75 considered in addition to MG) | BRCA: From age 30 …to age 75 | Consider MRI in case of a personal history of LCIS, ALH or ADH (if risk >20%): |
| National Comprehensive Cancer Network | Li-Fraumeni: From age 20 (20–29 alone and 30–75 in addition to MG) | Li-Fraumeni: From age 30 …to age 75 | Annual MRI since diagnosis (not before age 25)+Annual MRI since diagnosis (not before age 30) |
| (United States) | PTEN (Cowden): from age 30–35 or five years before youngest age at which a relative was diagnosed …to age 75 | PTEN (Cowden): from age 30 to 35 or five years before youngest age at which a relative was diagnosed …to age 75 | |
| Following chest radiotherapy received between 10 and 30 years of age: From 10 years after finishing it (not before age 25) | Following chest radiotherapy received between 10 and 30 years of age: Starting from 10 years after finishing it (not before age 30) | ||
| Family history: From 10 years before youngest age at which a relative was diagnosed with BC (not before age 25) | Family history: From 10 years before youngest age at which a relative was diagnosed with BC (not before age 30) | ||
| EUSOMA23 | From age 30 | From age 35 | |
| European Society of Breast Cancer Specialists | BRCA: From age 25 to 29 | Li-Fraumeni: no MG | |
| TP53: From age 20 | |||
| Following mantle-field radiotherapy at <30 years of age: eight years after finishing it | |||
| EUSOBI24 | From age 30 | From five years before youngest age at which a relative was diagnosed with BC | |
| European Society of Breast Imaging | (or five years before youngest age at which a relative was diagnosed with BC) | ||
| ESMO31 | BRCA: From age 25 …to age 75 | BRCA: From age 30 …to age 75 | |
| European Society for Medical Oncology | Li-Fraumeni: From age 20 …to age 75 | Li-Fraumeni: no MG | |
| PTEN (Cowden): From age 30… to age 75 | PTEN (Cowden): From age 30… to age 75 | ||
| Family history: From 10 years before youngest age at which a relative was diagnosed with BC | Family history: From 10 years before youngest age at which a relative was diagnosed with BC | ||
| SEOM36 | BRCA: From age 25 …to age 70 | BRCA: From age 30–35 …to age 75 | |
| Sociedad Española de Oncología Médica (Spanish Association of Medical Oncology) | TP53: From age 20 | TP53: From age 30 | |
| PTEN (Cowden): From age 30 | PTEN (Cowden): From age 30 | ||
| Family history: From age 25 | Family history: From age 40 or 10 years before youngest age at which a relative was diagnosed with BC | ||
| NICE37 | From age 30 ….to age 50 (maintained to age 70 if dense breasts) | From age 40 (from age 30–40, consideration of MG is also indicated) | |
| The National Institute For Health and Care Excellence | BRCA, TP53 or risk >30% due to family history: | …In BRCA: annual to age 70 | |
| (United Kingdom) | …In risk due to family history >30%: annual to age 60, and then population-based MG screening | ||
| Li-Fraumeni: no MG | |||
| NHS39 | BRCA or equivalent high risk without test: From age 30 | BRCA or equivalent high risk without test: From age 40 (starting from age 50, MG±MRI) | |
| Breast Screening Programme (NHSBSP) guidance and recommendations | TP53: From age 20 | TP53: From age 40 (starting from age 50, MG±MRI) | |
| (United Kingdom) | Following supradiaphragmatic radiotherapy before age 30: From age 30 | Following supradiaphragmatic radiotherapy before age 30: From age 40 (starting from age 50, MG±MRI) | |
| CCO38 | From age 30 …to age 70 | From age 30 …to age 70 | Also include MRI in case of a personal history of LCIS, ALH or ADH |
| Cancer Care Ontario (Canada) | BRCA, risk due to family history >25%, following chest radiotherapy at <30 years of age, eight years after finishing it | ||
We give the ages to start screening with MRI and introduce mammography (and ages to stop screening where specified), which show the variability among the recommendations in the different guidelines.
All the major American and European associations include patients who are carriers or first-degree relatives of carriers of a genetic mutation associated with a high risk of suffering from BC, women with a risk ≥20%–25% due to family history and patients who have experienced exposure to (mantle-field) chest radiotherapy at <30 years of age. However, some guidelines currently also include other patient subgroups in the recommendation (*).
The National Institute For Health and Care Excellence (NICE) guidelines specifies the subgroup of women with a risk due family history with a risk ≥30%.
(Abbreviations used: BC=breast cancer; ADH=atypical ductal hyperplasia; LCIS=lobular carcinoma in situ; ALH=atypical lobular hyperplasia).
It is estimated that there will be little increase in life expectancy when screening is started at age 25 instead of at age 30.32
A study by Tilanus-Linthorst et al.33 shows that the age at which BC is diagnosed partly depends on family history, and recommends taking into account the youngest age at which a family member was diagnosed with cancer to determine the age of onset.
Two studies that compare cost-effectiveness among different screening strategies in BRCA1/2 patients32,34 and that, curiously, recommend different ages to begin screening (age 2532 and age 3034) also indicated that the age to begin screening can be modified (age 25 or 30) depending on the youngest age at which a family member was diagnosed with cancer.
Based on this literature32–34 and an assessment of a compendium of the main guidelines, an optimal framework for action could be:
- •
In the BRCA subgroup (where a small number of patients have BC before age 30): start at age 25.17,23,31,35,36 Starting at age 30 could be considered if the youngest relative with BC was 40 or older when diagnosed.31
- •
In high familial risk cases: start at age 30.17,21–23,30,35,37,38 Starting at age 25 could be considered if the youngest relative with BC was 35 or younger when diagnosed.34
Exceptions:
- •
Patients who are carriers of the TP53 mutation, associated with Li-Fraumeni syndrome: start at age 20.23,29,31,37,39 At least one set of guidelines recommends not including MG, due to these patients’ high susceptibility to radiation.23,37
- •
A history of prior chest radiotherapy at an age under 30 years: start at age 25, or 8 years after finishing radiotherapy, whichever is the later of the two.17,23,27
The cohort of patients who undergo screening due to HR (BRCA1/2, rare syndromes, history of chest radiotherapy, high familial risk, etc.) is very mixed.
