To describe the technique of stereotactic body radiation therapy (SBRT) of lung lesions after the computed tomography (CT) guided placement of an internal fiducial marker and to assess the results, complications and secondary effects of these procedures.
Materials and methodsA series of 39 lesions (8 primary and 31 metastases) in 25 patients treated using this procedure were analyzed. A CT-guided percutaneous transthoracic puncture was performed for placing the internal marker in the lesion or near to it. The procedure did not require sedation.
The marker serves as a guide for the treatment of the lesion using SBRT with respiratory synchronism, which allows the movement of the tumor to be controlled and to decrease the radiation volume, giving high doses with precision to the tumor, and minimal to the surrounding healthy tissue.
ResultsThe only complication of the percutaneous fiducial placement was a pneumothorax in 6 (24%) patients. A pleural drain had to be placed in 3 patients. Local control was achieved in 96.7% of the lesions. The radiation produced a Grade 1 asthenia in 1 patient, a Grade 2 pneumonitis in one patient and a Grade 1 pneumonitis in the remainder.
ConclusionsThe CT-guided placement of internal markers in lung lesions is a safe technique that may be performed as ambulatory procedure. SBRT with respiratory synchronism allows the dose to the tumor to be increased, and reduces the volume of healthy lung treated, with few secondary effects.
Describir la técnica de radioterapia estereotáxica extracraneal (RTEE) de lesiones pulmonares tras colocar un marcador interno guiada por tomografía computarizada (TC) y valorar los resultados, complicaciones y efectos secundarios de estos procedimientos.
Material y métodoAnalizamos una serie de 39 lesiones en 25 pacientes (8 primarias y 31 metastásicas) tratadas mediante este procedimiento. Se realizó una punción percutánea transtorácica guiada por TC para la colocación de un marcador interno en la lesión o próximo a ella. El procedimiento no requiere sedación.
El marcador sirve de guía para el tratamiento de la lesión mediante RTEE con sincronismo respiratorio que permite controlar el movimiento del tumor y disminuir el volumen de irradiación administrando con precisión dosis altas al tumor y mínimas a los tejidos sanos circundantes.
ResultadosLa única complicación de las punciones transtorácicas fue el neumotórax en 6 pacientes (24%). Fue necesaria la colocación de un drenaje pleural en tres pacientes. Se consiguió el control local en el 96,7% de las lesiones. La irradiación produjo astenia grado 1 en un paciente, neumonitis grado 2 en un paciente y neumonitis grado 1 en el resto.
ConclusionesLa colocación guiada por TC de marcadores internos en las lesiones pulmonares es una técnica segura que se puede realizar de forma ambulante. La RTEE con sincronismo respiratorio permite aumentar la dosis al tumor y reducir el volumen de pulmón sano tratado con pocos efectos secundarios.
Surgery is the treatment of choice for early-stage lung cancer and it is described in the medical literature that partial lung resection to treat metastases prolongs survival and may result in healing in some patients with a limited number of metastasis.1 Some patients, due to associated comorbidities, fail to benefit from this treatment. Therapeutic options for these medically inoperable patients are limited and extracranial stereotactic radiation therapy (ESRT) comes up as an efficacious treatment alternative.
With the aim of increasing this technique's accuracy and safety, in our center we started ESRT by means of the “Exactrac Adaptive Gating” system of a Novalis (Brainlab) linear accelerator that allows, thanks to the use of internal markers, for tumor movement to be monitored during treatment and to be radiated in a concrete phase of the patient's respiratory cycle, thus damaging less pulmonary volume.
This study aims at describing the lung lesion ESRT technique after placement of a computed tomography (CT) guided internal marker and assessing the variation in size of these (primary and metastatic) lung lesions as response to the ESRT treatment and the complications and side effects of both the CT guided placement of internal markers and ESRT.
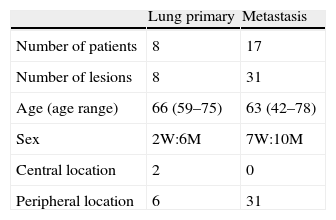
Materials and methodsIn this retrospective study from June 2008 to February 2010, 25 patients were included, of whom 17 presented pulmonary metastasis and 8 non-microcytic lung carcinomas. All the patients included signed a specific informed consent and the study was approved by our Hospital's Ethics Committee.
