This article reviews the Ovarian-Adnexal Reporting Data System Magnetic Resonance Imaging (O-RADS MRI) score for the characterization of indeterminate ovarian masses. We aim to provide sufficient information to enable readers to apply the score efficiently in clinical practice.
To this end, we review the indications of the O-RADS MRI score and the specific MRI protocol that must be applied. We review all the categories of the score, illustrating them through examples. Finally, we show the most common errors and pitfalls during the learning curve, providing the keys to avoiding them.
El propósito de este artículo es revisar la clasificación Ovarian-Adnexal Reporting Data System Magnetic Resonance Imaging (O-RADS RM) para caracterización de masas ováricas indeterminadas. Nuestro objetivo es que al final de la lectura de este artículo el lector tenga un conocimiento suficiente de dicha clasificación para poderla aplicar en la práctica clínica de forma eficiente.
Para ello, revisaremos las indicaciones de la clasificación O-RADS RM y el protocolo de RM específico que debe realizarse. Repasaremos todas las categorías de la clasificación aportando ejemplos ilustrativos. Por último, mostraremos los errores más frecuentes que aparecen durante la curva de aprendizaje y daremos las claves para subsanarlos.
The incidental finding of an ovarian mass is a common clinical problem and up to 30% are indeterminate by transvaginal ultrasound. Percutaneous biopsy, however, is contraindicated due to the risk of dissemination and poor diagnostic performance1. The result is that a significant number of patients who have benign adnexal masses but which are indeterminate by ultrasound, undergo potentially unnecessary aggressive surgery, leading to increased loss of fertility and higher morbidity and mortality. At the same time, women with malignant adnexal masses, also indeterminate on ultrasound, end up undergoing conservative, non-oncological surgery, with poorer outcomes in terms of disease progression, decreased survival times and the need for further surgery2. Moreover, 30% of the ovarian masses supposedly indeterminate on ultrasound correspond to non-ovarian pelvic disease, mainly subserosal fibroids.
Magnetic resonance imaging (MRI) plays a crucial role in the accurate characterisation and stratification of the preoperative risk of malignancy of indeterminate adnexal masses3. In 2013, Thomassin-Naggara et al. proposed the Ovarian-Adnexal Reporting and Data System Magnetic Resonance Imaging (O-RADS MRI) classification, a scoring system similar to the other RADS systems of the American College of Radiology (ACR), which aims to ensure correct risk stratification of the likelihood of malignancy of ovarian masses so they can receive the best possible management2. This risk stratification system was validated in a multicentre study in 20201.
The O-RADS MRI score divides ovarian masses into five different categories according to the risk of preoperative malignancy, and has recently been updated in consensus with the ACR4,5.
- -
SCORE 1 corresponds to normal ovarian cysts (follicles) and masses of extra-ovarian origin.
- -
SCORES 2−3 correspond to benign ovarian masses, with a negative predictive value for malignancy of 98%; these lesions can be safely operated on in non-cancer centres using minimally invasive surgery.
- -
SCORES 4–5 correspond to borderline or malignant tumours, with a sensitivity and specificity of 93%, and should be referred to a tertiary hospital for regulated oncological surgery.
In this article, we review the latest update of the O-RADS MRI risk stratification system so that after reading it the reader will have sufficient knowledge of the scoring system to be able to apply it efficiently in clinical practice.
MRI protocol necessary to correctly apply the O-RADS-MRI risk stratification systemThe MRI protocol for the characterisation of ovarian masses with the O-RADS MRI score requires a pelvic MRI to be performed with the sequences specified below5:
- -