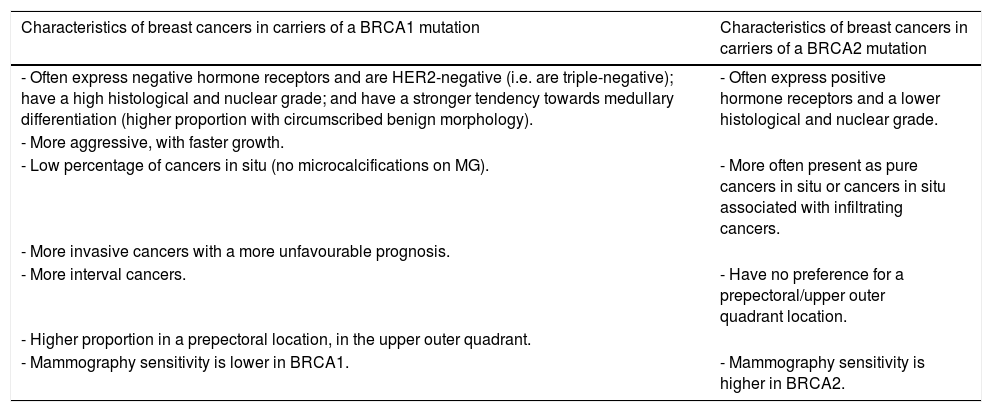
As early as 2010, Rijnsburger et al.40 indicated that it might be necessary to establish different strategies in different HR subgroups for different cancers and case histories. BCs in BRCA1 mutation carriers are different from those associated with BRCA2 mutations and sporadic mutations (Table 3).
Characteristics of breast cancers in subgroups of carriers of a BRCA1 or BRCA2 gene mutation.
| Characteristics of breast cancers in carriers of a BRCA1 mutation | Characteristics of breast cancers in carriers of a BRCA2 mutation |
|---|---|
| - Often express negative hormone receptors and are HER2-negative (i.e. are triple-negative); have a high histological and nuclear grade; and have a stronger tendency towards medullary differentiation (higher proportion with circumscribed benign morphology). | - Often express positive hormone receptors and a lower histological and nuclear grade. |
| - More aggressive, with faster growth. | |
| - Low percentage of cancers in situ (no microcalcifications on MG). | - More often present as pure cancers in situ or cancers in situ associated with infiltrating cancers. |
| - More invasive cancers with a more unfavourable prognosis. | |
| - More interval cancers. | - Have no preference for a prepectoral/upper outer quadrant location. |
| - Higher proportion in a prepectoral location, in the upper outer quadrant. | |
| - Mammography sensitivity is lower in BRCA1. | - Mammography sensitivity is higher in BRCA2. |
In BRCA1:
- •
They often express negative hormone receptors and are HER2-negative (triple negative), have a high histological and nuclear grade, and have a greater tendency towards medullary differentiation (which is why there is a higher proportion of BCs with benign morphology).
- •
They are more aggressive, with faster growth.
- •
There is a low percentage of DCIS (no microcalcifications on MG).
- •
There are more ICs with more unfavourable prognoses.
- •
There are more interval cancers.
- •
There is a higher proportion of cancers in a prepectoral location, in the upper outer quadrant.
- •
MG sensitivity is lower in BRCA1.
In BRCA2:
- •
They often express positive hormone receptors and have a lower histological and nuclear grade.
- •
They most frequently appear as pure DCIS or DCIS associated with IC.
- •
They lack a predilection for a prepectoral location/the upper outer quadrant.
- •
MG sensitivity is higher in BRCA2.
In patients with a history of lymphoma and chest radiotherapy:
- •
BCs in patients with a history of Hodgkin lymphoma treated with mantle-field radiotherapy have similar characteristics to those associated with BRCA2 mutations41 (smaller size and histological grade, higher proportion of positive hormone receptors and higher proportion of DCIS). Some authors (Ng et al.,41 Mariscotti et al.42 and Sung et al.43) have agreed that MRI sensitivity is lower than in other subgroups of HR patients, although this has not been corroborated by other studies (Tieu et al.44 and Freitas et al.45). Early BC detection in this subgroup may spare these patients post-mastectomy chest wall radiotherapy and cardiotoxic chemotherapy.
