To show the usefulness of magnetic resonance imaging in the anatomic and pathologic characterisation of the sellar region, emphasising the differential diagnosis of uncommon non-adenomatous tumors and pseudotumors studied in our institution.
ConclusionThe sellar region is a complex anatomic space with diverse types of tissues from which a wide spectrum of diseases can arise. Magnetic resonance imaging's high tissue resolution and ability to characterise the patterns of tumor growth and biological behaviour make it the best imaging technique to study this region.
El propósito de este artículo es destacar la utilidad de la resonancia magnética en la caracterización anatómica y patológica de la región selar haciendo hincapié en diagnósticos diferenciales de tumores no adenomatosos y pseudotumores poco frecuentes estudiados en nuestra institución.
ConclusiónLa región selar es un espacio anatómico complejo, con múltiples tipos de tejidos a partir de los cuales puede surgir un amplio espectro de patologías. La resonancia magnética es el mejor método por imágenes para estudiar esta región debido a su alta resolución tisular, la caracterización de los patrones de crecimiento tumoral y el comportamiento biológico.
The sellar region is a small space in the central nervous system (CNS), which includes the bony component of the sella turcica, the cavernous sinus, the suprasellar cistern and the pituitary gland. This gland is formed by two lobes, the neurohypophysis and the adenohypophysis. It develops