The recent financial crisis has led to a substantial reduction in public investment and technological renovation in Spain, resulting in a significant increase in the rate of obsolescence of diagnostic imaging equipment.
The Spanish Society of Medical Radiology, aware of the importance of maintaining appropriate technological measures to ensure the quality of health care, has elaborated a document with the aim of analyzing and promulgating the state of diagnostic imaging technology in Spain (depending on the imaging modality, between 32% and 59% of the equipment is more than 10 years old) as well as of establishing criteria and recommendations to guide the management of technology in medical imaging.
La reciente crisis económica ha provocado una sustancial disminución de la inversión pública y de la renovación tecnológica en España, que ha causado un incremento relevante del índice de obsolescencia de los equipos de diagnóstico por la imagen.
La Sociedad Española de Radiología Médica, consciente de la importancia de mantener unos medios tecnológicos apropiados para asegurar la calidad asistencial, ha elaborado un documento con el objetivo de analizar y difundir la situación de la tecnología de diagnóstico por la imagen en España (entre el 32% y el 59% de los equipos, según el tipo de modalidad, tienen más de 10 años) y para formular criterios y recomendaciones que sirvan de guía en la gestión de la tecnología de imagen médica.
Medical technology is an essential part of the healthcare process; it has been and continues to be a fundamental factor in achieving current levels of quality in healthcare.
Health technologies contribute to improving the efficiency of clinical processes, increasing their quality and increasing the safety of patients and professionals.
Specifically, diagnostic imaging (DI) technologies have improved their diagnostic accuracy, which makes it possible to detect many diseases at earlier stages and, therefore, treat them more quickly and effectively. The impact of the implementation of faster computerised tomography (CT) equipment and with less radiation doses represents only an example of efficiency and quality improvement in the healthcare process.
The constant development of physics, electronics and computing subjects DI technologies to permanent innovation cycles that provide new tools and resources, offering tangible benefits to healthcare processes. However, the rate of incorporation of these technological improvements to the public health centres of the Spanish National Health System (SNS) depends on the availability of resources of the autonomous communities (AC) and, specifically, of the investment plans of the centres, which determine the acquisition of new DI technology, as well as the renovation of the obsolete technology.
Several publications highlight a substantial decrease in public investment during the recent economic crisis,1 and a slowdown in technological renewal in Spain, which is significantly increasing the obsolescence, operational and technological index of DI equipment installed in health centres in Spain. This situation is much more evident if we compare Spain with the data from neighbouring countries such as those published by The European Coordination Committee of the Radiological, Electromedical and Healthcare IT Industry (COCIR), in which Spain appears among the last places in Europe because of the age of the DI equipment in use.2
This situation can be attributed to the current economic situation, but the lack of documentation on the life cycles of the technology, its costs of use, updating criteria, as well as the direct and indirect benefits that can be provided by renewing it with new more capable equipment, with lower consumption and greater diagnostic capacity also have an impact.
Given the evidence of a marked reduction in technological renewal and an increase in obsolescence and the comparative data with other European countries, the Sociedad Española de Radiología [Spanish Society of Radiology] (SERAM) considered it necessary to make a diagnosis as accurate as possible of the current situation.
Material and methodsThe situation in Spain was evaluated based on surveys carried out with the heads of the radiology department of public hospitals with more than 250 beds, using a form, sent and received by telematic means. The surveys were conducted from May to October 2017 and included a quantitative part, referring to the specific data of the equipment in their centre, and a second qualitative part that included the point of view of the heads of the department on the process of technological renewal in their setting.
In order to ensure greater objectivity, for the development of the surveys and their statistical analysis, the collaboration of the Fundación Signo was requested, which through its “Innovation and Technology” line of action has involved experienced professionals in this area to analyse the data.
During the summer of 2017, an analysis of the international literature that addresses the management criteria of the life cycle of the technology was carried out, mainly focused on official sources, from developed countries and with a similar health organisation. No exhaustive analysis has been attempted, instead just to find the basic indicators that define the renewal criteria of each country studied, knowing that they are indicators of recommendation and that, in no case, are they the norm or law.
At the same time, contributions and suggestions from different agents related to the technological acquisition and renewal process (managers, regional health services, medical physics departments, etc.) were collected through interviews.
The guide was published in October 2017.