In the BRCA1 subgroup, annual screening with MRI and MG is less effective: larger BCs with more affected positive axillary lymph nodes and a higher rate of interval cancers are found.15,46–48 Also, the overall sensitivity of screening with MRI and MG is lower (some studies have cited figures of 81.3%15 or even 60%48) (Fig. 1). It seems clear that the interval must be shortened in this subgroup. In all other subgroups (BRCA2, history of chest radiotherapy and high familial risk), the screening strategy with annual MRI and MG is beneficial, with most tumours detected being T1a/b or DCIS and with a sensitivity of screening with MRI and MG ≥90%.15,47,49,50
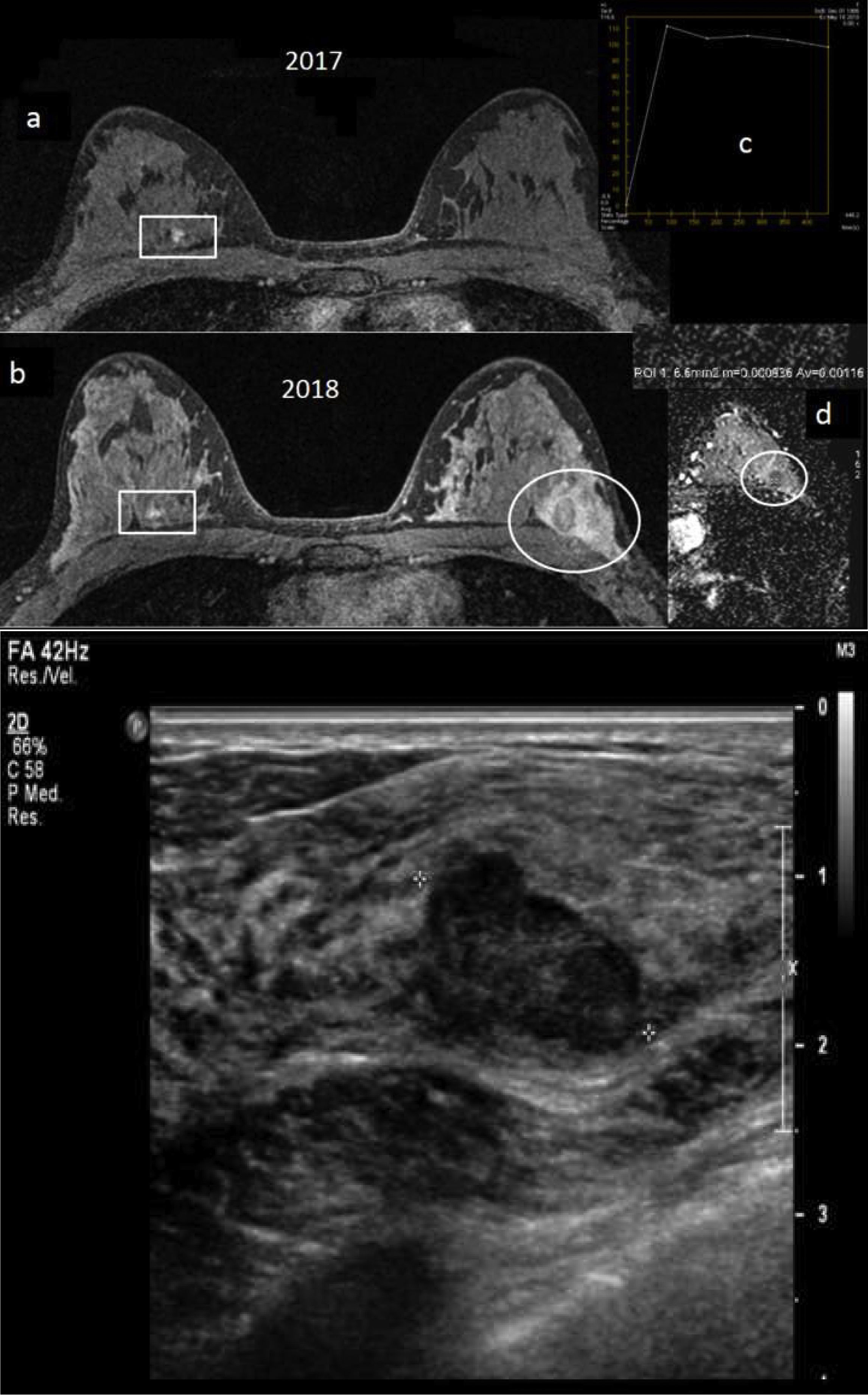
A 32-year-old BRCA1 patient, in whom a new nodule was palpated in her left breast a year after her most recent magnetic resonance imaging (MRI) screening, before her next annual follow-up MRI. (A) Breast MRI with intravenous contrast: delayed 3D axial sequence with fat saturation from 2017 (a) and from 2018/time of diagnosis (b), where the 2018 MRI shows a new, oval-shaped, lobulated nodule in the upper outer quadrant of the left breast which washed out the contrast relative to the surrounding tissue in that delayed 3D axial sequence (circle). The contralateral breast (rectangle) shows a circumscribed prepectoral nodule with uniform internal enhancement, already known, with prior biopsy with a result of fibroadenoma, stable. Uptake curve for the nodule in the left breast, type 3, (c) and map of axial diffusion with apparent diffusion coefficient (ADC)=1.1×10−3mm2/s for the nodule (d). The nodule in the left breast was not visible on the 2017 MRI. (B) Breast ultrasound: lobulated, oval-shaped, hypoechoic solid nodule, with not completely circumscribed margins measuring nearly 3cm, underlying a palpable nodule in the left breast. An ultrasound-guided core-needle biopsy was performed with a result of “grade 3 infiltrating ductal carcinoma”. Molecular subtype: triple-negative. This was the only case of interval cancer at our centre to date in a BRCA1 patient.