Axial and sagittal/coronal T2-weighted images with recommended slice thickness of 4 mm.
- -
Axial T1-weighted images with recommended slice thickness of 3 mm.
- -
Axial T1-weighted fat-saturated (FS) images with recommended slice thickness of 4 mm.
- -
Diffusion with b1000 and ADC map with recommended slice thickness of 4 mm.
- -
Dynamic contrast-enhanced sequence with recommended slice thickness of 3 mm.
- -
Post-contrast T1-weighted FS images with recommended slice thickness of 3 mm.
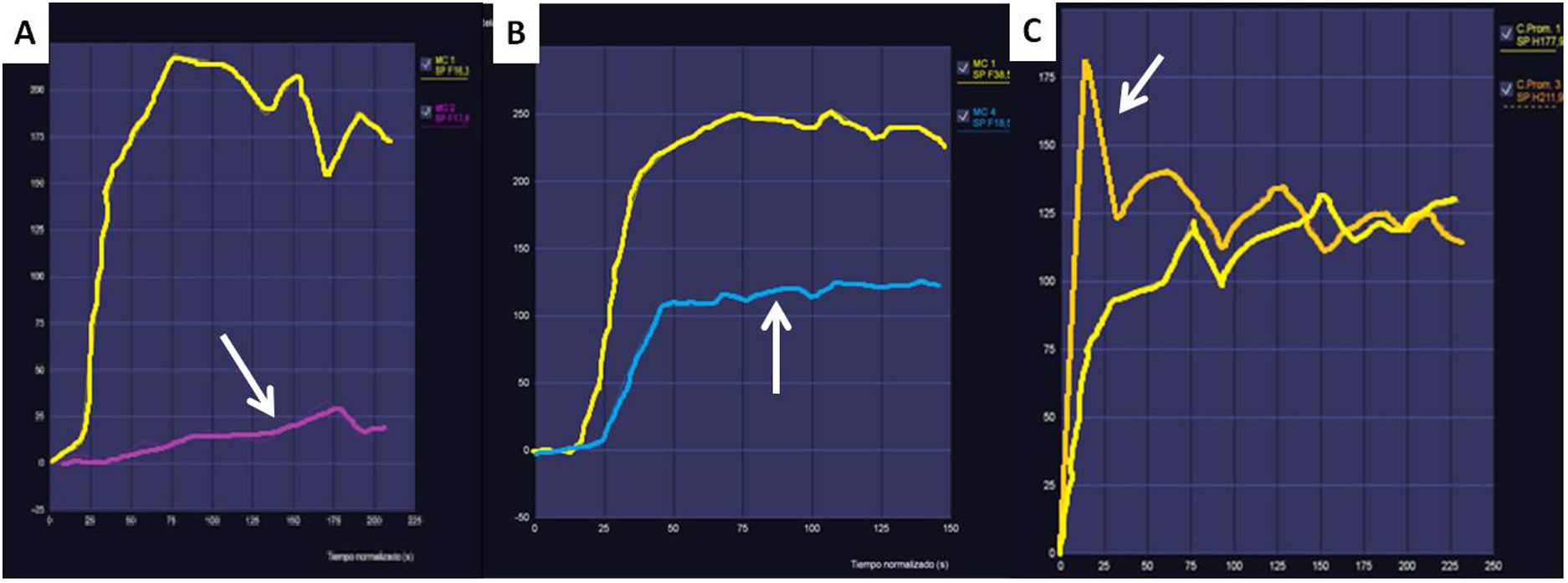
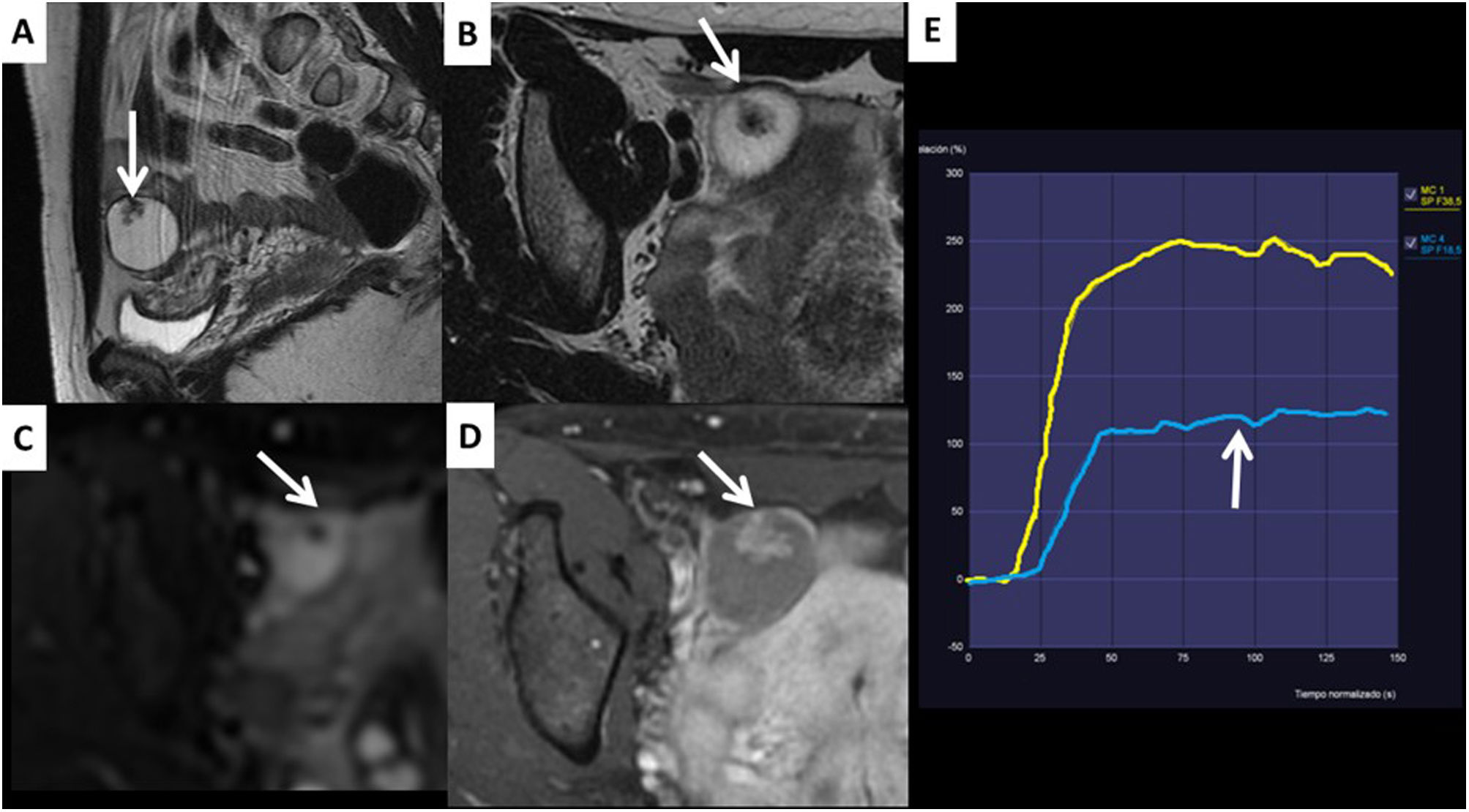
Apart from assessing the behaviour of ovarian masses in high-resolution anatomical and diffusion sequences, the O-RADS MRI system uses curves plotted from dynamic contrast-enhanced studies which assess time-intensity curves (TIC). With these curves, the behaviour of the contrast uptake by the solid tissue in the ovarian mass is compared with the uptake by the myometrium, yielding three types of curves which help us to stratify the risk of malignancy. Fig. 1 shows the three types of TIC and how they correlate with benign and malignant ovarian masses.
Examples of time-intensity curves (TIC) used in the O-RADS MRI score. A) The type 1 or low-risk curve shows a gradual increase in the curve without initial acceleration (arrow). It is associated with a low risk of malignancy. These masses do not require oncology surgery. B) The type 2 or intermediate-risk curve (arrow) shows an initial acceleration less than that of the myometrium (yellow curve in the 3 graphs). It is typical of borderline ovarian masses, which for practical purposes should be operated on in cancer hospitals due to their uncertain malignant potential. C) The type 3 or high-risk curve (arrow) shows an initial acceleration equal to or greater than that of the myometrium. This curve is characteristic of malignant ovarian masses.
The dynamic study with gadolinium is performed using fat saturated T1-weighted sequences and adding subtraction images, with a temporal resolution of less than 15 s and an ideal thickness of 3 mm, with the study starting 30 s before contrast injection and continuing for 4 min after. In the case of a large mass, it is better to increase the slice thickness and the field-of-view of the dynamic sequence with contrast, even temporarily spacing the acquisitions, if necessary, in order to be able to analyse the ovarian mass in its entirety and not omit solid parts from the study.
To create the TIC, a region of interest (ROI) is placed in the external part of the myometrium, choosing an area that has homogeneous uptake, avoiding fibroids, adenomyosis or other abnormal areas of the myometrium. The second ROI is placed in the area that is shown to have contrast enhancement on the subtracted T1 FS images.
Subtraction images should always be obtained in the dynamic study with contrast. They will enable us to correctly differentiate tissues hyperintense on T1, such as blood, from areas of uptake, helping to accurately locate the ROI of the TIC in the truly solid areas of the lesion.
If uptake curves cannot be created, the latest update of the O-RADS MRI risk stratification proposes comparing contrast uptake in the myometrium with that in the solid tissue of the masses 30–40 s after injection, although this is difficult to do in practice. A recent article shows the greater utility of contrast curves (TIC), so this criterion is not applied in our centre6.
Aspects to consider before applying the O-RADS MRI scoreThe O-RADS MRI risk stratification system should not be applied when a patient with an ovarian mass has acute, recurrent pelvic pain or symptoms of infection. Ovarian masses with associated pelvic pain are very often twisted; the presence of necrosis in these cases would make it difficult to create the contrast curves properly. Pyosalpinxes associated with pelvic inflammatory disease can simulate malignant ovarian masses by showing contrast uptake with high-risk curves and marked diffusion restriction due to the presence of pus7,8.