ResultsIn the analysis of the situation, 59 public hospitals of the SNS, which represent 48% of those consulted, have contributed their data by responding to the survey. 55% are hospitals with 250–500 beds; 21%, 500–1000 beds, and 24% have more than 1000 beds.
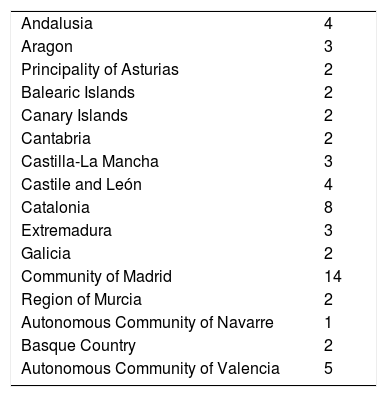
The ACs where the highest number of hospitals responded were Madrid and Catalonia (Table 1).
Distribution by autonomous community of hospitals that participated in the survey.
| Andalusia | 4 |
| Aragon | 3 |
| Principality of Asturias | 2 |
| Balearic Islands | 2 |
| Canary Islands | 2 |
| Cantabria | 2 |
| Castilla-La Mancha | 3 |
| Castile and León | 4 |
| Catalonia | 8 |
| Extremadura | 3 |
| Galicia | 2 |
| Community of Madrid | 14 |
| Region of Murcia | 2 |
| Autonomous Community of Navarre | 1 |
| Basque Country | 2 |
| Autonomous Community of Valencia | 5 |
From the statistical point of view, the sample obtained is sufficiently representative and with a distribution that covers practically the entire country; the biases and limitations are that the hospitals which are more aware of the problem may be oversized as the response was voluntary, that the survey focuses on a partial segment of the set of public health centres of the SNS (hospitals with more than 250 beds), although some include the teams of the speciality centres and health centres that depend on them, and that data are not collected from autonomous organisations that in some communities perform part of the activity, especially magnetic resonance imaging (MRI) and computed tomography (CT) scans, nor from those specifically dedicated to breast cancer screening. Also, the different degree of response per C could represent an additional bias.
However, in general, a good correlation is observed with the data elaborated by other agencies.
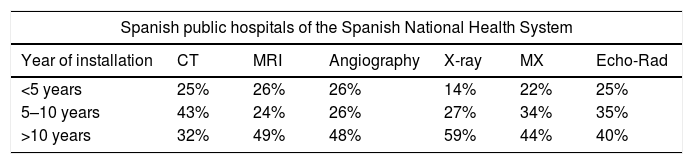
The results obtained related to the age profile are shown in Table 2.
Age of the equipment according to the survey data.
| Spanish public hospitals of the Spanish National Health System | ||||||
|---|---|---|---|---|---|---|
| Year of installation | CT | MRI | Angiography | X-ray | MX | Echo-Rad |
| <5 years | 25% | 26% | 26% | 14% | 22% | 25% |
| 5–10 years | 43% | 24% | 26% | 27% | 34% | 35% |
| >10 years | 32% | 49% | 48% | 59% | 44% | 40% |
CT: computed tomography; MR: magnetic resonance; MX: mammography; X-ray: conventional radiology.
The most relevant information is that a significant number of equipment of all types, between 32% and 59%, are more than 10 years old; data that agree enough with what was elaborated by the Federación Española de Empresas de Tecnología Sanitaria [Spanish Federation of Health Technology Companies] (FENIN), with values between 23% and 60%.
Other significant data are:
- •
49% of the MRI equipment, 32% of the CT and 48% of the angiographs installed in the radiology departments are more than 10 years old, which corresponds to an age level higher than the average of equipment installed in Spain, including public and private.2 It should be clarified that the data requested in the survey is the date of installation of the equipment; however, there is a growing number of MRI equipment and, to a lesser extent, CT equipment, which have been completely renewed in their hardware, firmware and software components, which can generate a distortion in their chronological classification. Therefore, it is probable that part of the documented equipment has been partially updated, which would allow reclassification of some of them.
- •
In conventional radiology, 59% of the equipment has been installed for more than 10 years; more than half is based on analogue technology to obtain the image, which greatly limits the implementation and use of new image management and distribution technologies. This is an aspect that should be taken into account for the technological update of conventional radiology systems.
- •
40% of the ultrasound equipment located in the radiology departments have been installed for more than 10 years and are older than the global average of ultrasound machines available in the centre, which is especially paradoxical, when the ultrasound scans located in radiology should have the greatest diagnostic capacity.