through a complex mechanism involving the diencephalon and stomodaeum around day 24 of gestation. Despite multiple tissue types, 80% of differential diagnoses include adenomas, meningiomas, aneurysms, astrocytomas, and craniopharyngiomas. In addition to clinical and laboratory analysis, magnetic resonance imaging (MRI) is the method of choice for reaching an accurate diagnosis, since it offers the best anatomical/tissue characterisation and establishes the tumor growth pattern through multi-planar acquisitions, thin slices, protocols for intravenous contrast with T1- and T2-weighted sequences, and demonstrates cellular behaviour with diffusion-weighted imaging sequences and apparent diffusion coefficient map (DWI/ADC).1–5
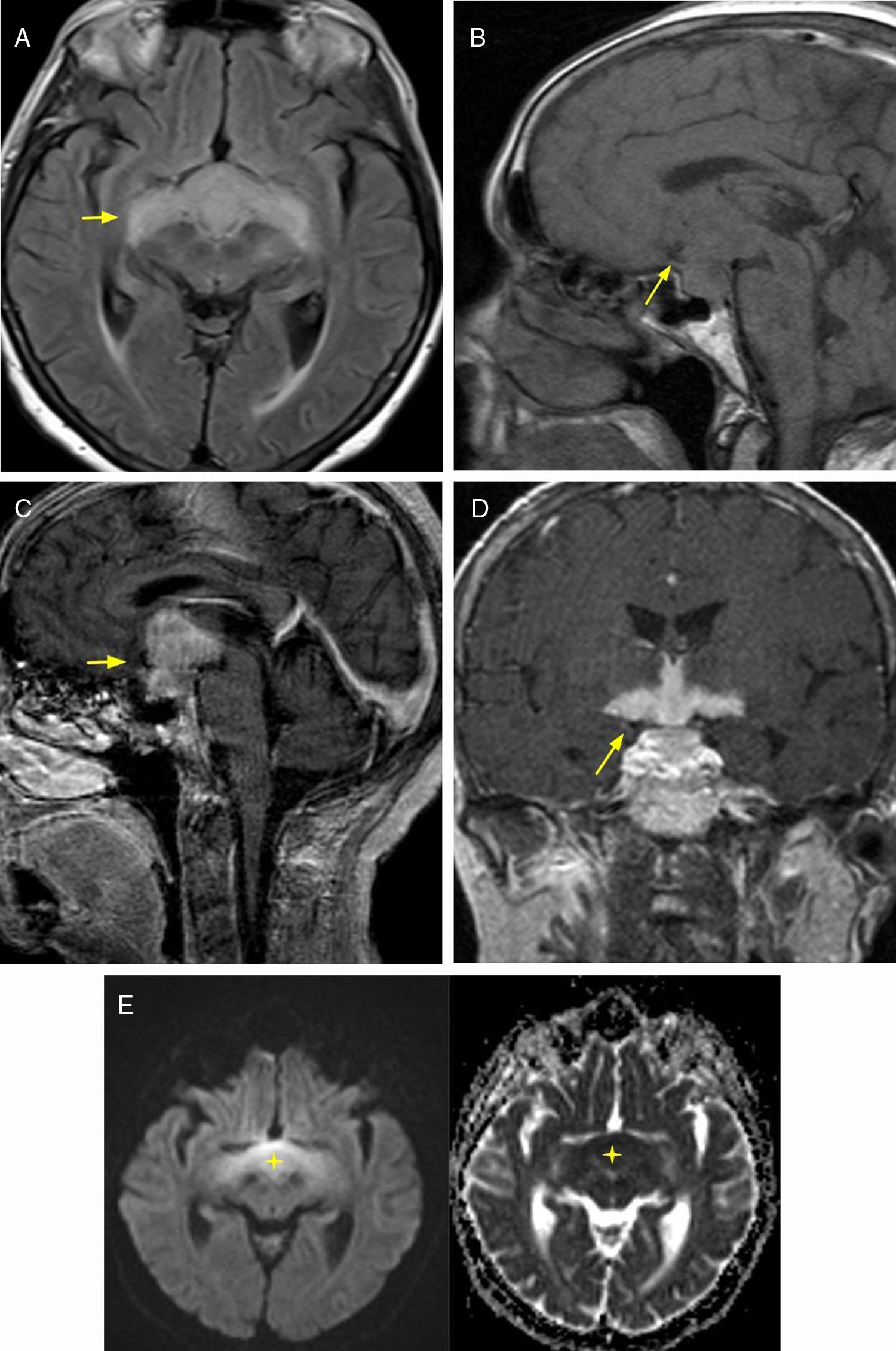
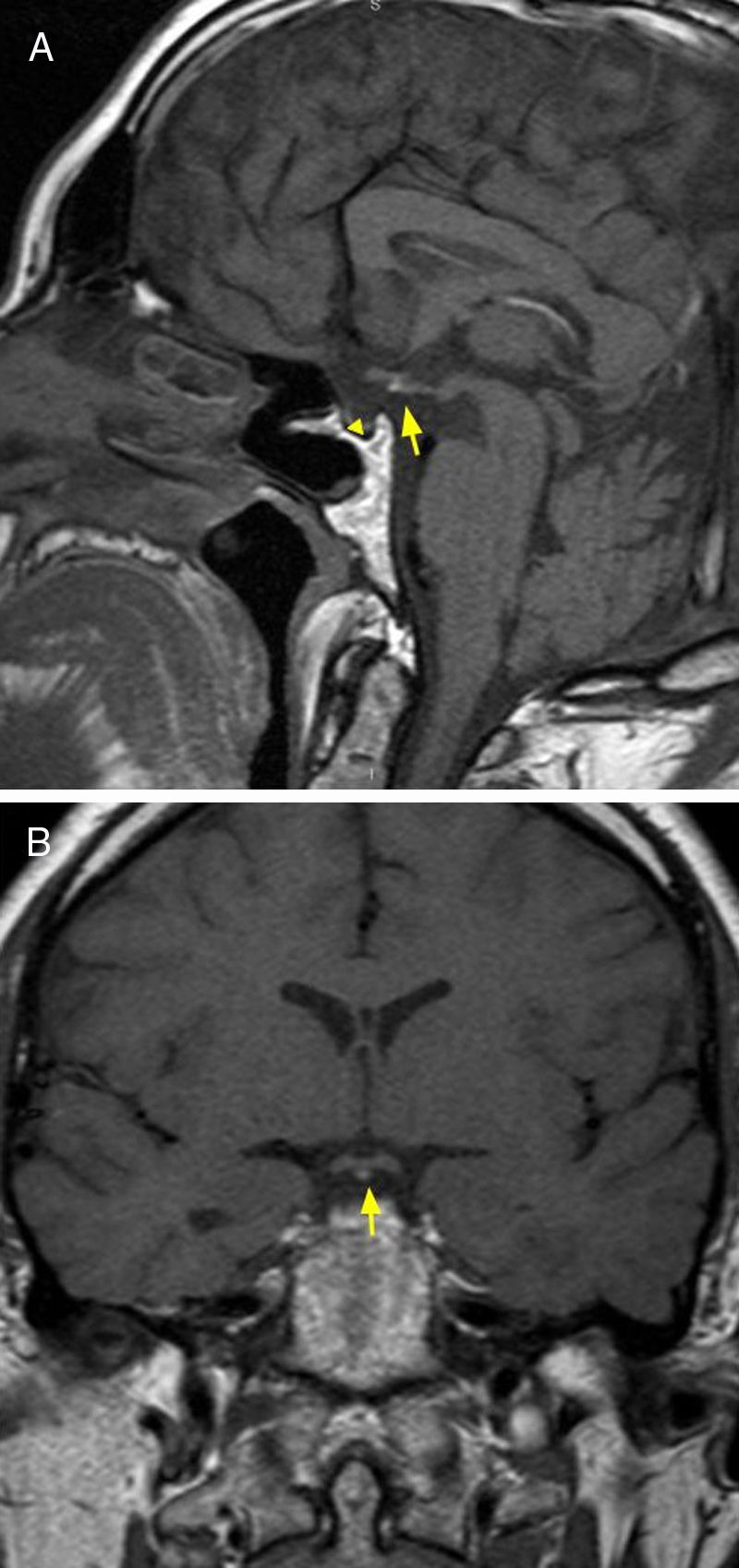
NeoplasmsPituicytomaA low-grade tumor, diagnosed between age 40 and 60 years. Clinical symptoms include visual disturbances due to compression of the optic chiasma, and headache. The MRI shows a lesion in the pituitary stalk and/or neurohypophysis, hypointense in T1-weighted sequences and hyperintense in T2-weighted sequences, with homogeneous enhancement after the administration of intravenous contrast6 (Fig. 1).
32-year-old patient with clinical diagnosis of diabetes insipidus, and by imaging, of pituicytoma. Brain MRI, axial T2 sequence (A) showing thickening of the pituitary stalk (arrow). T1 coronal and sagittal plane with intravenous contrast (B and C) where intense enhancement is observed.