Some masses have sufficiently specific radiological characteristics to be diagnosed without the need for O-RADS MRI classification; in these cases, pathognomonic radiological findings prevail, as occurs, for example, in endometriomas, dermoid cysts, ovarian oedema, ovarian fibromatosis, granulosa cell tumours and cystadenofibromas9,10.
Table 1 shows the checklist that serves as a guide for applying the O-RADS MRI scoring system. There is also an "app" available on the O-RADS MRI page of the ACR website which can be of help for applying the system effectively11.
O-RADS MRI checklist.
| 1. Can we see any pelvic mass on MRI? |
| – No, end |
| – Yes, continue |
| 2. Is the mass ovarian or extra-ovarian? |
| – It is extra-ovarian, it corresponds to an O-RADS 1 lesion, end |
| – It is ovarian, continue |
| 3. Does the patient have pelvic pain or fever? |
| – Yes, suspect ovarian torsion mass or pelvic inflammatory disease, end |
| – No, continue |
| 4. Does the ovarian mass correspond to a physiological structure? |
| – Yes, it corresponds to an O-RADS 1 lesion, end |
| – No, continue |
| 5. Does the ovarian mass have associated peritoneal carcinomatosis? |
| – Yes, O-RADS 5 |
| – No, continue |
| 6. Does the ovarian mass have a radiological image which allows a specific diagnosis? |
| – Yes, O-RADS characterisation is not necessary |
| – No, continue |
| 7. Does it have solid part? |
| – No, it will correspond to an O-RADS 2−3 lesion, see classification in Fig. 2 |
| – Yes, continue |
| 8. ¿Does it have fat? |
| – Yes, if it has a Rokitansky nodule it corresponds to an O-RADS 2 lesion, any other type of solid tissue associated with a mass with fat is O-RADS 4 |
| – No, continue |
| 9. Is it hypointense on T2 and hypointense on b1000? |
| – Yes, it is a fibroma, O-RADS 2, end |
| – No, continue |
| 10. Is it a lesion with a low-risk curve? |
| – Yes, O-RADS 3, end |
| – No, continue |
| 11. Is the ovarian mass suspected of being of mucinous lineage? |
| – Yes, suspect metastasis, look for primary, operate at third level hospital |
| – No, continue |
| 12. Does the ovarian mass have specific borderline tumour morphology? |
| – Yes, always consider O-RADS 4 even if the solid part has a low-risk curve |
| – No, continue |
| 13. Does the ovarian mass have an intermediate-risk uptake curve? |
| – Yes, O-RADS 4 |
| – No, continue |
| 14. Does the ovarian mass have a high-risk uptake curve? |
| – Yes, O-RADS 5 |
| – No, if the mass does not meet any of the above requirements, perform only a radiological description of the lesion |
Lastly, it is very important that we all speak the same radiological language when describing ovarian masses. It is therefore absolutely essential to be conversant with the terminology (lexicon) of MRI findings in ovarian masses before starting to work with the O-RADS system. We recommend reading the ovarian mass lexicon articles available in the literature and which we have cited here5,12.
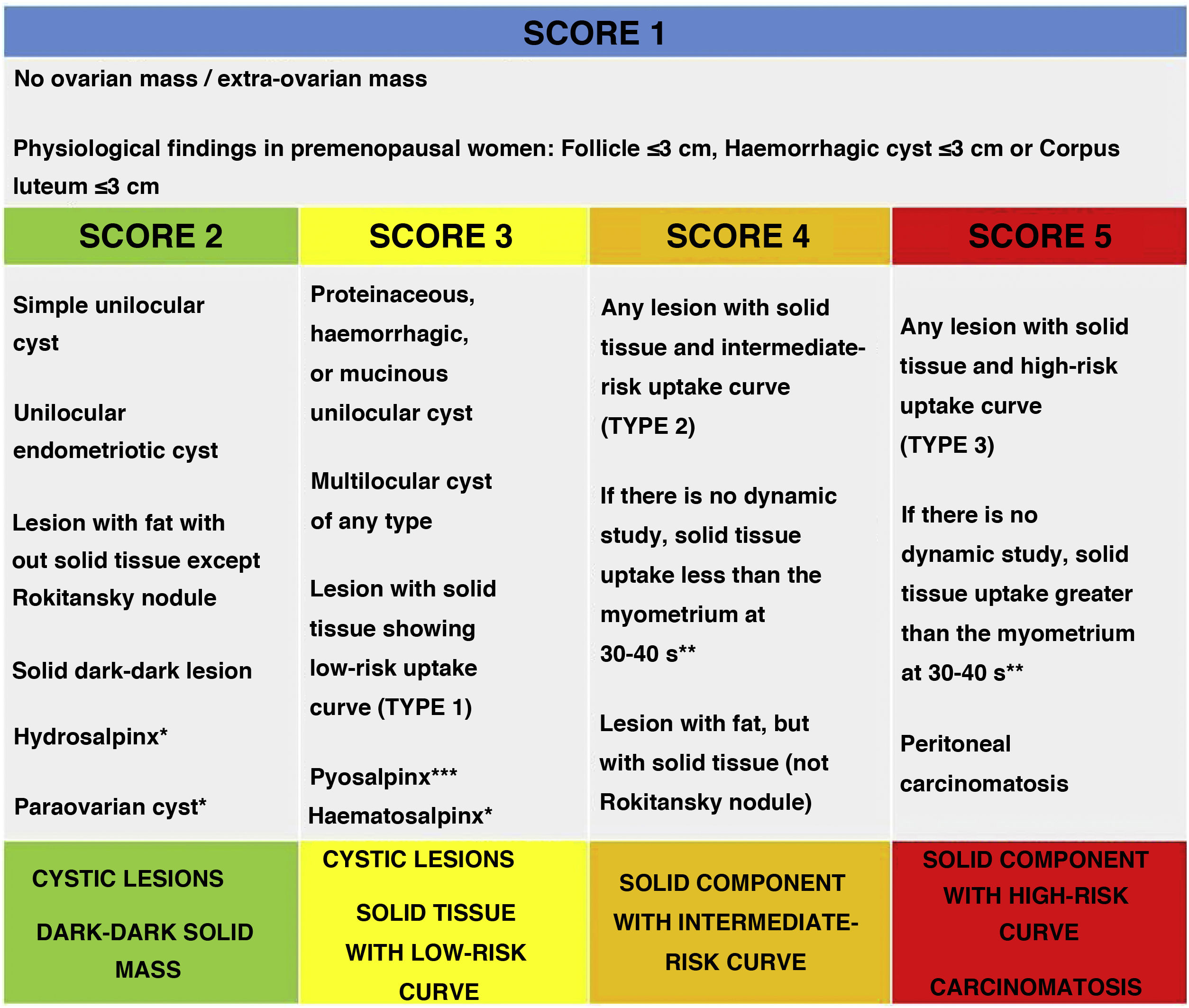
O-RADS MRI risk stratification systemThe O-RADS MRI risk stratification system, including the consensus modifications with the ACR, can be consulted in the article by Sadowsky et al.5. A summary of the characteristics of the update, which we describe in detail below, is provided in Fig. 2.