- •
19% of the plain radiology rooms, 15% of the radiosurgical equipment, 29% of the laptops and 36% of the ultrasound scanners do not have a maintenance contract, so they do not receive preventive checks and maintenance of the original characteristics with which they were acquired. In our opinion, this is an important factor in the life cycle of the technology, given that a regulated maintenance that covers the manufacturer's recommendations has a relevant influence on the maintenance of the equipment's useful life, especially when the basic functions are linked to rapidly evolving technologies such as information and communication technologies.
The survey also included aspects related to the management of DI technology. The most significant data were:
- •
Hospitals that have a complete inventory database that incorporates the information related to the acquisition, installation, maintenance, healthcare activity, technological update and use costs that would allow a comprehensive management of the DI technology are exceptional.
- •
Only 8% of the hospitals surveyed had a structured process (formal document) for planning the renewal of their DI technology.
- •
Satisfaction with the current process of technology renewal was 3.62 out of 10 points, which indicates a high level of dissatisfaction.
- •
Only 12% of hospitals or health ministries have a guide or protocols for managing the life cycle of their DI equipment.
- •
The degree of importance of having a guide for the management of technology has reached in the survey to the heads of department an average rating of 9.30 points out of 10, which indicates the high interest in developing this documentation.
A wider discussion and analysis of the data obtained in the survey and its comparison with the European data are available in Annex 1 of the SERAM guide for the renewal and technological update in radiology,3 available at: http://seram.es/modules.php?name=webstructure&idwebstructure=207.
After the SERAM guide, in December 2017, FENIN published a document on the technological profile of hospitals, obtained by adding data from their associated companies, with results that matched considerably with ours: between 23% and 60% of DI equipment in public hospitals, according to modalities, were more than 10 years old.4
DiscussionIn addition to the analysis of the situation, the SERAM guide aims to help health organisations determine when and how to update or replace DI medical technology equipment or add new emerging technologies through the analysis of the useful life cycles of these technologies.
There are different ways of approaching the management of DI technology, which depend mainly on the health system model (financing and provision) where the services are developed, the purpose of the institution providing the service and the macroeconomic and social environment where the activity is developed.
In private insurance systems, in which the incorporation of technological innovation represents a competitive advantage to attract more patients and increase healthcare activity, a business model is developed that adjusts the renewal criteria to the expectations of the return on investment (ROI) made.
The concept of life cycle changes in public insurance systems, where benefits are defined a priori and there are no economic incentives that justify the concept of ROI. In them, investment in technology is interpreted more as a necessary cost than as an investment in itself, and the renewal of the equipment is based above all on the life cycles of DI technology, although in the private provision systems the return differences that are established depending on the age of the equipment are important. Therefore, it is in the public provision systems where the best references for our case are found.
The most complete reference is Lifecycle Guidance for Medical Imaging Equipment in Canada 2013,5,6 published by the Canadian Association of Radiologists and which has served as a reference for the preparation of this guide for its extensive documentary review and selection of the most relevant data to document the criteria used in the United States, Canada and Australia.
Canada has an excellent bibliographic base based on the complete monitoring of usage indicators and renewal guides in the different provinces. It has established some recommendations for the planning of the equipment that include the definition of the criteria for the prioritisation of the replacement or life cycle management, defined as a formal process of planning the renewal of the DI equipment, and monitors the degree of application and compliance. In 2016, the conclusions document on the practice in the renewal of DI equipment, developed by the Canadian Agency for Drugs and Technologies in Health (CADTH), was published.7
The recommendation on life expectancy of the equipment of the Canadian guide introduces a relevant element, such as incorporating the degree of use to the life cycle criterion, which is assessed according to the number of exams or the daily time of use (8h/16h/24h), defining three bands according to the level of use: high, medium or low.5
In the United States, there are multiple publications that address the criteria of renewal or useful life of equipment.8,9 The American Hospital Association has published several reviews of its guide ‘Estimated Useful Lives of Depreciable Hospital Assets’.10 This guide is used as a reference for the assessment of the state of the technological resources from the healthcare providers Medicare and Medicaid. The U.S. Department of Veterans Affairs published a guide in 2012 with an exhaustive list of life expectancy times of health technology equipment in which it states that “the life expectancy (cycle) must not be the only criterion for replacing equipment, but it must be used together with other factors such as technological obsolescence, failure rate, usage costs, maintenance problems, suspicions of lack of safety, etc.”.