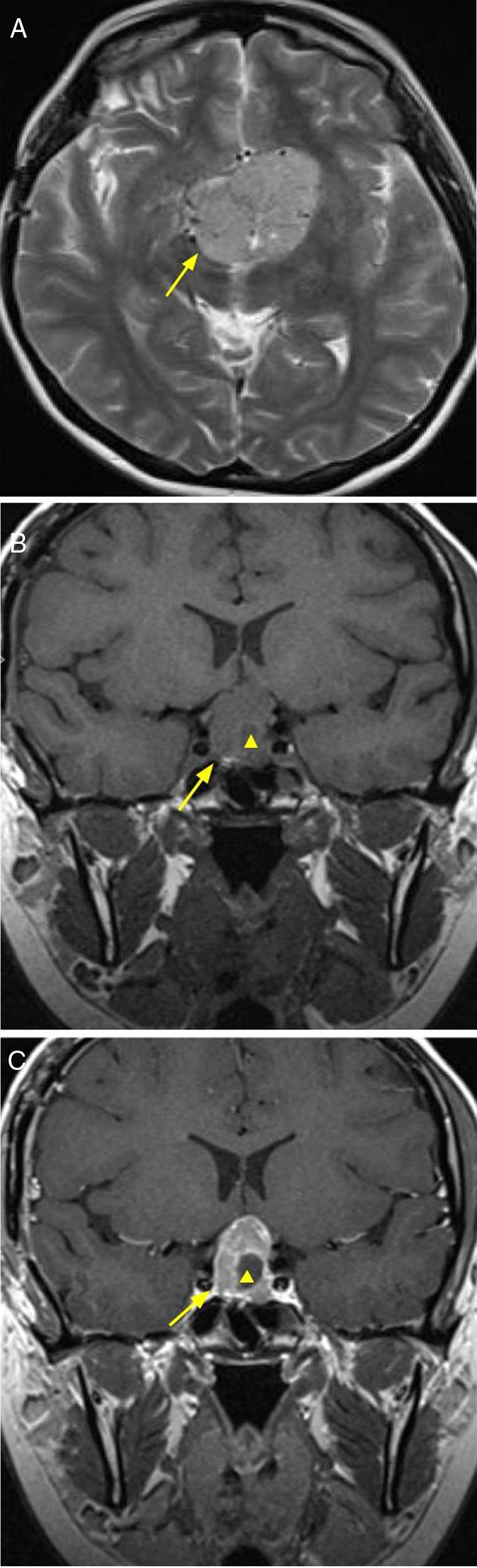
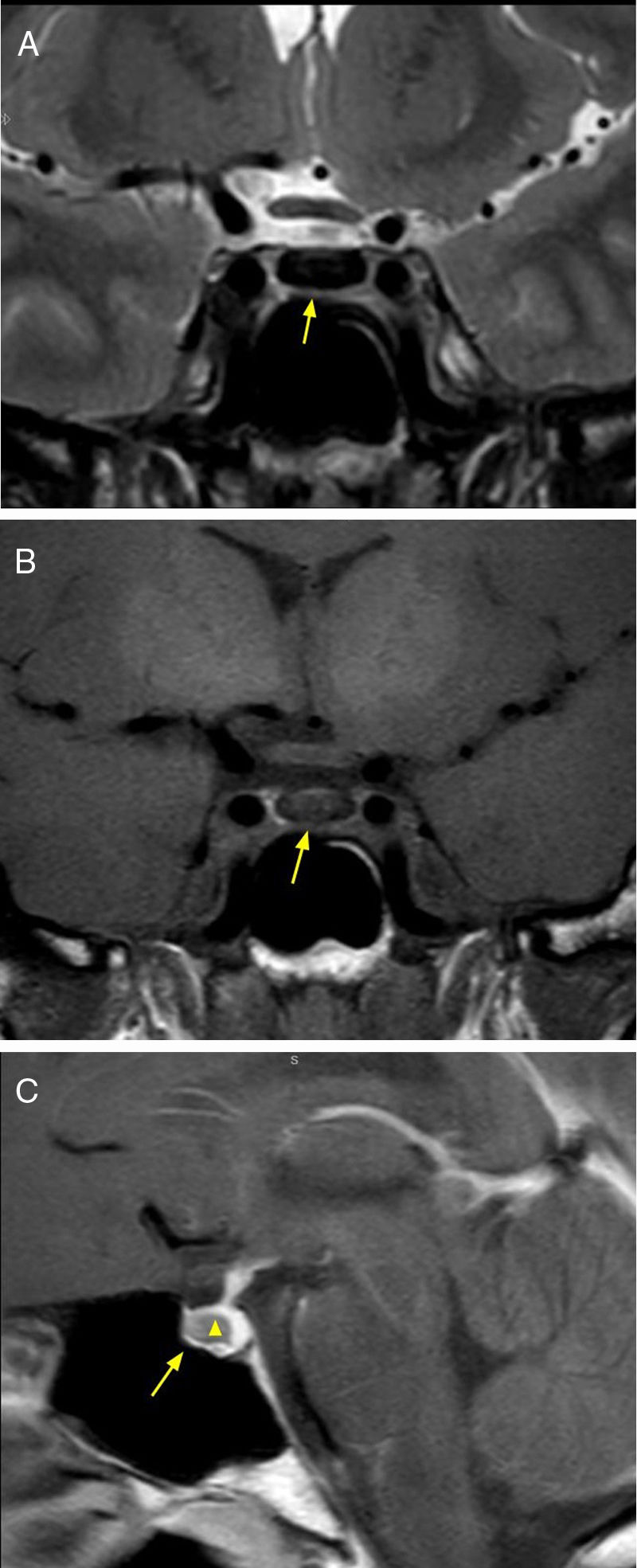
A rare neoplasia, which accounts for 1% of all intracranial tumors. Derived from germ cells that failed to migrate to the gonads. It affects children and adolescents, with a higher prevalence in women. It has a wide range of clinical symptoms, among which diabetes insipidus is the most frequent. This type of lesion exhibits suprasellar involvement with extension from the pituitary stalk and hypothalamus towards the third ventricle. The MRI shows one or several tumors, hypointense in T1-weighted sequences and hyperintense in T2 sequences, with enhancement after the administration of intravenous contrast7,8 (Fig. 2).
