Immunotherapy is a new treatment in advanced lung cancer that works by modulating the immune response against malignant cells. One aspect that is challenging for radiologists in the evaluation of the response to immunotherapy is the phenomenon of pseudoprogression, in which the infiltration of inflammatory cells causes lesions to increase in size or new lesions to appear and then decrease in size or disappear. Pseudoprogression actually represents a response to treatment. We aimed to determine the frequency of pseudoprogression in patients with advanced stages of lung cancer treated with nivolumab.
Patients and methodsWe included 56 patients with advanced stages of lung cancer treated with nivolumab as a second-line or later treatment. We analyzed CT studies done while patients were undergoing nivolumab treatment. Tumor pseudoprogression was defined as an increase in the size of lesions or appearance of new lesions followed by a decrease in size or disappearance of these lesions on follow-up CT studies 4–8 weeks later. We did a descriptive analysis.
ResultsIn 15 patients, it was impossible to evaluate possible pseudoprogression because a second CT study was unavailable due to change of treatment or death. Tumor pseudoprogression was observed in 5 (12.2%) of the 41 patients, in most cases within 12 weeks of treatment initiation (in the fourth cycle). A second episode of pseudoprogression occurred in 2 (40%) of the 5 patients with an initial episode; the second episode occurred more than 12 weeks after treatment initiation.
ConclusionTumor pseudoprogression occurred in 12.2% of patients with advanced stage lung cancer treated with nivolumab. An increase in lesion size or the appearance of new lesions must be assessed over time to avoid mistaking pseudoprogression for true progression of disease.
La inmunoterapia es un nuevo tratamiento en estadios avanzados del cáncer de pulmón de célula no pequeña (CPCNP) que modula la respuesta inmunitaria frente a células malignas. Un reto para el radiólogo es la valoración del fenómeno de la pseudoprogresión, en la que se observa un aumento o aparición de lesiones por infiltración de células inflamatorias, con posterior disminución o desaparición de estas, hecho que traduce en realidad la respuesta al tratamiento. El objetivo fue determinar la frecuencia de pseudoprogresión en pacientes con CPCNP en estadios avanzados tratados con nivolumab.
Pacientes y métodosSe incluyeron 56 pacientes con CPCNP en estado avanzado tratados con nivolumab en segunda línea o posterior. Se analizaron las tomografías computarizadas (TC) realizadas durante el período de tratamiento. Se consideró pseudoprogresión tumoral al aumento o aparición de lesiones con posterior disminución o desaparición en una TC de control realizada a las 4–8 semanas. Se realizó un análisis descriptivo.
ResultadosNo se pudo valorar la existencia de pseudoprogresión en 15 pacientes porque no se disponía de al menos dos TC (por cambio de tratamiento o fallecimiento). Se observó pseudoprogresión tumoral en un 12,2% (5/41) de los pacientes, en la mayoría de los casos antes de las 12 semanas del inicio del tratamiento (en el 4.° ciclo). Un 40% (2/5) de los pacientes con pseudoprogresión presentó un segundo episodio, que se produjo de forma tardía, a partir de las 12 semanas del inicio del tratamiento.
ConclusiónSe observó pseudoprogresión tumoral en un 12,2% de los pacientes con CPCNP tratados con nivolumab. El aumento o aparición de lesiones debe valorarse evolutivamente para evitar diagnosticar erróneamente progresión de la enfermedad.