Australia has a universal health system financed by the federal government, although the responsibility for management lies with the six states of the nation. In 2003, the state of Victoria published the document ‘Managing medical equipment in public hospitals’,11 prepared by the Office of the Auditor General of Victoria, which has been an important documentary reference on the management of healthcare technology. Some of the conclusions of the audit are to have a record of the technology with its classification and essential documentation and the need for hospitals to plan their needs for medical equipment on a long-term basis (indicates 5 years), including regular monitoring of their life cycle and the conditions of maintenance and use.
A more recent publication12 focuses the audit report on the analysis of high-value equipment, specifically MRI and CT. This document once again emphasises the importance of planning, in the medium-to-long term, the needs for the renewal of technology in such a way as to avoid improvisation.
The comprehensive management of the life cycle of medical technology has been addressed in a very detailed way by the Department of Health of the State of Victoria, to the point of developing a comprehensive protocol of medical technology management that addresses from the planning of the replacement, the increase of resources, the acquisition process, the usage with its maintenance and control, until the uninstallation and discarding, which also includes the specific programme of technological renewal for the period 2018–2019.13
The United Kingdom has generated multiple documents related to the management of health technology and, specifically, of DI. Thus, in 2015, the Medicines and Healthcare products Regulatory Agency published a guide for the management of medical technology: ‘Managing Medical Devices’,14 which addresses the comprehensive concept of the life cycle, from procurement planning to uninstallation, and which reinforces the concept of maintaining a single documentary record with the complete information of each team, both economic and technical as well as regulatory.
The Auditor General for Scotland has published a more comprehensive study on the management model of medical technology, ‘Equipped to care’,15 in which it describes and recommends the model of management and activity of health technology in the centres of the UK National Health Service (NHS). In its subsequent review, in 2004, ‘Better Equipped to Care?’,16 it evaluates the profile of the technology in Scottish hospitals, indicating, in annex 9 of the document, the replacement periods of certain technologies.
In Spain, in 2013, the Ministry of Health, Consumption and Social Welfare published a document on quality standards and recommendations in the diagnostic and image processing healthcare units in which the management of equipment, planning, hiring, commissioning and maintenance is analysed, and in which several estimates of the useful life of different imaging modalities are included.17
COCIR, which integrates the main European manufacturers and suppliers of DI technology, launched the ‘Golden Rules’ a few years ago, in which the age of the equipment is related to its ability to incorporate current and innovative technology; three rules are proposed:
- •
At least 60% of the equipment installed in a centre must be less than 5 years old.
- •
At most, 30% of the equipment must be between 6 and 10 years old.
- •
The available technology older than 10 years old will be limited to a maximum of 10%.
From the international literature that gathers the experiences, criteria and indicators of the most developed countries and the suggestions and recommendations received from the different agents related to the technological acquisition and renewal process, SERAM considers it necessary to make the following recommendations:
- •
It is necessary to develop and maintain a complete record of all DI equipment. The collection of data from the survey of the guide illustrates the difficulties in documenting the inventory of the equipment of each hospital, and highlights the dispersion of the information of each of them, date of acquisition, number of work shifts, annual activity, types of maintenance performed, quality controls and corrective measures in equipment that uses ionising radiation, inventory of breakdowns, etc., data that in many cases are available, but in different spread out records. It seems evident that to carry out an active management of these resources, it is essential to begin by having a documentary record that is as complete as possible.
- •
The basic part of these records must be publicly accessible. The current demands for greater transparency and patient access to all the information that affects them make it advisable that at least the basic information of this record be of a public nature; essential, on the other hand, as a tool to control resources and their status, as well as when preparing status reports and reliable statistics.
- •
It is necessary to establish objective criteria for the renewal of equipment. There are multiple factors that influence the life expectancy of equipment, but, from the practical point of view, in most institutions the parameter “time of use” is used as a renewal criterion. Although the life cycle is different for each type of image modality, there is an important coincidence between the different publications, which establish periods of obsolescence that range between 7 and 10 years.
Among the criteria analysed, we recommend using those developed by the Canadian Association of Radiologists, which are the most widespread and that add another relevant element to the temporary parameters of renewal, such as the degree of use.