In the MRI and MG binomial, the most sensitive technique is MRI,9,12,13,15,16 regardless of age, risk group or breast density13 (Fig. 2). MG adds little to MRI and does not increase sensitivity much.12,13,16 Cancers detected by MG alone represent a very low percentage and are largely DCISs (and low-grade cancers).11,12 Several studies have reported that around 30% of cancers12,16,49 are detected by MRI alone (some studies have reported figures as high as 45%50 and 50%11). MG uses ionising radiation and has lower sensitivity. Furthermore, in young patients, calcifying DCISs are rare. Therefore, EUSOMA23 has maintained its recommendation not to introduce MG until age 35 and never before age 30, whereas the main American guidelines continue to recommend introducing MG at age 30.17,21,30
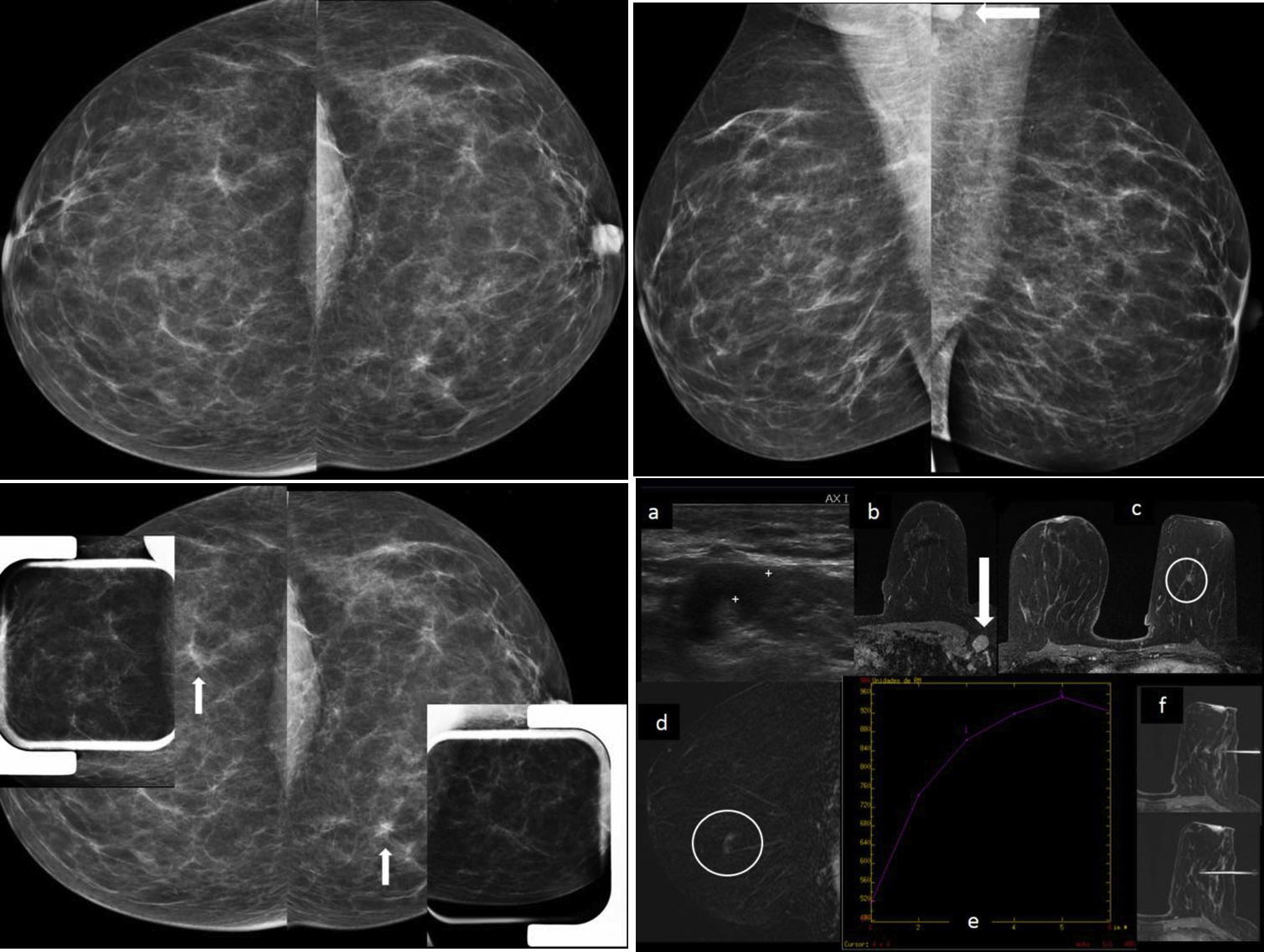
Case of cancer detected in a screening study in a 47-year-old BRCA2 patient. Pathological axillary lymph node detected on magnetic resonance imaging (MRI) and on post-MRI ultrasound with a fine-needle aspiration biopsy (FNAB) positive for malignant cells with findings of metastatic carcinoma and millimetric cancer (8mm) in the ipsilateral breast, only visible on MRI as a millimetric non-circumscribed pseudonodular focal enhancement between the lower quadrants. An MRI-guided biopsy yielded a result of “grade 2 infiltrating ductal cancer+moderate-grade ductal carcinoma in situ”. There was no mammographic translation of the cancer between the lower quadrants of the left breast (measuring 8mm) detected on MRI, despite the predominantly fatty density. This patient's previous breast MRIs, three and two years earlier, had been difficult to assess as a result of multiple enhancements due to breast-feeding, and there was no MRI study in the year before the diagnosis. (The patient was the only case at our centre with axillary macrometastases.) (A) Bilateral mammography, craniocaudal projections: “ACR A” breasts, with no obvious lesions (normal). (B) Bilateral mammography, mediolateral oblique projections. “ACR A” breasts, with no obvious lesions (normal). Axillary lymphadenopathy is partially seen on the left mediolateral oblique projection (arrow). (C) Mammographic focal compressions over asymmetries of outer quadrants of right breast and inner quadrants of left breast of baseline craniocaudal projections (arrows), which did not persist, leading to the deduction that these were areas of overlapping tissue. (D) Left axillary ultrasound (a): pathological left axillary lymphadenopathy, with widespread cortical thickening with FNAB: “positive for malignant cells with findings of metastatic carcinoma”. Breast ultrasound showed no obvious findings. Breast MRI with intravenous contrast: on delayed axial 3D with fat saturation (b) pathological axillary lymphadenopathy is seen (arrow), and on delayed 3D axial with fat saturation (c) non-circumscribed pseudonodular focal enhancement measuring 8mm in the lower quadrants of the left breast is seen (circle); early digital subtraction (d): non-circumscribed pseudonodular focal enhancement measuring 8mm in the lower quadrants of the left breast (circle); uptake curve (e): type 1 (this was the only obvious intramammary enhancement, arousing suspicion of malignancy); and MRI-guided vacuum-assisted biopsy, 3D axial with fat saturation (e): non-circumscribed pseudonodular focal enhancement measuring 8mm between the lower quadrants of the left breast with no ultrasound translation subjected to an MRI-guided biopsy, with a result of “grade 2 infiltrating ductal carcinoma+moderate-grade ductal carcinoma in situ”. Molecular subtype: luminal B.