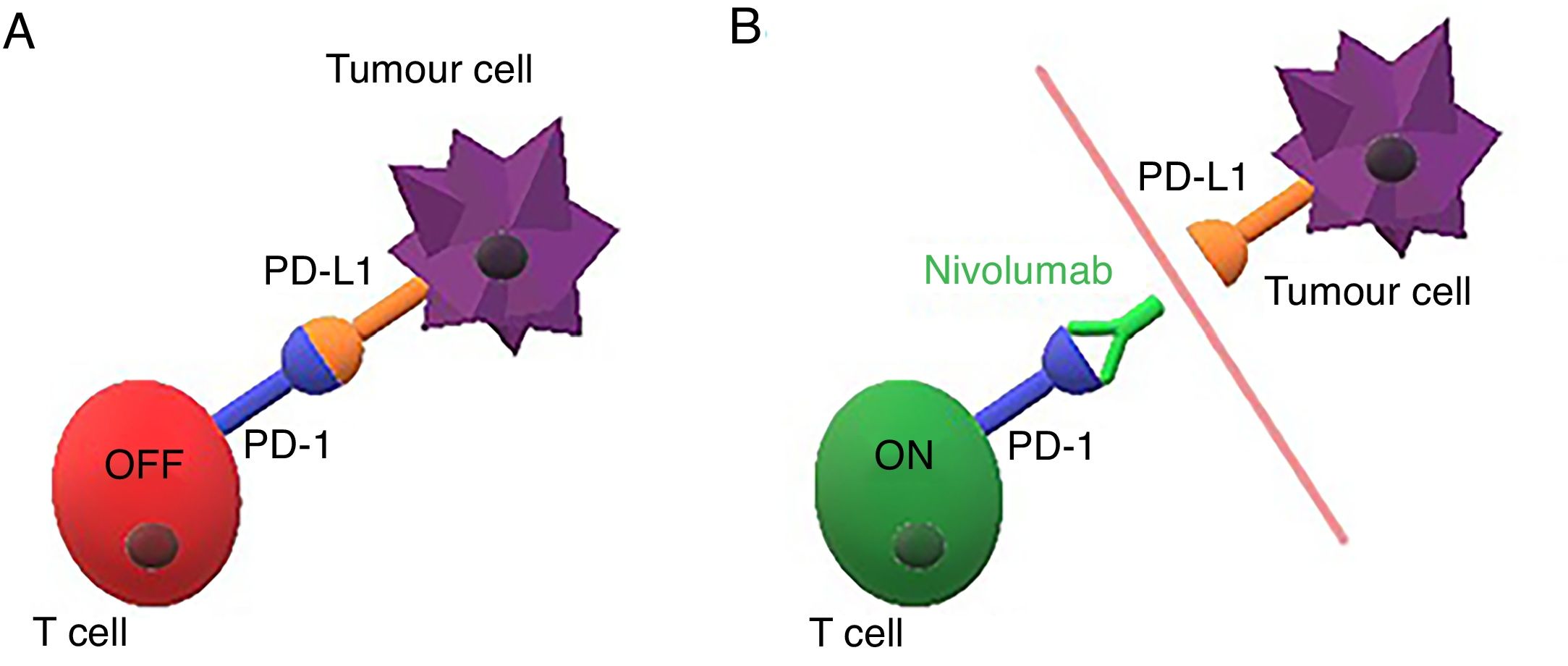
Immunotherapy is a new cancer treatment that modulates the immune response against malignant cells. The programmed cell death-1 receptor (PD-1) and its programmed cell death-1 ligand (PD-L1) are new therapeutic targets that have proven useful in malignancies such as melanoma, kidney and urothelial cancer, and non-small cell lung cancer (NSCLC).1–4 Lung cancer is the second most common cancer in both women and men, but the leading cause of cancer mortality in both sexes.5 Immunotherapy drugs targeting PD-1 (such as pembrolizumab and nivolumab) or PD-L1 (such as atezolizumab) have become a crucial alternative treatment in metastatic NSCLC due to their capacity to increase overall survival compared to conventional chemotherapy treatments.6–10 PD-1 (expressed by activated T cells) is blocked by PD-L1 (expressed by tumour cells), thus inhibiting the immune response (Fig. 1 A). Nivolumab is a monoclonal antibody that binds to PD-1 and blocks its interaction with the PD-L1 ligand, which restores the antitumour immunity (Fig. 1 B).1,7 This blockade of the mechanism that inhibits the immune response produces the desired antitumour effect, but can lead to excessive activation of the immune system and cause immune-mediated adverse effects or tumour response patterns that differ from conventional chemotherapies, and are not yet fully understood.3,4
There are three patterns of response to treatment with immunomodulators in computed tomography (CT).11 The tumour can decrease in size or disappear; the disease may stabilise; or the lesions may increase in size, possibly secondary to the increase in the number of tumour cells, that is to say, tumour progression, or secondary to infiltration by inflammatory cells, which is not considered disease progression but is not distinguished by CT. The decrease in size of these tumours in a follow-up scan would confirm the existence of an inflammatory component in the previous CT. This phenomenon is known as tumour pseudoprogression (PsP), and is currently interpreted as a response to immunotherapy.4,12 In recent years, different criteria for evaluating tumour response to immunomodulatory therapy (irRC, irRECIST and iRECIST) have been put forward,12–15 since this cannot be categorised in the guidelines developed for conventional chemotherapy.
Given the absence of clinical or biological markers that determine the activity of immunomodulatory drugs, radiological evaluation is of vital importance in the clinical management of these patients. For this reason, radiologists must be able to interpret the PsP phenomenon in order to avoid misdiagnosing disease progression. It has been estimated that up to 10% of cancer patients receiving immunotherapy develop PsP.3 However, the proportion of NSCLC patients presenting with PsP with nivolumab is still unclear; the few studies published to date have estimated it to be between 0% and 5%.3,16–18 In consideration of the above, the aim of this study was to determine the frequency of PsP in patients with advanced stage NSCLC treated with nivolumab.