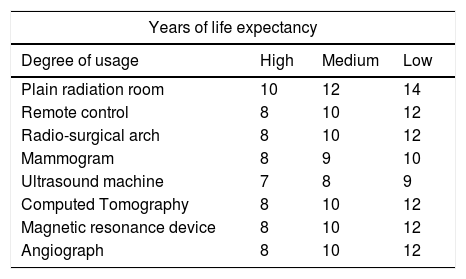
To determine the degrees of use, it is possible to use the number of studies that appear in the Canadian guide or the number of daily working shifts, which is low when they work in a single shift, medium when they work in two, and high in the cases of emergency equipment or that work in three daily shifts (Table 3).
Years of life expectancy of the equipment based on their use.
| Years of life expectancy | |||
|---|---|---|---|
| Degree of usage | High | Medium | Low |
| Plain radiation room | 10 | 12 | 14 |
| Remote control | 8 | 10 | 12 |
| Radio-surgical arch | 8 | 10 | 12 |
| Mammogram | 8 | 9 | 10 |
| Ultrasound machine | 7 | 8 | 9 |
| Computed Tomography | 8 | 10 | 12 |
| Magnetic resonance device | 8 | 10 | 12 |
| Angiograph | 8 | 10 | 12 |
Although life expectancies are essential when planning the needs for the renewal of technology, this should not be the only criterion for replacing a piece of equipment, but should be used in conjunction with other factors that may advise moving forward with the renewal, such as technological obsolescence, lack of safety, improvement of productivity, cost of use, failure rate, maintenance problems, availability of spare parts, etc. An example is the tomographs for paediatric patients, in which the lower radiation dose of the new equipment advises to move forward their renewal.
- •
The equipment must have adequate maintenance. The maintenance of the equipment has a decisive influence on its life expectancy. Non-existent or incorrect maintenance reduces the useful life, but can also affect the reliability and safe use. It is essential to ensure, throughout the life cycle, a complete maintenance that preserves the functionality of the equipment in its original specifications.
- •
Assessing the need to perform updates during the life of the equipment. Inside a piece of equipment, the computer components (hardware and software) have a shorter life cycle than the rest of the equipment (around 5 years). Updates are often recommended by replacing these components or incorporating new ones that can add value by improving patient safety or examination quality.
- •
It is necessary to plan the renewal needs in the medium term. Once the replacement criteria have been defined, it is convenient to establish a formal process for planning the renewal of the DI equipment, which defines the equipment, technological band and necessary financial resources. In order to avoid improvisation, prioritise objectively the most necessary investments and improve efficiency in the use of resources, in all the references, special emphasis is placed on the necessary medium-term planning; the most common is to plan for 5 years.
- •
At present, for the replacement of some types of equipment, it is necessary to consider whether it is more appropriate to acquire a new one as opposed to the major update (which replaces a relevant part of the equipment), which can be perfectly technically valid and more economical (e.g. update of the hardware, software and firmware of an MRI device maintaining the magnet).
- •
Maintaining the diagnostic reliability and safe use are essential premises. Pieces of equipment that have exceeded their life cycle should follow, until their renewal, an exhaustive control process that more frequently monitors and regulates compliance with their functionality and calibration through appropriate preventive maintenance and certification of functionality and safety by a qualified and accredited technical service.
Although some of the recommendations make it necessary to recover at least the level of investment and replacement prior to the economic crisis, many of them are organisational and refer to the need to develop a comprehensive, objective and transparent DI equipment management process.
Authorship- 1.
Responsible for the integrity of the study: MATG and ILP.
- 2.
Study conception: MATG and ILP.
- 3.
Study design: MATG and ILP.
- 4.
Data collection: MATG and ILP.
- 5.
Analysis and interpretation of data: MATG and ILP.
- 6.
Statistical processing: N/A
- 7.
Literature search: MATG and ILP.
- 8.
Drafting of the paper: MATG and ILP.
- 9.
Critical review of the manuscript with intellectually relevant contributions: MATG and ILP.
- 10.
Approval of the final version: MATG and ILP.
The authors declare that they have no conflicts of interest.
I. López Parrilla is currently the director of institutional relations at Philips Ibérica.
Please cite this article as: Trapero García MA, López Parrilla I. Guía de la SERAM para la renovación y actualización tecnológica en radiología. Radiología. 2019;61:35–41.