Given the low sensitivity of MG for detecting cancers in patients with a BRCA mutation (especially BRCA1) and the potential risk in them of carcinogenesis due to radiation, the possibility of eliminating MG has been raised, especially in screening women under 40. It has been proposed that MG be delayed to age 4051 or even omitted in BRCA1 mutation carriers.52 A study by Objeijn et al.53 even concluded that omitting it under this age was cost-effective.
Some studies have recommended refraining from MG in young women (<40 years of age), even in all subgroups,48,54 and many studies have called into question the role of MG in screening in HR cases in general,15,16,49,50,55,56 based on the limited additional value of MG in diagnosis, consistent with other prior studies.11–13 The fact that the recommendation to add MG to screening with MRI in HR cases remains in the guidelines is essentially due to the data contributed by two significant studies: a meta-analysis by Phi et al.,57 based on results from six multi-centre studies, and a study by Heijnsdijk et al.,47 which also combined results from three studies. Both suggested that MG may be of value, especially in BRCA2.
At present, some guidelines recommend delaying until age 40 the introduction of MG in HR BRCA1 patients. The 2018 ACR update17 already accepted that this “can be considered”, but stated that more evidence is needed to determine the role of MG in BRCA1, and that it is more beneficial in BRCA2. Like the rest of the American guidelines, it continues to recommend introducing MG at age 30 in all other subgroups. In reality, virtually all the literature mentioned supports the limited value of MG in BRCA1 patients under 40. This may be considered to be clear evidence in itself. Some guidelines in European countries (such as the United Kingdom39 and Germany; Table 435) already consider delaying the introduction of MG to age 40 in HR cases. Therefore, within the existing variability of guidelines and practice at different centres with respect to the time to add MG to screening in HR cases, a gradual trend towards delaying it may be discerned. The future role of MG in the screening of HR patients has yet to be seen. Given the current trend, MG might even end up being omitted except in patients in whom MRI cannot be performed due to some contraindication.
High-risk follow-up programme with magnetic resonance imaging. German Consortium of Hereditary Breast and Ovarian Cancer (GC-HBOC), Germany.35
| Established | In 2005, recommendations updated in 2013 |
| Screening centres | At present 15 specialised university centres in Germany, which also provide genetic counselling and testing for high-risk women |
| Funds | Funded through special comprehensive healthcare agreements between payers and participating centres |
| Admission criteria | Carriers of a known mutation of a breast cancer-susceptible gene or at high risk based on family history |
| Age range covered (years) | |
| • BRCA1/2 mutation carriers | Age 25–70 |
| • Carriers of other moderate-risk genes | Age 30–70 |
| • Women at high risk with no known mutation | Age 30–50 |
| MRI | Every year |
| Ultrasound | |
| -> BRCA1/2 mutation carriers | Every six months |
| -> All others | Every year |
| Mammography | Personalised decision, for example, every 1–2 years from age 40 |
Adaptation to Spanish based on the high-risk follow-up programme with MRI from the German Consortium of Hereditary Breast and Ovarian Cancer (GC-HBOC), Germany.35 These guidelines add ultrasound to the protocol with the argument that an ultrasound performed after the MRI and clinical examination may increase the MRI's specificity, may reduce callbacks and patient anxiety, and is generally well accepted by patients as part of screening programmes. Ultrasound is offered to patients with a genetic mutation at six-month intervals and in those cases the ultrasound coinciding with the annual examination serves as an important comparison for the ultrasound examination done every six months on its own.
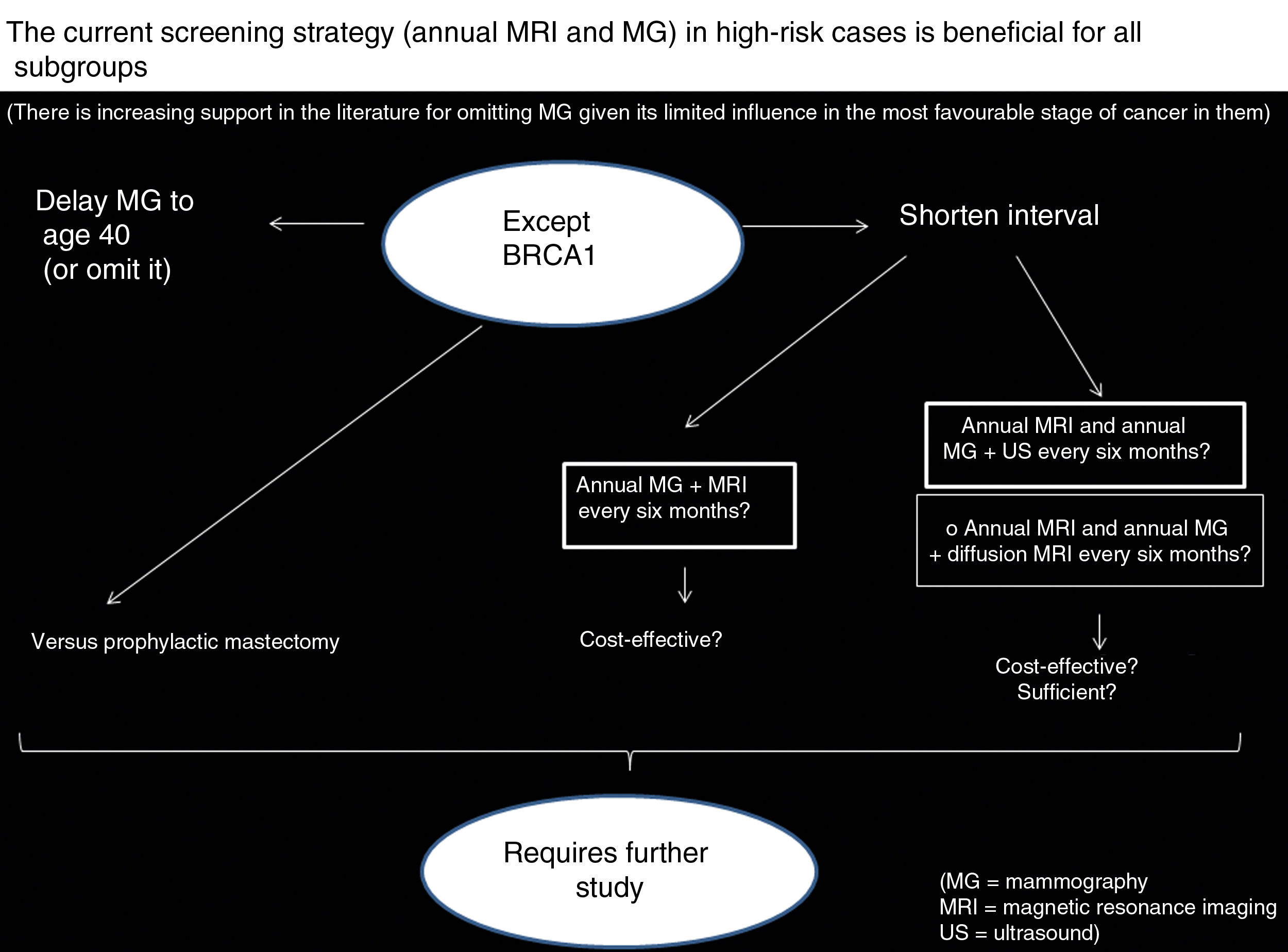
In summary, in all HR subgroups the current screening strategy is beneficial, and the literature increasingly supports omitting MG given its limited influence on the most favourable stage of cancer in them, but it is not so beneficial in the BRCA1 subgroup, in which it seems clear that the protective effect of screening with MRI is less. Therefore, it has been suggested that it is necessary to shorten the interval in BRCA1. How can it be done? (Fig. 3):