13-year-old female patient with clinical diagnosis of diabetes insipidus, and diagnostic imaging of germinoma. Brain MRI, T2 sequence, coronal and axial (A and B) planes, showing hyperintense 11mm tumor in the suprasellar region affecting the tuber cinereum and the pituitary stalk (arrow). T1 sequence without intravenous contrast, sagittal plane (C) where hypointense neoplasm in the third ventricle is observed (arrowhead) and that mentioned previously (arrow). T1 sequence with intravenous contrast, sagittal plane (D), showing enhancement of both lesions. Resective surgery was performed and the anatomopathological result confirmed the diagnosis of germinoma.
Tumour located in the midline and derived from remains of notochord. Affects the sacrum (60%) and also located in the sphenoid bone (25%). Accounts for 1–4% of primary malignant bone tumors. It is more frequent in men, and in the fourth decade of life. The brain MRI is very useful for assessing the extraskeletal extension, with hypo/isointense behaviour in T1 sequences and hyperintense in T2 sequences; may contain calcifications and internal septa and acquire a lobulated configuration9 (Fig. 3).
77-year-old female patient with visual disturbances and diagnostic imaging of chordoma. Brain MRI, FLAIR sequence (A) and T2 (B), axial plane, showing expansive and lobulated, hyperintense lesion (arrow) originating in sphenoid bone and compressing adjacent structures. T1 sequence with intravenous contrast, axial (C) and sagittal plane (D), showing low peripheral and heterogeneous enhancement (arrowhead). Treatment was surgical and the anatomical pathology report confirmed the diagnosis of chordoma.
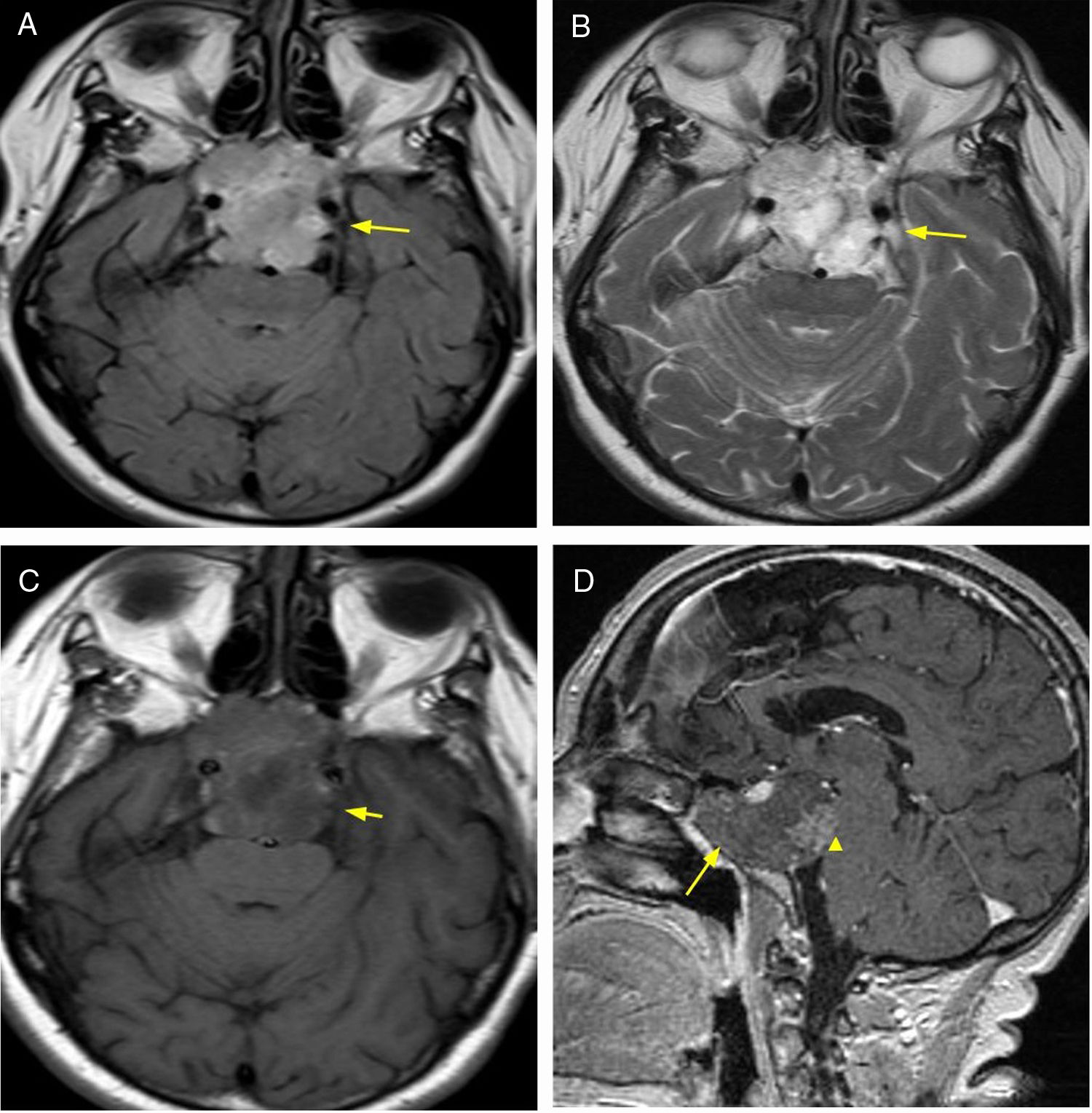
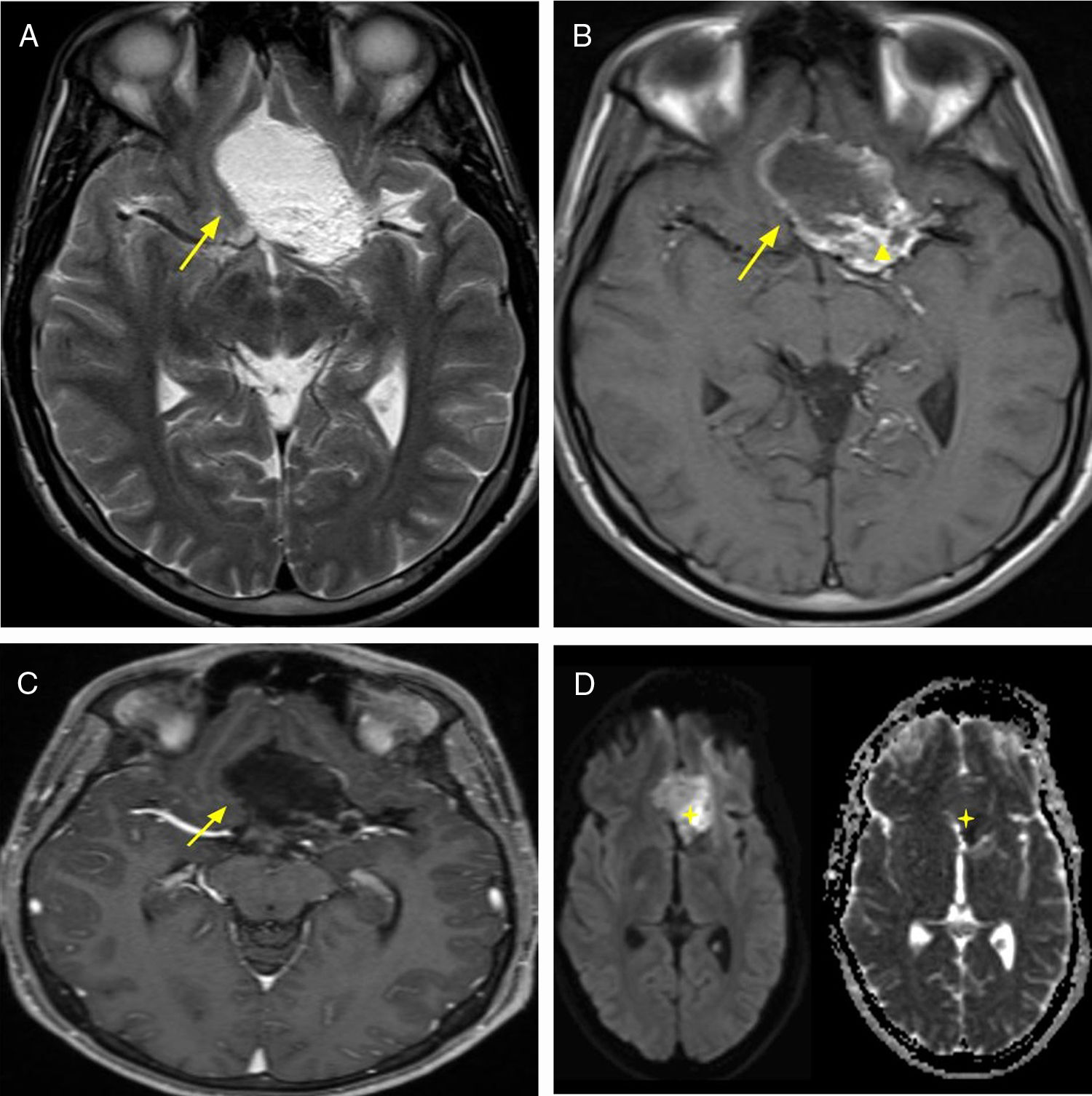
Primary central nervous system lymphoma accounts for 3–5% of all brain tumors. It is of the non-Hodgkin variant, mainly of B-cell origin. The MRI findings include T1 sequence hypointense signal, iso- to hyperintense signal in T2, and intense enhancement after administration of intravenous contrast. It manifests high cellularity, which allows for characterisation with DWI/ADC sequences when restriction is evidenced. It does not exhibit central necrosis. Among the associated findings, vasogenic oedema is mentioned, although in immunodeficient individuals, appearances tend to be more heterogeneous (revealing absence of enhancement/central necrosis and/or haemorrhage)10 (Fig. 4).