Our treatment protocol included the following stages.
Patient selectionOur center's lung cancer commission evaluated all the cases of lung lesion. The inclusion criteria appear in Table 1.
Inclusion criteria.
| – Patients with histologic diagnosis of non-microcytic lung carcinoma or extrapulmonary lung metastasis. |
| – Inoperable patients or rejection of surgery. |
| – Patients without node-positive injury. |
| – The patients with non-microcytic lung carcinoma had to comply with the M0 criterion. |
| – In the patients with metastasis, extrapulmonary disease (primary or metastatic) had to be potentially treatable. |
| – Central tumors must not present infiltration of major vessel or bronchi. |
| – Patients above 18 years of age. |
| – Number of lesions equal to or less than three. |
| – Lesion size equal to or less than 5cm. |
| – Lung function FEV1>40%. |
| – Karnofsky greater than or equal to 70. |
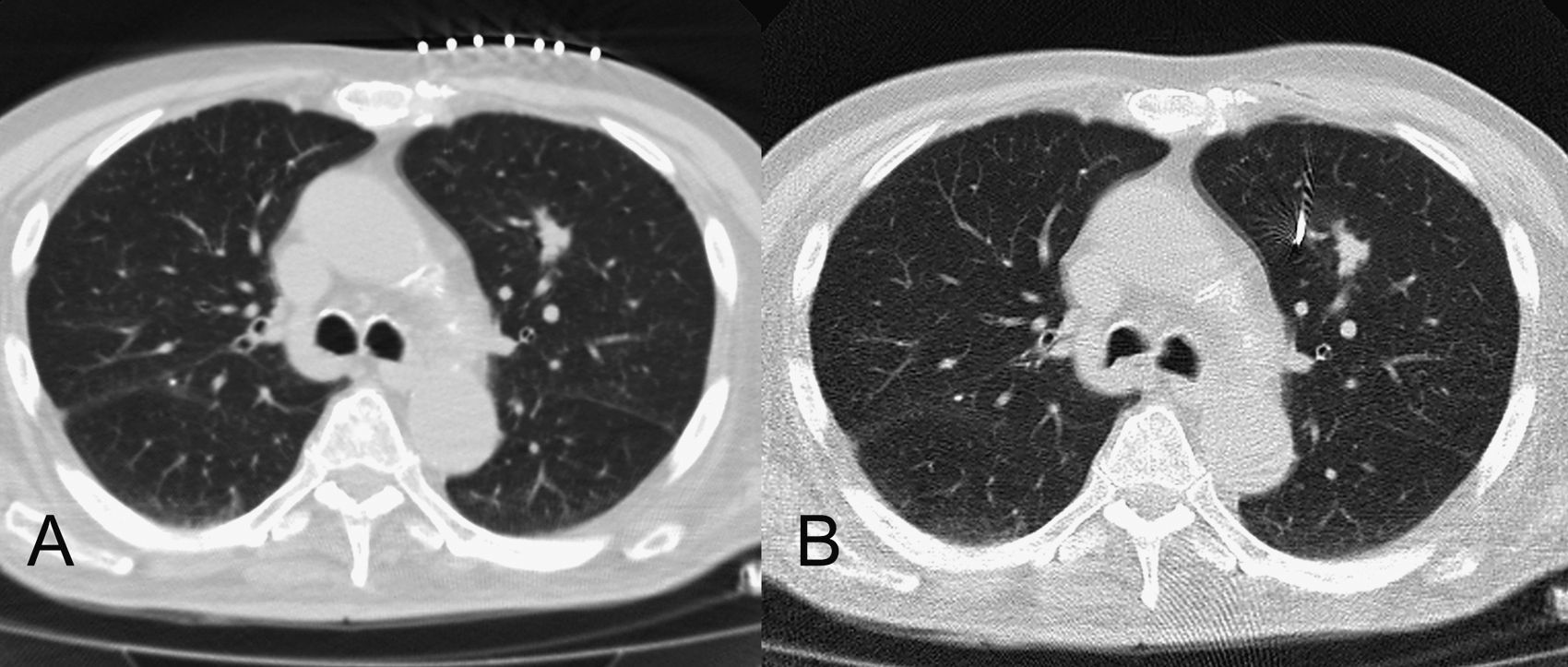
An internal marker was placed in all the patients so as to control the tumor's movement during radiation. These procedures, as well as the subsequent radiologic control of the lesions and complications, were performed by three radiologists with 20, 6 and 3 years of experience. The technique was carried out by means of a CT guided transthoracic tap in an Aquilion (Toshiba, 64 detectors), without scopy. After determining the most adequate trajectory to reach the lesion, an 18 G needle was introduced preloaded with an internal marker, a 30mm×0.75mm gold coil, (Visicoil®, Core Oncology, CA, USA) which was subsequently released in the lesion or near it (Fig. 1). The procedure did not require sedation, but local anesthesia was applied (1% lidocaine).