O-RADS MRI score according to the ACR update.
* Hydrosalpinx, paraovarian cyst and haematosalpinx can be considered O-RADS 1 lesions as they are not ovarian masses.
** Theoretical statement without studies to support it hitherto.
*** O-RADS should not be applied when pelvic inflammatory disease is suspected.
Source: Sadowski et al.5
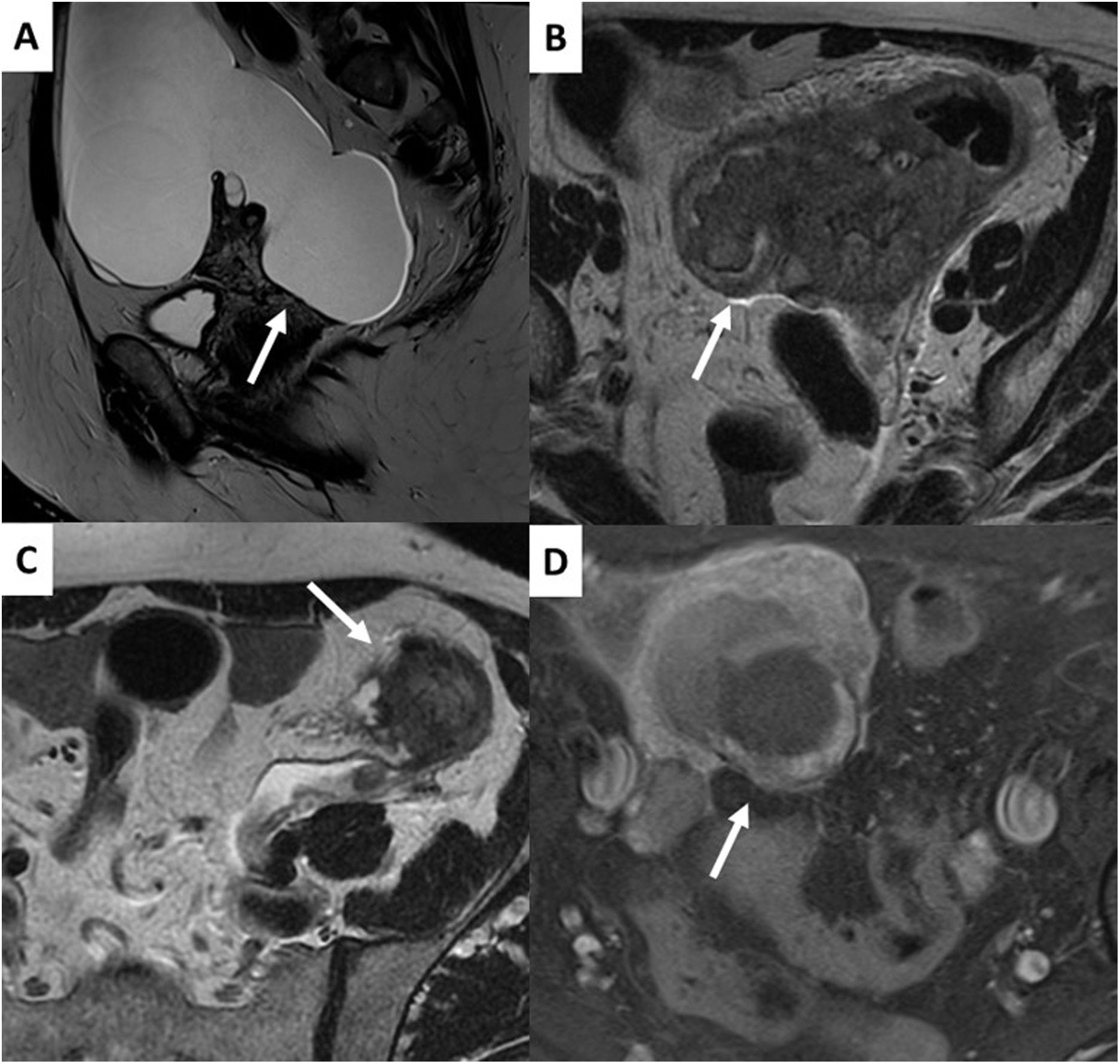
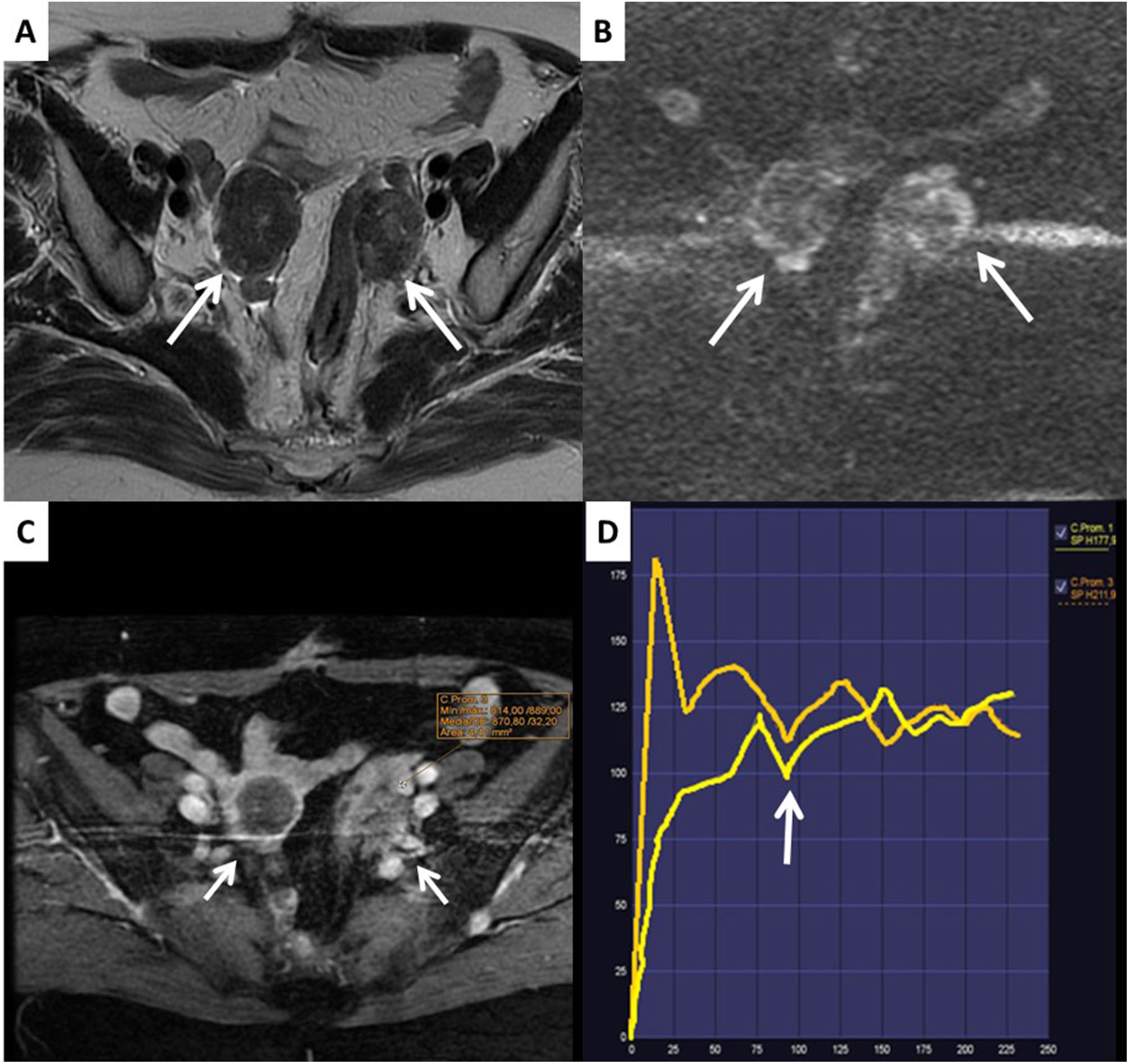
Score 1 lesions correspond to normal ovarian cysts (follicles or corpora lutea) and masses which are not of ovarian origin (Fig. 3).