64-year-old female patient with headache and visual hallucinations. Diagnostic imaging: lymphoma. Brain MRI, FLAIR sequence (A) axial plane and T1 (B) sagittal plane showing expansive, infiltrative lesion originating in the hypothalamus and hypophysitis with perilesional oedema (arrowhead). T1 sequence with intravenous contrast axial plane (C) and coronal plane (D) with intense enhancement (arrow). DWI/ADC sequences (E) with signs of cell restriction. Biopsy was performed and diagnosis of lymphoma was confirmed though anatomical pathology.
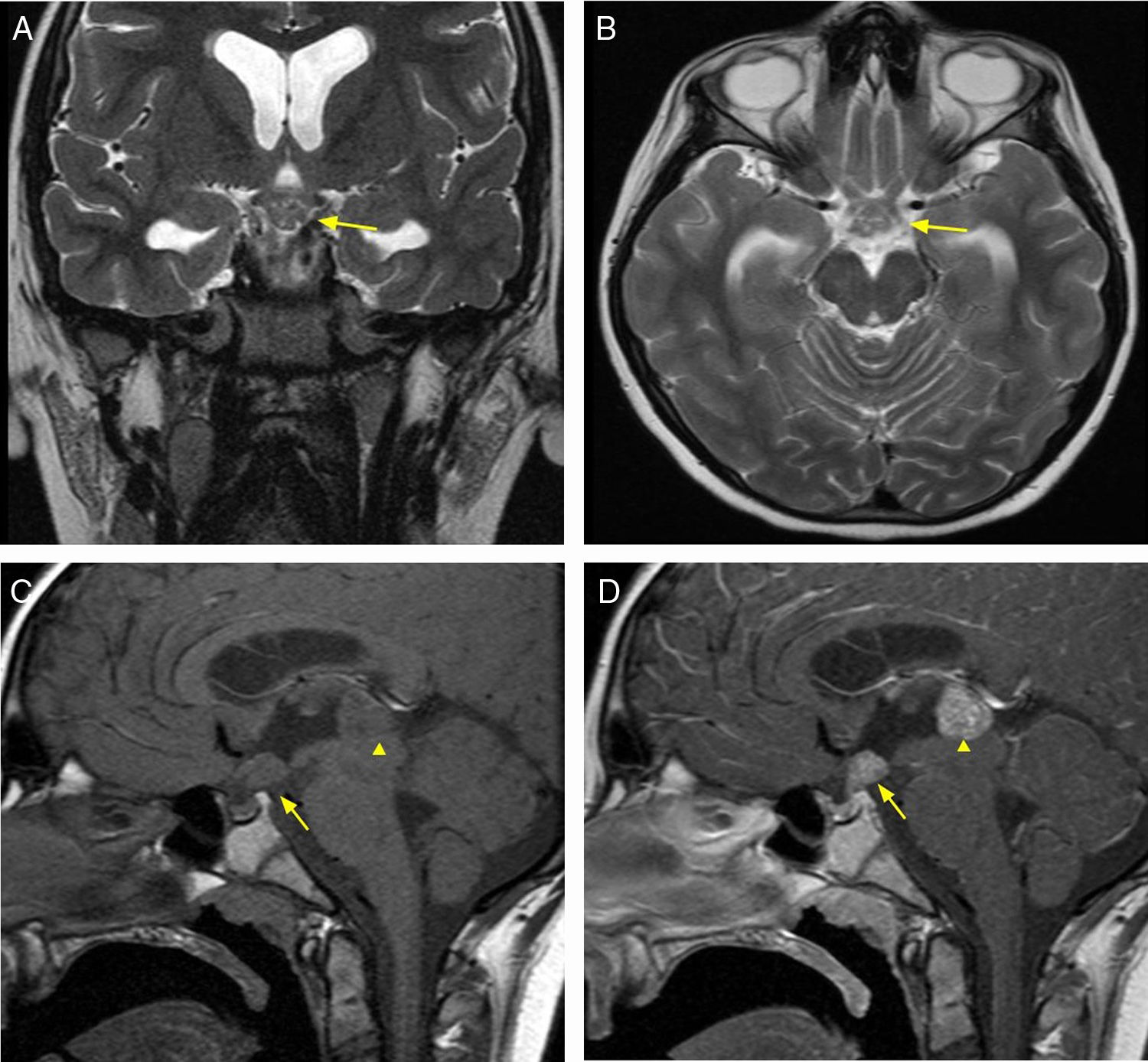
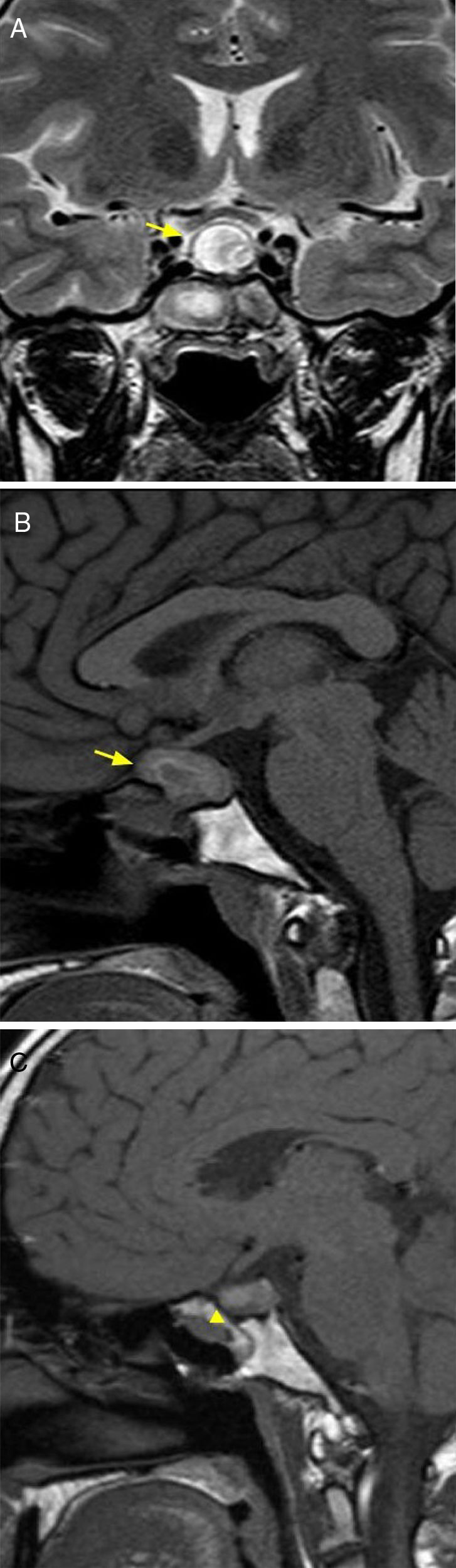
A benign, encapsulated tumor with slow and progressive growth consisting of Schwann cell proliferation. It accounts for 8–10% of all primary intracranial tumors, and the age range of appearance is wide (20–70 years). Intrasellar schwannomas are extremely rare and may occur sporadically or as manifestations of neurofibromatosis type 2 or Schwannomatosis (third major form of neurofibromatosis).11 MRI findings include an expansive, mixed lesion (solid and cystic component), with hypointense signal in T1 sequences with homogeneous enhancement, while the T2 sequences reveal a hyperintense signal and it rarely calcifies12,13 (Fig. 5).
40-year-old female patient with amenorrhoea of 11 years of evolution, elevated serum levels of prolactin and chronic headache. Diagnostic and anatomical pathology imaging of schwannoma. Brain MRI, axial T2 sequence (A) showing expansive, suprasellar lesion (arrow). T1 sequence without intravenous contrast, coronal plane (B), and with intravenous contrast, coronal plane (C), with areas of cystic/necrotic degeneration (arrowhead) and moderate enhancement (arrow).