Axial plane CT images obtained during the placement of the internal marker. (A) A lung nodule is observed in the upper left lobe. The lesion is located and the needle's trajectory is determined by means of radiopaque markers on the patient's skin. (B) The internal marker has been located near the lesion.
Once the procedure was finished, a CT without contrast was performed limited to the lesion to determine the correct location of the marker and to detect possible immediate complications (pneumothorax, hemorrhage, marker migration). A thorax radiography was obtained 6h after the procedure to rule out the presence of delayed pneumothorax.
Treatment simulation and planningThe treatment simulation was performed in a CT Somaton Sensation (Siemens, 16 detectors). The patient's position during simulation was supine with a thorax immobilization system (inclined plane or Medtec vacuum mattress). Five points were tattooed on the patient's skin and a refringent sphere (detectable by the accelerator's infrared system) was placed on each. The simulation was performed the day after the tap if during marker placement a pneumothorax had not occurred. In the cases in which it did occur, the simulation was put off until it was completely resolved. The CT image acquisition was performed with suspended breathing.
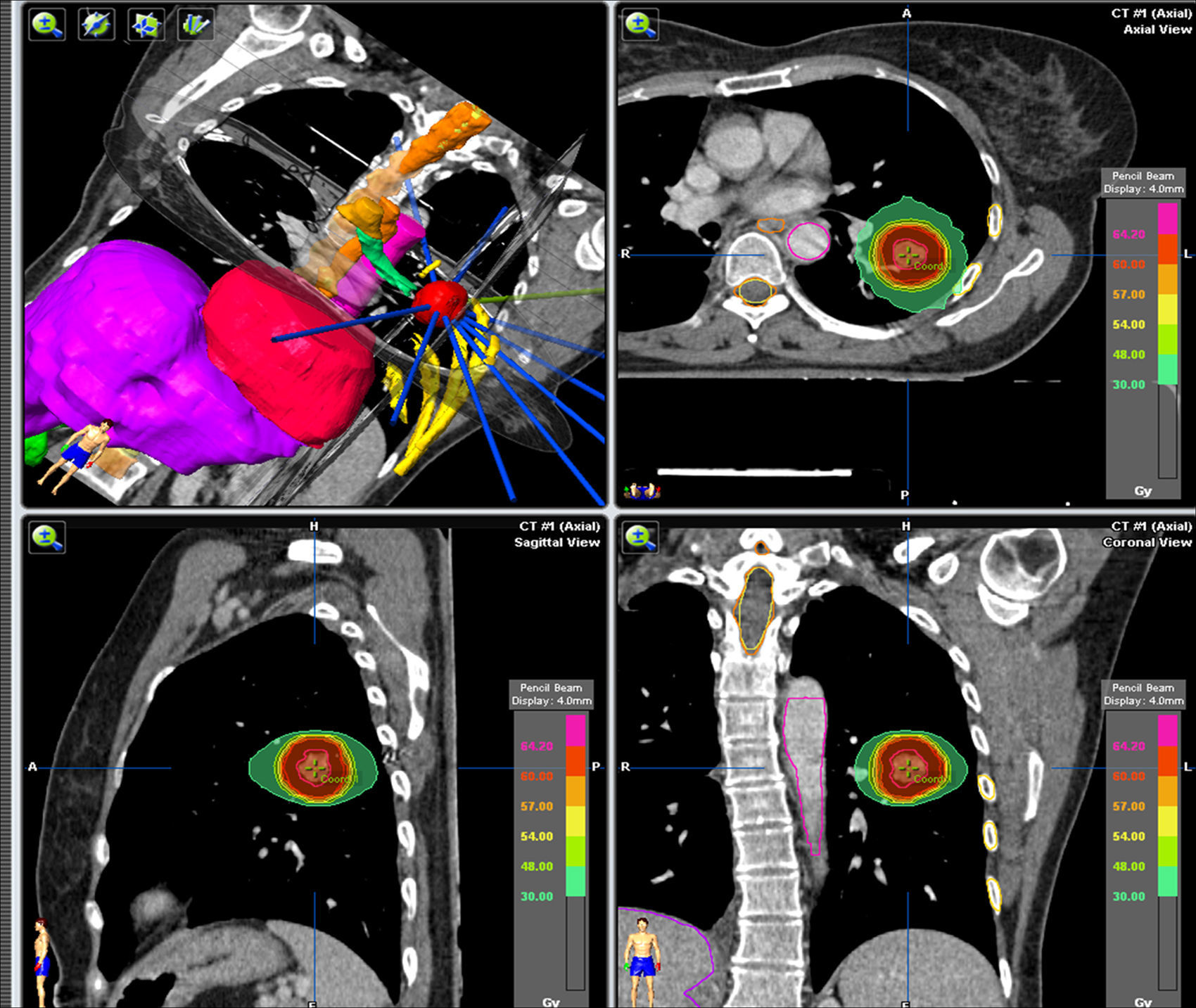
In order to plan the treatment, an iPlan 4.0.1 (BrainLab) planner was used. The planning volume (PV) consisted in the tumor being macroscopically visible in the CT lung window (1.500/−500 window/level) plus some 5mm expansion margins in all the planes (Fig. 2). The structures near the LV were outlined as critical organs and the limit doses admitted in these structures were: lung V20 (lung volume receiving 20Gy) 15%, and the maximum doses in the heart and the large vessels <30Gy, spinal marrow <18Gy, skin <21Gy and ribs <30Gy in 10cc.