It must be borne in mind that the corpus luteum can have thick, irregular walls and septa which show contrast uptake, restricted diffusion and FDG uptake on PET imaging and should not be confused with an ovarian mass.
It is important to know that healthy ovarian parenchyma shows restricted diffusion and contrast uptake with high-risk TIC curves and that healthy ovarian tissue can be mistakenly interpreted as corresponding to solid tissue from an adjacent ovarian mass. The fact that the ovaries restrict diffusion can be of great help in ruling out the ovarian origin of a pelvic mass. Other radiological signs that help us to classify pelvic masses as ovarian or non-ovarian are by following the ovarian veins or the round ligaments, as well as their intra- or extraperitoneal location or their position with respect to the distal ureters.
The pelvic masses confounded with ovarian masses on ultrasound are mostly subserosal myomas, easily characterised by MRI if the two ovaries are viewed separately. Fibroids have characteristic signs, such as a marked hypointense signal on T2-weighted images or so-called “bridging vessels” going to the fibroid from the uterus, which are a pathognomonic sign of the myometrial origin of the mass being studied.
O-RADS MRI score 2−3Score 2−3 lesions correspond to benign masses which can be operated on by non-oncology gynaecologists in non-specialised hospitals.
Score 2 lesions are mostly serous or haemorrhagic cysts and fatty cysts. The even uptake in the wall is not considered to correspond to solid tissue in the ovarian cyst in any of these three cases. In the case of a unilocular haemorrhagic cystic lesion showing hypointense areas in its interior on T2-weighted images, which correspond to blood products accumulating over time (“shading sign”), the diagnosis will be endometrioma.
The only ovarian masses with solid content considered to be Score 2 are those which are homogeneously hypointense on T2 and hypointense on the b1000 diffusion images, known as "dark-dark" lesions; these signs are pathognomonic for ovarian fibromas. Although not necessary for diagnosis, fibromas usually have a low-risk curve (Fig. 4).
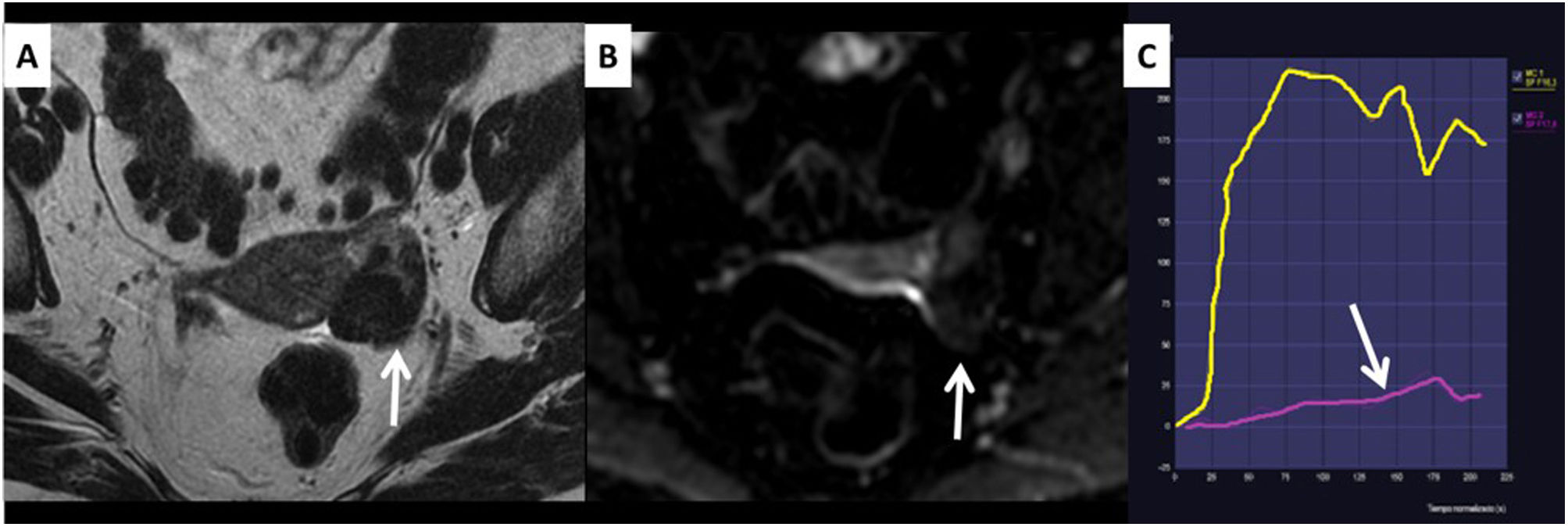
Ovarian fibroma with dark-dark characteristics, O-RADS MRI Score 2. The left ovarian lesion is hypointense on T2 (dark) and does not restrict diffusion on b1000 (dark), arrows in images A and B. Fibromas are the lesions that cause most diagnostic problems by ultrasound and for which the O-RADS MRI score will be most useful. C) This fibroma also has a low-risk curve (arrow).