By doing an MRI every six months?A very recent study by Guindalini et al.58 assessed intensive screening using MRI with intravenous contrast every six months and MG annually. Its authors concluded that MRI every six months may lower the stage of BCs in BRCA1 without an excessive impact on callbacks and biopsies. They obtained a higher positive predictive value and fewer false positives in BRCA1 patients than in other subgroups; all BCs detected measured less than 1cm and were without positive axillary lymph nodes, and there was no interval BC. Their outcomes were achieved despite having diagnosed aggressive, fast-growing tumours and dedifferentiated grade 3 tumours in three-quarters of cases. They deduced that this strategy is more beneficial for BRCA1 and also concluded that MG can be omitted because it does not increase detection of ICs, consistent with the abundant literature in this respect described above. Whether it is cost-effective must be evaluated in future research.
Kuhl et al.59 suggest a strategy for improving access to MRI every six months in BRCA1 mutation carriers, specifically abbreviated MRI (with comparable detection, but a lower cost given its shorter machine time and reading time, as explained in part 1 of this update), since it would decrease the financial burden. These authors argue that it could be prudent to perform an initial MRI with a full protocol and then perform follow-up studies, or at least mid-year studies, with an abbreviated protocol. They point out that studies investigating the cost-efficacy relationship of this MRI every six months must assess not only direct and indirect costs of MRI and biopsies generated, but also opportunities to decrease costs in terms of MGs saved and the impact of decreased systemic treatments and years of life lost.
By adding ultrasound every six months to annual MRI and MG?Two studies (Bosse et al.60 and Cortesi et al.61) haveindicated that ultrasound (twice yearly, every six months) does indeed give added value to screening with annual MRI and MG, with a detection rate of 11.1% and 18.8% of additional BCs, although ultrasound somewhat increases numbers of biopsies and false positives.
Bick et al.’s HR follow-up programme (German Consortium of Hereditary Breast and Ovarian Cancer (GC-HBOC); see Table 4) offered “patients with a genetic mutation” ultrasound every six months in addition to annual MRI and MG (with MG starting at age 40)35, with one ultrasound at the same time as the annual MRI and MG and the other ultrasound six months later. They found ultrasound's contribution to BC detection rate to be low, with very few cancers detected by ultrasound alone in their 10 years of experience16 (2 out of 221 BCs, or 0.9%, were detected by ultrasound alone).
We also found a study by van Zelst et al.48 that found that “automated breast ultrasound” (ABUS) every six months did not offer any added value to annual MRI and MG screening. However, a close reading of this study revealed that two of its 16 BCs appeared on a prior ABUS, and were false negatives as they were two circumscribed, hypoechoic nodules that were mistaken for cysts. This represents a rate of additional BCs of 12%, consistent with the figures from the above-mentioned authors who did find ultrasound to be of added value.
In a small subgroup of patients from the German “EVA-trial” prospective study, no additional value in BC detection was found in those who underwent ultrasound every six months.11
It would also be necessary to assess whether an additional ultrasound every six months is cost-effective and whether it is still insufficient “because it is clear that its detection rate would be lower than that of MRI every six months”.
By adding diffusion MRI to annual MRI and MG?Although we have found no studies examining the possible benefits of adding diffusion MRI every six months to annual MRI and MG, we base our proposal for this on the value of diffusion MRI in tumour detection. The regimen could be, if an “alternate” protocol is used, adding diffusion MRI at the same time as the MG six months after the annual MRI with contrast (MRI with contrast which may entail an additional diffusion sequence). Different studies have reported diffusion MRI sensitivity to be 45%–94%.62–67 Diffusion MRI has a higher rate of detection of additional BCs than ultrasound (8‰ versus 4‰). In addition, ultrasound takes a great deal of time and has very low specificity when used for screening.65 Implementing diffusion MRI in clinical practice still has many limitations that must be overcome. To achieve this, it is necessary to:
- •
Adjust/standardise suitable sequences, to increase diagnostic acuity.