The dose prescribed for the LV depended on the proximity of the lesion to the main air way and to the large vessels. The peripheral lesions (located at >2cm of the carina) received higher doses (10 fractions of 7.8Gy or 3 fractions of 15 or 20Gy). The central lesions received lower doses (5 fractions of 10Gy or 10 fractions of 5Gy).
The treatment planning was performed by means of 3D conformed radiation therapy using a single isocenter for each lesion with multiple coplanar fixed fields conformed with micromultilaminates and using a power of 6MV.
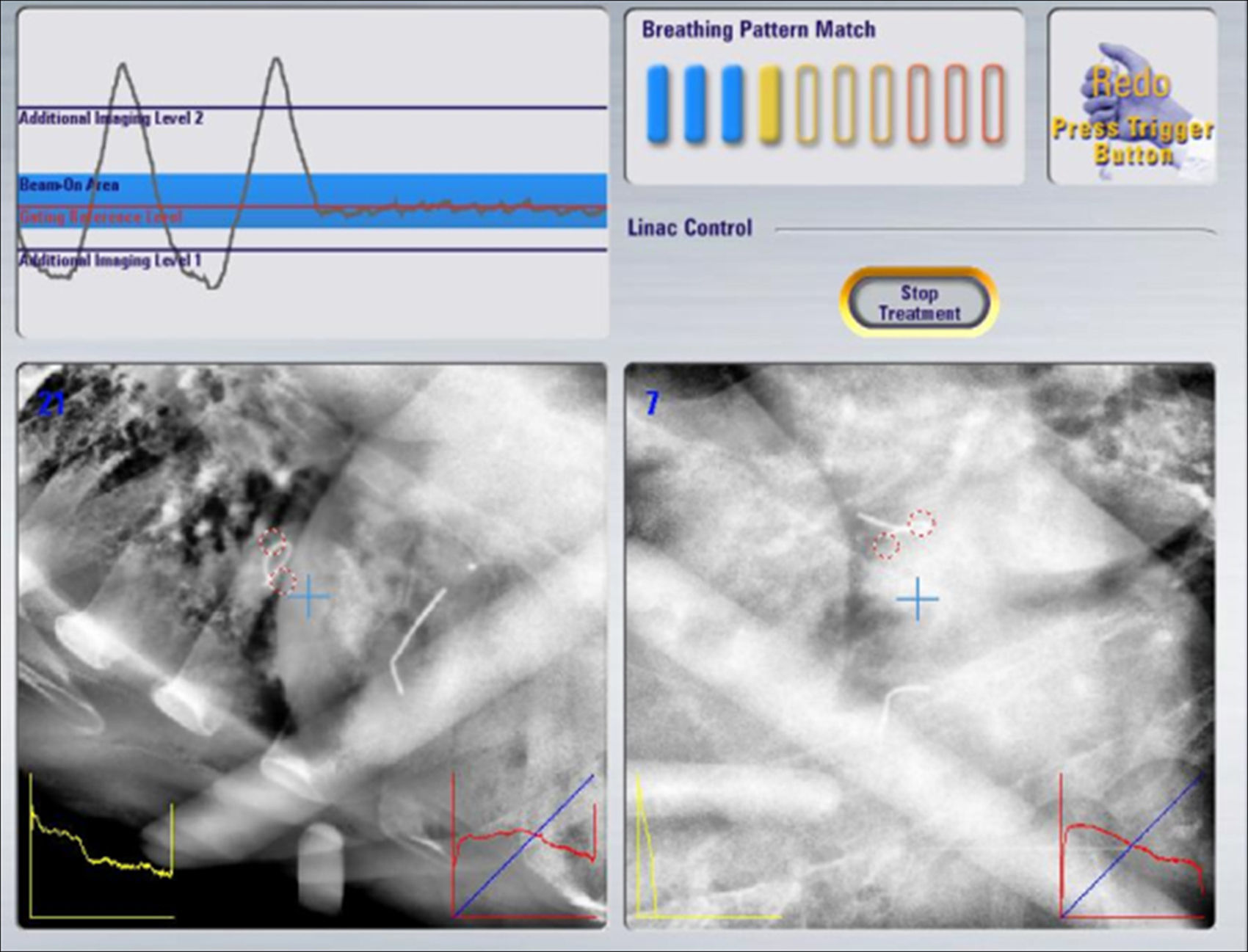
Treatment verification and administrationThe high precision verification system of the accelerator Novalis (Brainlab) called “Exactrac Adaptive Gating” was used. This system incorporates two X-ray tubes, an infrared system and a robotic table, and it allows for the tumor's movement to be controlled and radiated on a given stage of the respiratory cycle (Fig. 3).
Follow-upThe patients were evaluated clinically and analytically and by image every three months. The assessment of the response by image was carried out by means of a CT study with intravenous contrast measuring the maximum size of the lesions in the axial images with a lung window. A period of three months was established as the minimum necessary to evaluate the tumor's radiologic response to treatment. It was defined as (a) local control, the tumor's disappearance or decrease in volume greater than 30% in relation with the pre-treatment study; (b) local relapse, the tumor's growth when greater than 20% or an increase in size after an initial decrease, and (c) stability, the lesions that do not comply with the previous criteria.