It should be noted that dermoid cysts/teratomas may contain solid tissue, but Score 2 lesions will only be considered if this solid tissue is associated with the Rokitansky nodule, which is easily identifiable by the presence of hair and calcifications, including in the form of teeth, in its interior. If the solid tissue associated with the fat mass does not correspond to a Rokitansky nodule, Score 4 lesions will be considered, regardless of the uptake curve. In such cases, due to the possibility of an immature teratoma or other malignant form of ovarian tumour with fat, it is better to operate in a cancer centre.
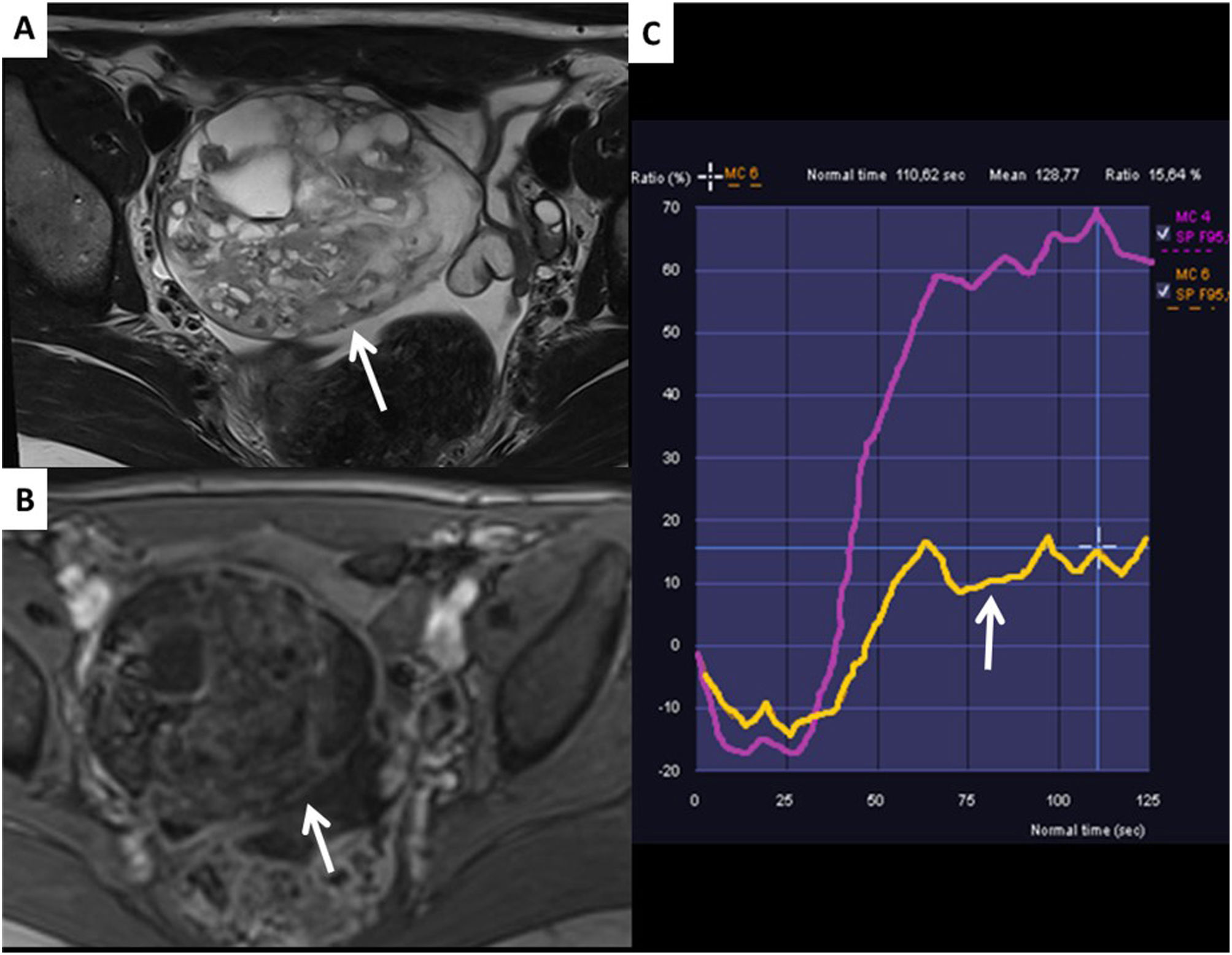
When the lesions are multicystic, heterogeneous and without solid tissue, they will be considered to be Score 3 lesions. Subtraction images of the dynamic sequences with contrast are very useful in masses of this type to differentiate true contrast enhancement from hyperintense areas in non-enhancing T1-weighted sequences. It is important to stress that smooth septa, as well as smooth walls, are not considered to be solid tissue. If readers would like to take a more in-depth look at O-RADS MRI Score 2−3 cystic lesions, we recommend the article by Assouline et al13.
The O-RADS Score 3 group also includes ovarian masses with a solid component which have a low-risk uptake curve. These masses are usually cystadenofibromas, in which the solid area shows very characteristic hypointensity (Fig. 5).