- •
Improve spatial resolution, since it may interfere with the detection of small BCs.
- •
Resolve technical artefact problems.
- •
Train readers.
All this means that diffusion MRI is not a viable alternative in practice.
In summary, a larger-scale study is required to determine the best way to shorten the follow-up interval in patients with a BRCA1 mutation (more intensive screening), although it seems that MRI every six months could be the most suitable and effective option. It would even be necessary to compare this option to prophylactic mastectomy and analyse the long-term outcomes of each.
Alternate versus synchronous MRIAnother matter of debate is whether annual MRI and MG should be synchronous or alternate. It seems that alternating them may be beneficial, given that:
- •
Some studies featuring analysis with mathematical models comparing alternatives have indicated that it is more effective.32,34
- •
Some studies have reported cancers detected on an intermediate MG with a normal prior MRI six months earlier68–70 or on an MRI with a normal prior MG six months earlier.71 They have deduced, therefore, that it must entail some benefit. What is not known is whether that mammographic abnormality detected on an intermediate MG would have been seen six months earlier if the MG had been done with the MRI or whether an MRI at the time of that MG would have also detected that BC, or vice versa if the abnormality had been detected on an intermediate MRI.
Moreover, a study by Othman et al.72, which compared the two modes (synchronous and alternating), found the rate of false positives to be similar in both strategies and a sensitivity of 92% when they were employed in an alternating mode71 (this range was similar to that in series using a synchronous mode). The authors of this study concluded that both alternatives are valid, and alluded to the need to conduct multi-institutional studies to compare them and determine the optimal protocol — although they admitted that these studies would need to recruit many patients and would have a high cost.
Our centre made the switch to an alternating mode a little over a year ago, because we believe that it offers a slight possible benefit compared to a synchronous mode. We added an ultrasound at the same time as an MG performed six months after a normal MRI in an attempt to increase sensitivity compared to MG alone, with a particular view to the BRCA1 subgroup, which suffers from more interval cancers. We thought that we obtained a potential benefit compared to a synchronous mode, backed by the literature finding that ultrasound every six months contributes value when added to annual screening with MRI and MG, such as the studies by Bosse et al.60 and Cortesi et al.61 These studies emphasised the characteristics of the tumours detected in patients with a BRCA mutation on ultrasounds performed six months after an MRI. Most were infiltrating, T1 and asymptomatic cancers.
With this alternating mode, we added a screening ultrasound to the MRI in the first round of screening only (as it increases the MRI's specificity, which is lower in the first round due to pre-existing benign lesions).
Ceiling (upper age limit) for MRI and MG (until when?)Whether MRI should continue to be performed over the age of 50 has also been a topic of particular controversy. But we will analyse the evidence:
- •
Some studies of screening with MRI and MG have examined detection by age and found a high incidence in those over 50. For example, in an Italian multi-centre study,12 60% of cancers detected were in those over 50, and 27% were in those over 60.
- •
Multiple studies have shown that MRI detects cancers that MG does not in those over 5012,47,73,74 (much higher sensitivity).
- •
Some studies/meta-analyses have indicated that adding MRI to MG in screening patients over 50 increases cancer detection to an extent similar to that seen in younger patients — both BRCA mutation-carrier patients75 and non-carrier HR patients.49 Surprisingly, in BRCA mutation carriers, MG sensitivity was not higher in those over 50 than in younger patients,75 but was indeed higher in non-carrier HR patients.49
Therefore, the following considerations are put forward:
- •
It should be equally justifiable to offer MRI to patients over 50 and to younger patients, as MRI manages to detect additional cancers in both subgroups.
- •
The argument in favour of screening with MG alone in patients over 50 with a BRCA mutation75 is that older patients are expected to show less radiographic density. That would suggest that the MG sensitivity increases with age. However, this was not seen in a meta-analysis of patients carrying this mutation. While one meta-analysis of non-carriers49 did find a slight increase in MG's sensitivity with age, 32% of the cancers in women over 50 were detected on MRI alone.
- •
In patients carrying a BRCA mutation, tumours grow twice as fast as in non-carriers, with no evidence of changes in the biology of the tumour as a result of reaching age 50 or age 60.
It seems logical and justifiable, therefore, to continue screening with MRI past age 50 and up to at least age 7035,36,38 (level of evidence: IIA36) and to continue annual MG up to age 7536 (level of evidence: also IIA36), or to maintain annual MRI and MG up to age 75.17,29–31 Currently, most of the major guidelines recommend one of those two options (see Table 2). Some guidelines which place a ceiling for screening with MRI and MG at age 70 justify this by considering factors such as a decrease in relative risk of cancer with age in patients with a hereditary risk, the fact that few patients over age 69 have been enrolled in studies and the observation that BC screening has not shown a benefit in terms of mortality in women over 70 in the general population.38
Follow-up after risk-reduction surgeryMany centres do not offer specific guidelines for patients after risk-reduction surgery (RRS).76 Prospective data are needed to determine the most effective regimen for post-RRS follow-up in women with a BRCA mutation.
Today it is known77 that the incidence of BC after RRS is low: 0.7% per patient and 0.35% per breast (attributed to the presence of fibroglandular tissue). In addition, we also know that of the main RRS techniques today (nipple-sparing mastectomy and skin-sparing mastectomy), MRI is the technique that enables the most robust and reproducible measurement/quantification of fibroglandular tissue. It detects fibroglandular tissue in 20% of cases (50% in nipple-sparing mastectomy versus 13% in skin-sparing mastectomy), not only in the retroareolar area, but also in more peripheral areas of the breast (31% in a nipple-sparing mastectomy versus 13% in a skin-sparing mastectomy). It also detects higher proportions of fibroglandular tissue in a nipple-sparing mastectomy (7.9%) than in a skin-sparing mastectomy (1.7%).