The clinical assessment of tolerance and possible toxicities derived from the treatment was conducted in the Radiation Therapy Oncology Office (anamnesis, physical examination, hemogram and full biochemistry). Secondary toxicity to radiation therapy was classified according to the National Cancer Institute Common Criteria of Terminology for Adverse Event V4.0.2
Pneumonitis was classified into Grade 1 (asymptomatic), Grade 2 (symptomatic, medical treatment required), Grade 3 (serious symptoms limiting everyday activities and need for oxygen therapy), Grade 4 (life-threatening symptoms) and Grade 5 (death). Asthenia was classified into Grade 1 (mild), Grade 2 (moderate), Grade 3 (severe) and Grade 4 (hospitalization).
ResultsOur patients’ characteristics are included in Table 2. The patients diagnosed with lung cancer were on Stage I. The patients’ primary tumors with metastatic lesions were rectum carcinoma in 9, colon carcinoma in 6 and soft-tissue sarcomas in 2.
After the percutaneous placement of the markers pneumothorax occurred in 6 patients (24%). In three patients it was necessary to place a pleural drainage tube. They were all resolved in less than ten days and there were not any clinical repercussions in the patients. There occurred marker migration in one patient (it was expectorated).
All the patients completed the radiation therapy treatment without interruptions.
The patients’ median followup was 8.15 months (range: 1–19 months).