Extremely rare tumor with only 4 cases of intrasellar localisation reported up until 2017 in English publications. Of congenital origin and benign, they account for 0.04–0.6% of intracranial tumors. They have a capsule with dermal appendages such as hair follicles, glands and fat. In MRI they exhibit a hyperintense signal in T1 sequences with hypointensity to a mixed signal in the T2-weighted sequences, due to the abundance of lipids in the cysts. Other characteristics include the absence of peripheral oedema and reinforcement with the administration of intravenous contrast with avascular pattern14 (Fig. 6).
25-year-old male patient, with chronic headache. Diagnostic and anatomopathological imaging of dermoid cyst. Brain MRI, T2 sequence, axial plane (A) showing expansive heterogeneous tumor in anterior fossa (arrow). T1 sequence, axial plane (B), where fat signal (arrowhead) and absence of perilesional oedema is observed. T1 FAT-SAT sequence axial plane with intravenous contrast (C), with low peripheral enhancement. DWI/ADC sequence (D) showing focal cellular restriction (star).
This entity should be considered as a differential diagnosis of the expansive lesions of the sellar region. There are four types: lymphocytic, granulomatous, xanthomatous and plasmacytic. The first is the most common and is of autoimmune origin. The MRI reveals an enlarged gland in diffuse form with suprasellar extension, thickening of the pituitary stalk, absence of clear differentiation between the anterior and posterior lobes, and intense postcontrast enhancement. Treatment includes replacement for functional pituitary deficiency and the use of steroids. Surgical treatment is reserved for those patients with a lack of response to conservative treatment15,16 (Fig. 7).
29-year-old female patient with secondary amenorrhoea and diagnostic imaging of hypophysitis. Brain MRI, T2 sequence, coronal plane (A), and T1 sequence, sagittal plane (B), where lesion with cystic appearance with heterogeneous content is observed (arrow). FLAIR sequence, sagittal plane (C) showing reduction in size after treatment with steroids. Anatomopathological report confirmed diagnosis of autoimmune hypophysitis of the lymphocytic type.