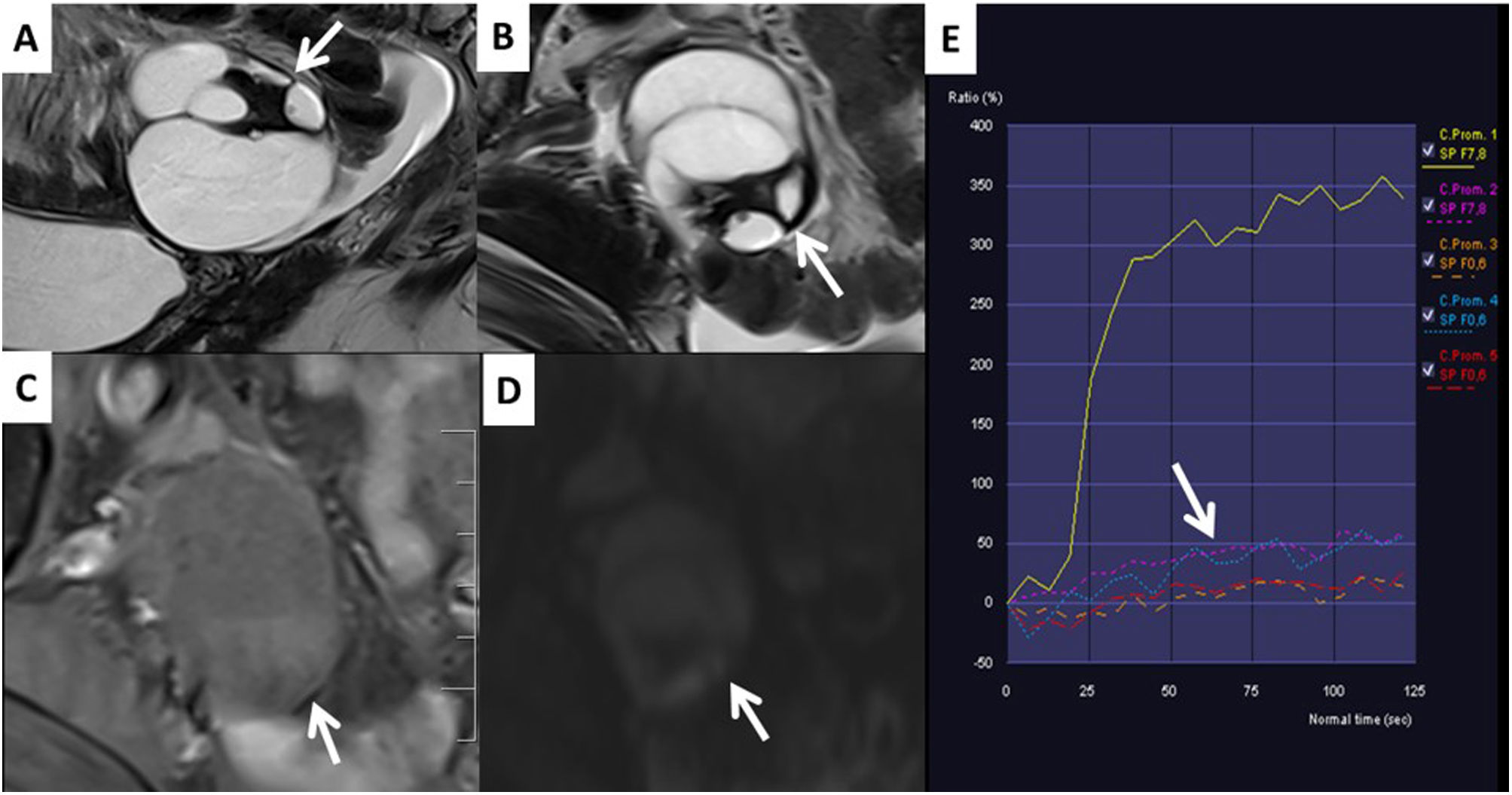
Cystadenofibroma. A) T2 sagittal. B) T2 axial. C) Post-contrast T1 FS. D) b1000. E) Low-risk curve. Right ovarian solid cystic lesion (arrows) with hypointense solid tissue on T2, which does not restrict diffusion and shows a low-risk contrast uptake curve (arrows). It corresponds to an O-RADS SCORE 3 lesion and is pathognomonic of cystadenofibroma.
Borderline tumours may also have low-risk curves, but the characteristic morphology of their solid part enables us to make the distinction correctly. We recommend becoming familiar with the typical findings of borderline tumours by reading the articles cited below14,15 (Fig. 6).
Borderline tumour with low-risk curve. A) Post-contrast T1 FS. B) T2 sagittal. C) Low-risk curve (arrow). The images show bilateral ovarian masses with minimal contrast uptake which have tree-like morphology and papillary mural growths (arrows) typical of borderline tumours. A reminder that it is better to operate on borderline tumours in cancer centres due to uncertainty as to whether or not they are malignant.
Finally, it must be taken into account that some borderline or malignant mucinous tumours have virtually no solid tissue and may be incorrectly classified as Score 3 lesions. The morphology of mucinous lesions is also very typical and whenever suspected, mucinous tumours of another origin (for example, colon, appendix) should be ruled out10. Additionally, it is not uncommon for mucinous tumours, even benign ones, to have small tears and mucinous fluid around them, and this has to be differentiated from peritoneal carcinomatosis. In general, when a mucinous tumour is suspected, with a characteristic image, the gynaecologist needs to be warned of this possibility (Fig. 7).
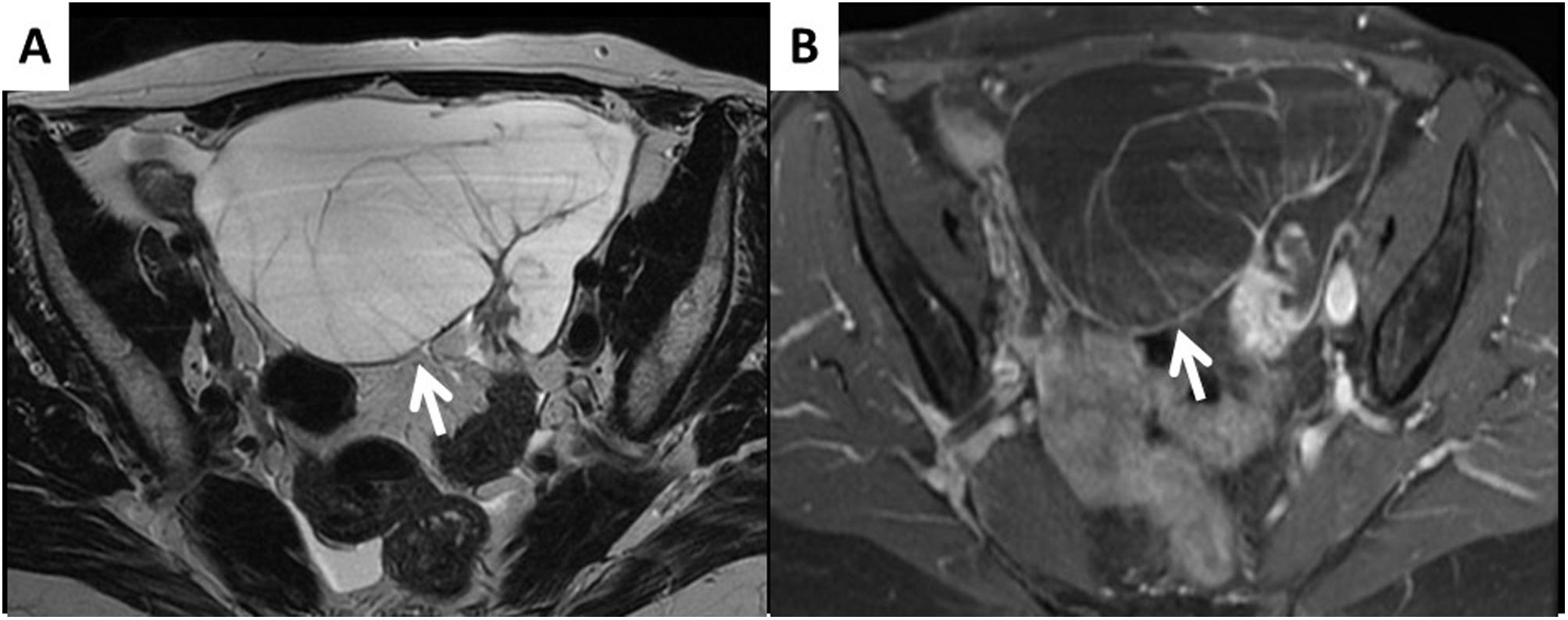
Appendiceal mucinous tumour metastasis. A) The T2-weighted image is very typical of mucinous tumours; they are multi-septate cystic masses with practically no solid component (arrow). B) The post-contrast T1 FS image shows only even uptake in the walls of the septa. With tumours of these characteristics, the oncologist should be warned that it may be a primary or metastatic malignant mucinous lesion.