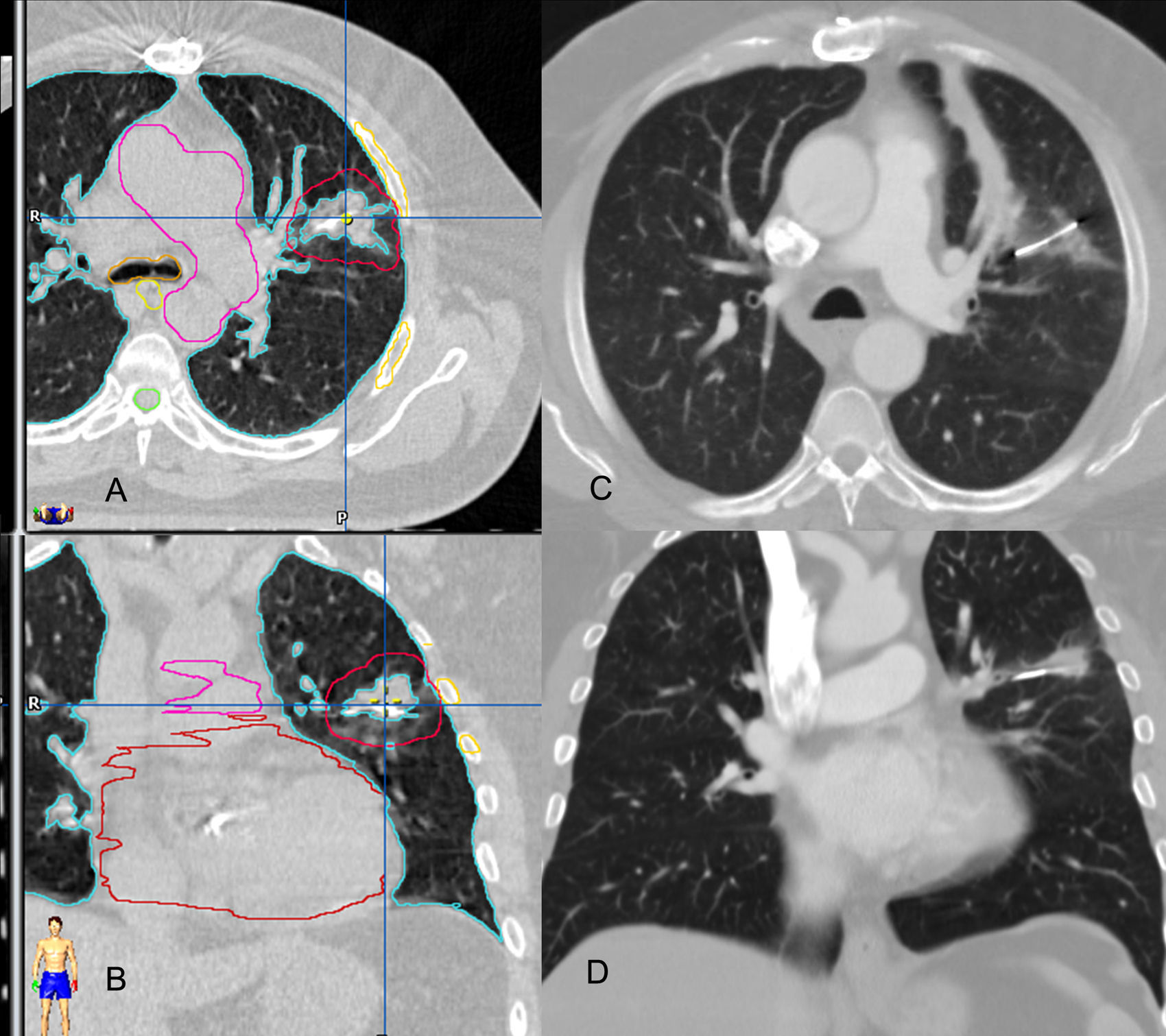
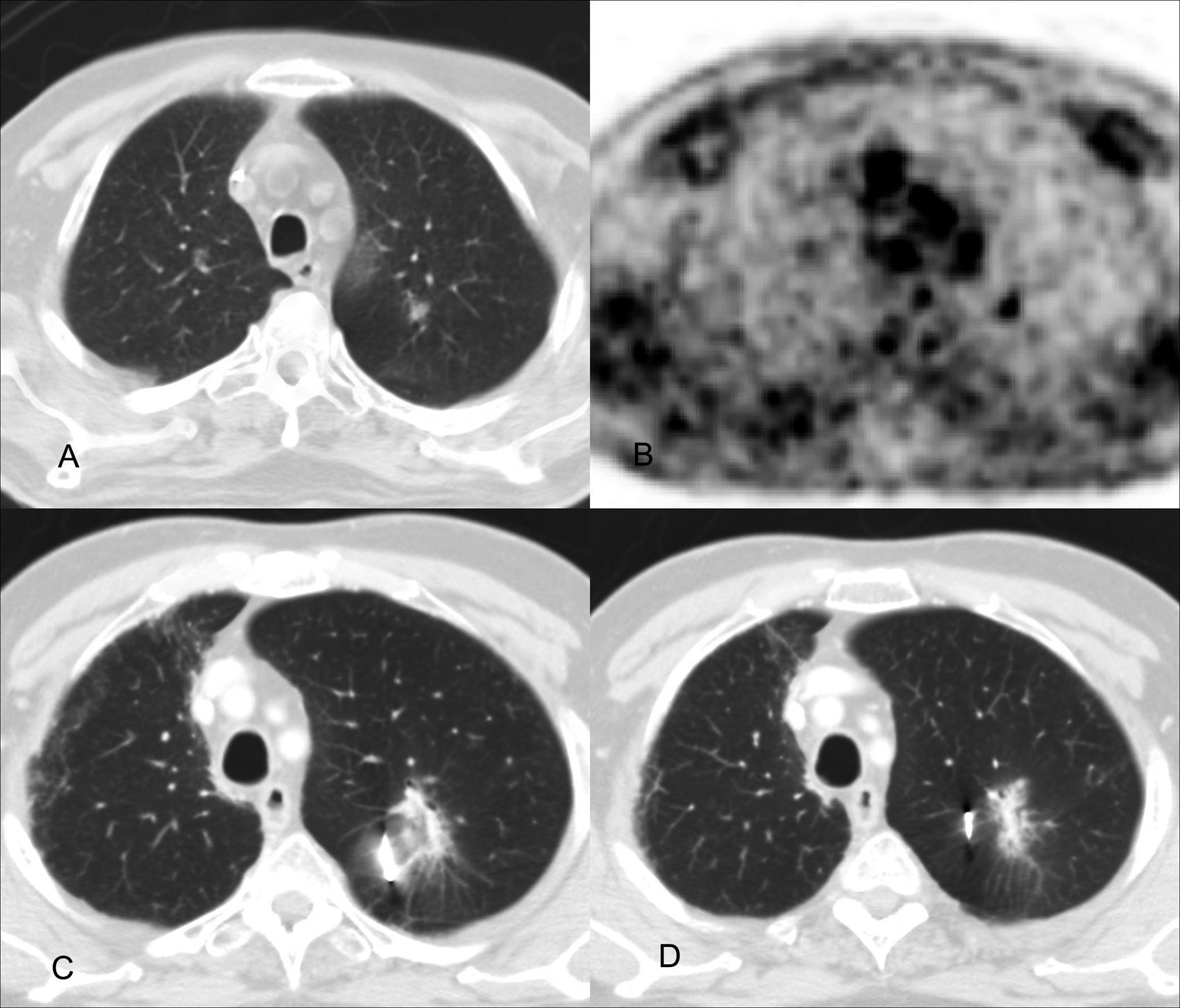
At the moment when the 39 lesions treated were analyzed, 31 were radiologically evaluable. The remaining 8 lesions could not be evaluated because followup was less than three months. Local control was confirmed in 30 lesions (96.7%) (Fig. 4) and local relapse in one lesion.
Local control: images of the planning study of the treatment on the axial (A) and coronal planes (B) and correlation with CT control on the axial (C) and coronal planes (D), three months after the end of the treatment. The placement of the internal marker in the lesion is observed. There is a significant decrease of the tumor's volume.
Acute toxicity due to radiation included a Grade 1 asthenia in one patient, Grade 2 pneumonitis in one patient, and Grade 1 pneumonitis in 24 patients (Fig. 5). In all the TC studies of the subsequent followup, radiologic findings were observed in relation with asymptomatic pneumonitis.