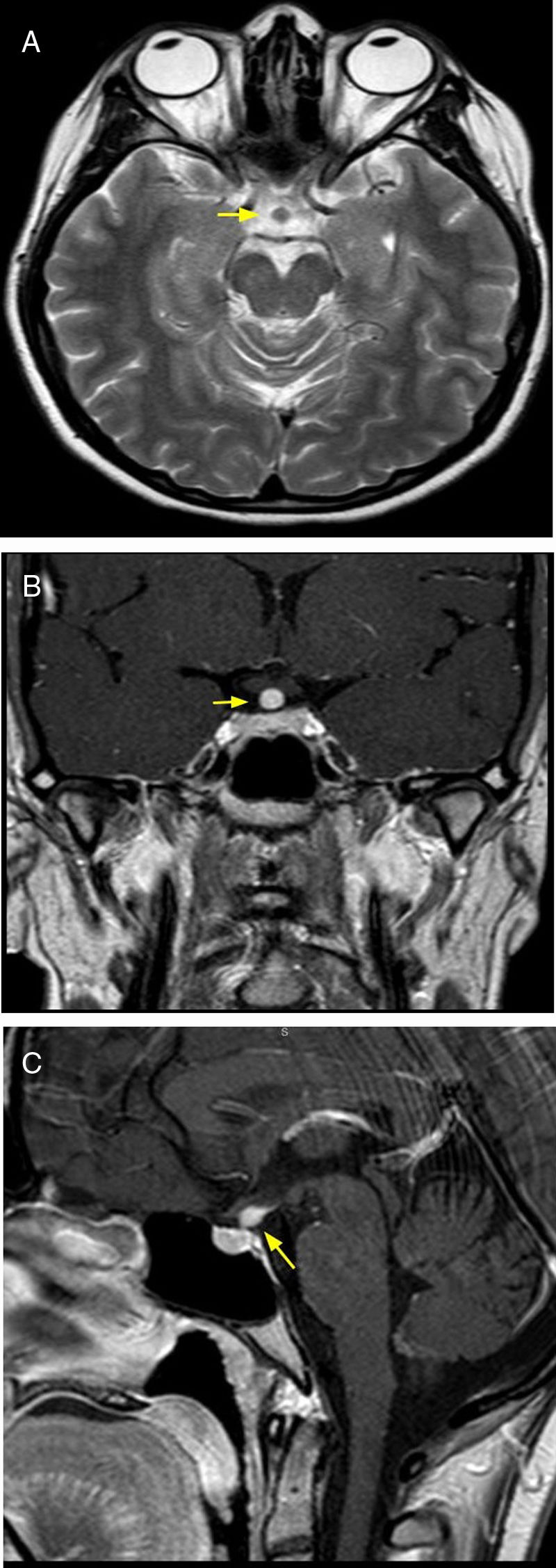
A congenital anomaly that can manifest as panhypopituitarism or partial hormone deficiency. Other congenital abnormalities of the CNS may coexist. It is the result of a growth deficiency and fusion of the neurohypophysis with the adenohypophysis. It manifests with absence of spontaneous hyperintensity of the neurohypophysis and with hyperintensity of signal in the T1 sequence in the median eminence (floor of the third ventricle) or along the infundibular stalk, instead of its normal posterior position. The infundibular stalk may be small or absent17 (Fig. 8).
20-year-old male patient with symptoms of panhypopituitarism and diagnostic imaging of ectopic neurohypophysis. Brain MRI, T1 sequence, sagittal (A) and coronal plane (B), showing hypoplastic adenohypophysis (arrowhead), pituitary stalk agenesis and ectopic neurohypophysis (arrow).
An autosomal recessive disease different from the classic adult disorder, caused by mutations in two genes. Iron begins to accumulate early in life, and before 30 years of age symptoms are observed; hypogonadotropic hypogonadism and cardiac involvement are the leading symptoms in the clinical syndrome. The MRI shows an enlarged pituitary gland with iron deposits, represented as a low signal intensity in T1 and T2 sequences18 (Fig. 9).
27-year-old female patient with symptoms of hypogonadotropic hypogonadism and elevated serum ferritin. Brain MRI, T2 sequence (A) and T1 sequence, coronal plane (B), where hypointense and enlarged pituitary gland is observed (arrow). T1 sequence, sagittal plane with intravenous contrast, showing absence of enhancement (arrowhead), compatible with haemosiderin deposits. The diagnosis of juvenile haemochromatosis was confirmed due to hepatic and cardiac involvement.
- •
MRI plays a key role in the evaluation of tumors of the sellar region and pseudotumoral pathology.
- •
Diffusion-weighted imaging sequences/apparent diffusion coefficient map (DWI/ADC) contribute to tumor delimitation, in the infiltration of adjacent structures such as lymphomas, or in the characterisation of dermoid cysts.
- •
The sellar region is a complex anatomical space with different embryological origins. 20% of its pathology is accounted for by rare neoplasms and pseudotumoral lesions such as pituicytoma, germinoma, chordoma and autoimmune hypophysitis, among others.
- 1.
Responsible for the integrity of the study: DA, FO, JMV, JF and MP.
- 2.
Study conception: DA, FO, JMV, JF and MP.
- 3.
Study design: DA, FO, JMV, JF and MP.
- 4.
Data collection: DA, FO, JMV, JF and MP.
- 5.
Data analysis and interpretation: DA, FO, JMV, JF and MP.
- 6.
Statistical processing: N/A.
- 7.
Literature search: DA, FO, JMV, JF and MP.
- 8.
Drafting of the paper: DA, FO, JMV, JF and MP.
- 9.
Critical review of the manuscript with intellectually relevant contributions: DA, FO, JMV, JF and MP.
- 10.
Approval of the final version: DA, FO, JMV, JF and MP.
The authors declare that they have no conflicts of interest.
Please cite this article as: Adri D, Olivera F, Villegas JM, Funes J, Pietrani M. Región selar: evaluación mediante resonancia magnética de lesiones tumorales y pseudotumorales de baja frecuencia. Radiología. 2019;61:467–476.