O-RADS Score 4–5 ovarian lesions are essentially characterised by the fact that they contain solid tissue and this solid tissue does not have dark-dark characteristics or a low-risk curve, is not associated with a Rokitansky nodule and nor, after imaging, is it suspected of being a borderline tumour or a cystadenofibroma.
The O-RADS MRI score distinguishes between the different types of solid tissue according to their morphology to standardise the language used to describe ovarian masses. It makes the distinction between the following types of solid component: papillary projection; mural nodule; irregular septa; irregular wall; solid-cystic mass; and predominantly solid mass. Thin wall septa, clots, debris, fibrin, fat, hair, calcifications, and other typical elements of Rokitansky nodule are not considered to be solid tissue5.
If the mass has an intermediate-risk contrast curve in its solid component, it will be classified as O-RADS MRI Score 4 (Fig. 8), and if it has a high-risk contrast curve, it will be classified as O-RADS MRI Score 5 (Fig. 9). In both cases, the ovarian mass needs to be operated on in a cancer centre.
Bilateral ovarian metastases, intestinal primary. A) T2 axial. B) b1000. C) Post-contrast T1 FS. D) Type 3 uptake curve (arrow). When we see bilateral solid lesions which restrict diffusion and show a high-risk uptake curve, malignancy should be suspected even though they are hypointense on T1 (arrows).
It should be remembered that in borderline tumours with specific radiological characteristics and fatty tumours with a different morphology from the dermoid cyst, even if they have contrast uptake with a low-risk curve, it is better to consider them O-RADS MRI Score 4 and perform the surgery in a specialised oncology centre (Fig. 10).
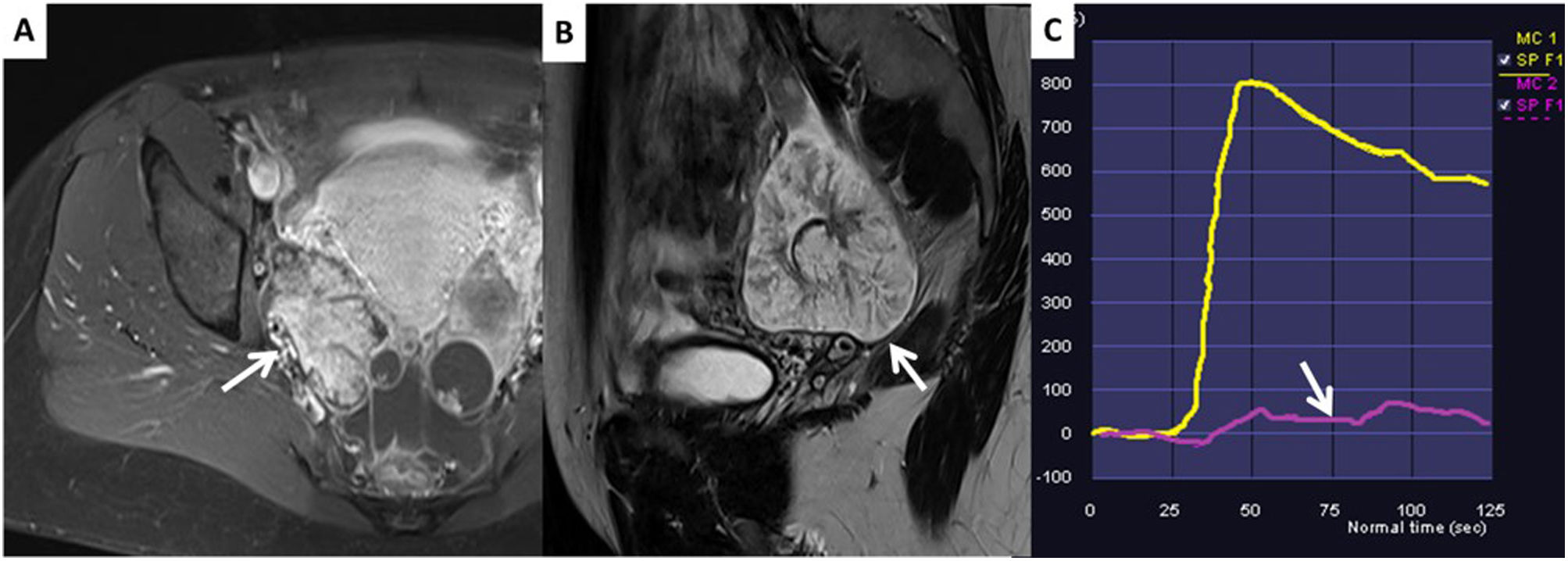
Immature teratoma. The images show a solid/cystic lesion with enhancing areas which have an intermediate risk curve (C) and hyperintense areas in T1 (no image) and T2 (A), which are suppressed in the post-contrast T1-weighted FS sequence (B), consistent with fatty tissue. It should be remembered that any solid lesion with fatty tissue which does not have the typical characteristics of the Rokitansky nodule should be considered malignant and operated on in a cancer centre with an intraoperative pathology consultation to rule out malignancy.
Solid lesions which are hypointense on T2 but with restricted diffusion and a high-risk curve should not be considered fibromas and should be suspected of being ovarian metastases, particularly if they are bilateral (see Fig. 9).
If peritoneal implants are detected in the ovarian mass characterisation MRI study, the ovarian mass will be considered malignant, regardless of its characteristics, i.e. an O-RADS MRI Score 5 lesion. The diffusion sequence is very useful in these cases for detecting and locating implants difficult to visualise in anatomical sequences.
ConclusionsThe O-RADS MRI risk stratification system is very useful for effectively classifying ovarian masses which are indeterminate by transvaginal ultrasound. In order to correctly use this scoring system, we need to adhere to a strict protocol and be familiar with the specific characteristics, the most common errors and the exceptions.
Authorship- 1
Responsible for study integrity: CS.
- 2
Study conception: CS, PF and MM.
- 3
Study design: N/A.
- 4
Data collection: N/A.
- 5
Data analysis and interpretation: N/A.
- 6
Statistical processing: N/A.
- 7
Literature search: LLC, CS and MM.
- 8
Drafting of the article: CS and LLC.
- 9
Critical review of the manuscript with intellectually relevant contributions: CN, PF and MM.
- 10
Approval of the final version: CS, LLC, CN, PF and MM.
The authors declare that they have no conflicts of interest.