Pneumonitis: image of the PET-CT study at the diagnosis (A and B) and control stages at 6 months (C) and 12 months (D) after the end of treatment. A 1cm nodule is observed in the diagnosis in the upper left lobe (A) with an increase in 18FDG uptake in PET image (B). At 6 months of treatment, the internal marker is observed and there is an increase of attenuation of the parenchyma surrounding the nodule, with poorly delimited outline, with pneumonitis. At 12 months, the lung injury has diminished.
In our series, the local control of lung lesions (primary tumors and metastasis) has been excellent, with few complications during placement of internal markers and low toxicity rates of the ESRT treatment.
Our system for placing these markers is performed with CT guided transthoracic pulmonary tap. It has the advantage that the patient does not need to be sedated (just local anesthesia) and, if no complications arise, the patient is released in a few hours.
The only complication that aroused during the internal markers’ placement was pneumothorax (6 patients, 24%). Of these, three required placement of a pleural drainage tube. These complication rates are lower than the ones reported in the literature, which describe up to 45% of pneumothorax,3–6 similar to the diagnostic lung biopsies.7 This may be explained because our image verification system only needs one internal marker, while other systems need at least 4 markers.5
In our series there occurred only one marker migration, which lodged near the main bronchus and was expectorated by the patient. This migration rate is also lower than the one reported in medical bibliography (9.1–19%)4,6 and it may probably be explained by the fact that the marker we used is a coil which, when released, it gets stuck in the lung parenchyma, which makes its movement difficult.
The local control of lung lesions that we have attained is excellent (97.8%) and it corresponds with that described in the bibliography (88–98%).3,5,8–12 Respiratory synchronicity during radiation allows administering very high doses to the tumor with the certainty that toxicity will not increase in healthy tissues. ESRT treatment toxicity rates were low. Only one patient required medical treatment (corticoids) due to a Grade 2 pneumonitis, and the patient did not need hospitalization, another patient presented Grade 1 asthenia. The rest of the patients developed Grade 1 pneumonitis. These results correspond to those reported in the bibliography.10,12
Our study has several limitations. The number of patients included is small and we do not have a control group. Given the limited number of lesions we have neither considered inter- and intraobserver variability to measure the lesions nor the clinical evaluation. The followup time is also short (8.1 months as an average) and delayed toxicity cannot be evaluated. However, it must be considered that a treatment with results is being offered as an alternative to surgery in patients who, due to their comorbidity, cannot be operated on with optimal results in the local control of the disease. In patients with a limited number of lung metastasis, this local control of the disease may prolong survival.1
In sum, ESRT with respiratory synchronicity allows us to perform high precision treatments increasing the dose in the tumor and reducing the healthy lung volume treated, with few side effects. The placement of CT guided internal markers is a safe technique that may be performed ambulatorily. Taking into account the favorable results that are being published, that may be comparable to those of the surgical series, the objective of the present clinical trials in Stage II is to assess the efficacy of ESRT as an alternative to surgery in operable patients.13
Authors- 1.
People responsible for the study's integrity: MFP, CRR and MTS.
- 2.
Conception of the study: MFP, CRR, MTS and OHR.
- 3.
Design of the study: CRR and MTS.
- 4.
Data acquisition: MFP, CRR, OHR, MAK, ESS.
- 5.
Data analysis and interpretation: MFP, CRR, MTS, OHR, MAK, ESS and AMF.
- 6.
Statistical treatment: it is not necessary.
- 7.
Bibliographic search: MFP, OHR, MAK, ESS and AMF.
- 8.
Writing of the paper: MFP, CRR and MTS.
- 9.
Critical revision of the manuscript with relevant intellectual contributions: MFP, CRR, MTS, OHR, MAK, ESS and AMF.
- 10.
Approval of final version: MFP, CRR, MTS, OHR, MAK, ESS and AMF.
The authors declare that they have no conflict of interests.
Please cite this article as: Fernández-Velilla Peña M, et al. Tratamiento de lesiones pulmonares mediante radioterapia estereotáxica extracraneal tras colocación guiada por tomografía computarizada de marcador Visicoil®: experiencia inicial. Radiología. 2013;55:225–32.