In prophylactic surgery, compared to other surgeries, it is crucial to remove as much fibroglandular as possible. A preoperative MRI should be performed six months before the surgery to rule out BC. This MRI should also aid the surgeon in not leaving behind any fibroglandular tissue. A postoperative MRI should also be done to determine whether any fibroglandular tissue remains (fibroglandular tissue measurement): if not, follow-up could be done with ultrasound; if so, follow-up with MRI should be proposed, and patients who might benefit from a second surgery should be identified. Surgeons must report fibroglandular tissue measured because it represents a persistent risk of cancer.
Nipple-sparing mastectomy has been accepted as just as safe as skin-sparing mastectomy and aesthetically better and, although there may be some controversy with regard to the risk of developing a new cancer or a recurrence of one that still exists, recent studies have supported the notion that nipple-sparing mastectomy is safe.78
Screening in high-risk malesRegarding high-risk males, at present there is insufficient evidence to justify screening with imaging tests.31 At least one set of guidelines recommends, with a very low level of evidence (IIIC), considering MG at age 40, especially in males with gynaecomastia and BRCA2 mutation carriers.36 However, a very recent study showed a potential benefit of annual MG in HR males, with higher rates of cancer detection than in women, of cancers undetected during examination and in early stages. Further studies are needed to validate those results and offer more definitive recommendations on when and how to evaluate HR men.79
ConclusionIn patients with a high risk of BC (absolute lifetime risk of suffering from BC ≥20%-25%), screening with annual MRI and MG is recommended, and with the evidence from the aforementioned literature, our proposed protocol would be as follows:
- •
Start of screening: age 25 or 30.
- a)
In BRCA subgroup: start at age 25, although starting at age 30 could be considered if the youngest relative with BC was 40 or older when diagnosed. Also in carriers of other genes associated with HR (see Table 1).
- b)
In familial HR: start at age 30, although starting at age 25 could be considered if the youngest relative with BC was 35 or older when diagnosed.
- c)
Exceptions:
–Li-Fraumeni: start at age 20, with the recommendation that MG not be included, due to these patients’ high susceptibility to radiation.
–A history of prior chest radiotherapy, start at age 25, or eight years after finishing radiotherapy (whichever is the later of the two).
- a)
- •
Introduction of MG: at age 35, as a general rule (following European group/EUSOMA directives).
- a)
In familial HR: it may be introduced at age 40 or 10 years before the youngest age at which a relative was diagnosed, though not before age 35.36
- b)
Delay MG to age 40:
- 1.
or omit MG in BRCA1 patients?
- 2.
or omit MG in patients under 40?
- 3.
or omit MG in the future?
- 1.
These three options (1, 2 and 3) may require further study, although the evidence is gradually going in that direction.
Ages 25–35 (up to introducing MG): annual MRI and ultrasound.
- a)
- •
Synchronous versus alternate MRI and MG protocol: alternating may be beneficial.
- •
It may be beneficial to shorten the interval in BRCA1 (by performing MRI every six months? by adding ultrasound every six months? etc.): this requires further study, although it seems that MRI every six months may be optimal and will also require assessment of its cost-effectiveness.
- •
Maintain MRI up to at least age 70. Stop screening at age 75.
It is advisable to perform MRI in the second week of the cycle (to optimise specificity).
In patients in whom MRI is contraindicated due to claustrophobia, contrast allergy, incompatible implants or other reasons, screening would be performed with MG and ultrasound (MG every year and ultrasound every six months);61 prophylactic surgery might even need to be considered in BRCA1 patients. In pregnant patients, screening would be performed with ultrasound alone, although it may be reasonable to offer them ultrasound at shorter intervals.35
MRI is not contraindicated in breast-feeding and, although background parenchymal enhancement may represent a limitation, MRI continues to detect cancers and satellite lesions. Supplementary subtraction images can be used to decrease background parenchymal enhancement and facilitate diagnosis.80
MG with contrast is a promising new technique that may be an option at centres not equipped with MRI and in women in whom MRI is contraindicated. Like MRI, it provides a functional evaluation of tissue neovascularisation following an injection of iodinated contrast.81
FundingThis review received no specific grants from public agencies, the commercial sector or non-profit organisations.
Patient informed consent and dataThis study has the approval of the ethics committee at our centre. We have written informed consent forms for patients whose images from different imaging tests are shown in this article.
Authorship- 1.
Responsible for study integrity: SAR.
- 2.
Study concept: SAR.
- 3.
Study design: SAR.
- 4.
Data acquisition: SAR, ABD, JAL, BCC and ALR.
- 5.
Data analysis and interpretation: SAR, ABD, JAL, BCC and ALR.
- 6.
Statistical processing: SAR and JAL.
- 7.
Literature search: SAR.
- 8.
Drafting of the article: SAR.
- 9.
Critical review of the manuscript with intellectually significant contributions: SAR, ABD, JAL and BCC.
- 10.
Approval of the final version: SAR, ABD, JAL, BCC and ALR.
The authors declare that they have no conflicts of interest.
We would like to express our gratitude to all the other departments at our hospital centre that play a role in the management of breast pathology (Gynaecology, Oncology, Pathology and Nuclear Medicine) for their efforts and close, continuous collaboration with us (because the fruits of our professional and scientific labour are rooted in the great work done by everyone and strong multidisciplinary ties and cohesion, as well as in always trying to do our best with the resources that we have, without ever losing sight of the main objective: the benefit of the patients).
As the first author, I would like to thank my family for their patience and for the time that my dedication to science takes from my dedication to them.
I would also like to thank Ann Marsden for her kind help in translating the abstract into English.
Please cite this article as: Alonso Roca S, Delgado Laguna AB, Arantzeta Lexarreta J, Cajal Campo B, López Ruiz A. Cribado en pacientes con riesgo incrementado de cáncer de mama (parte 2). ¿Dónde estamos? Controversias actuales del cribado con resonancia magnética. Radiología. 2020. https://doi.org/10.1016/j.rx.2020.04.009