Material and methodsPatientsThis is a retrospective study approved by the hospital’s ethics committee. Informed consent was obtained from all participants. The study inclusion criteria were: all patients with advanced NSCLC treated with nivolumab in the second line or later from July 2015 to September 2017 (n=56). Patients without two or more CT studies to evaluate tumour evolution were excluded. All patients underwent follow-up CT every four to eight weeks as part of their standard care during the period of treatment with nivolumab. Follow-up lasted from the start of treatment with nivolumab (date of the first dose) to the last CT scan performed before February 2018, or to the last CT scan prior to a change in treatment or death. The following clinical data were collected for each patient: age at the start of treatment with nivolumab, sex, tumour histological subtype, tumour stage, evolution of the cancer, and duration of follow-up. The percentage of patients positive for PD-L1 was not collected, since this parameter is not a prerequisite for starting treatment with nivolumab in the second line or later in advanced stage NSCLC.
Acquisition and interpretation of CT imagesCT studies were performed in two multidetector computed tomography (MDCT) devices, according to availability (Emotion 16 and Somaton AS definition, Siemens Healthcare, Erlangen, Germany). The standard acquisition protocol for patients with lung cancer was followed (chest and upper abdomen CT with 100ml of intravenous contrast in the arterial phase and CT from the upper abdomen to the iliac crests in venous phase), with a slice thickness of 5mm and increments of 5mm.
The CT images were analysed independently by two thoracic radiologists with 10–20 years of experience, and disagreements were resolved by consensus between them or by the intervention of a third expert thoracic radiologist. CT studies were evaluated using RECIST criteria. PsP was defined as a 20% or more increase in tumour size or the unequivocal appearance of lesions with subsequent decrease in size or disappearance in a CT follow-up performed after four to eight weeks. In patients presenting with PsP, a third consensus reading was performed to confirm the PsP. The PsP episode was classified as early or late, depending on whether it occurred before or after the first 12 weeks of treatment, respectively.
Statistical analysisA descriptive analysis of the data was performed. Absolute frequencies and percentages were used to show the distribution of the different variables, and the mean and standard deviation (SD) were calculated for the age and duration of follow-up variables.
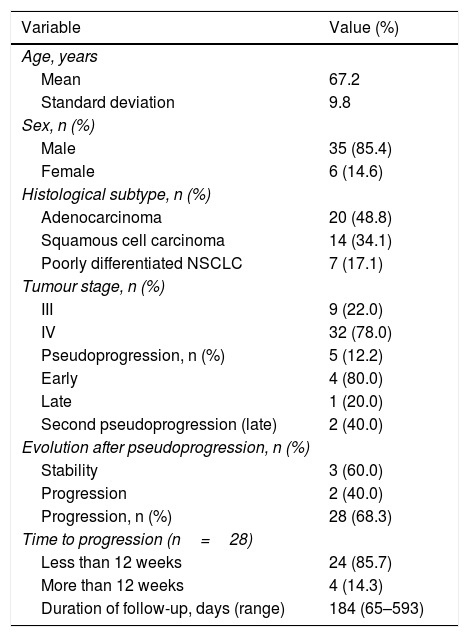
ResultsMedian follow-up time was 184 days (65–593 days). The presence of PsP could not be evaluated in 15 patients who did not undergo at least two CTs (due to change of treatment or death) to evaluate tumour evolution. The distribution of the demographic values of the 41 patients finally analysed are shown in Table 1. Mean age was 67.2 years (SD 9.8 years). Most (85.4%) (35/41) of patients were male. The most common histological type was adenocarcinoma, which accounted for 48.8% (20/41) of cases. Tumours in 22.0% (9/41) of patients were stage III at the start of treatment with nivolumab, and stage IV in 78.0% (32/41). (Fig. 3).
Demographic characteristics, histological subtype, tumour stage and response to treatment (n=41).
| Variable | Value (%) |
|---|---|
| Age, years | |
| Mean | 67.2 |
| Standard deviation | 9.8 |
| Sex, n (%) | |
| Male | 35 (85.4) |
| Female | 6 (14.6) |
| Histological subtype, n (%) | |
| Adenocarcinoma | 20 (48.8) |
| Squamous cell carcinoma | 14 (34.1) |
| Poorly differentiated NSCLC | 7 (17.1) |
| Tumour stage, n (%) | |
| III | 9 (22.0) |
| IV | 32 (78.0) |
| Pseudoprogression, n (%) | 5 (12.2) |
| Early | 4 (80.0) |
| Late | 1 (20.0) |
| Second pseudoprogression (late) | 2 (40.0) |
| Evolution after pseudoprogression, n (%) | |
| Stability | 3 (60.0) |
| Progression | 2 (40.0) |
| Progression, n (%) | 28 (68.3) |
| Time to progression (n=28) | |
| Less than 12 weeks | 24 (85.7) |
| More than 12 weeks | 4 (14.3) |
| Duration of follow-up, days (range) | 184 (65–593) |
NSCLC: non-small cell lung cancer.
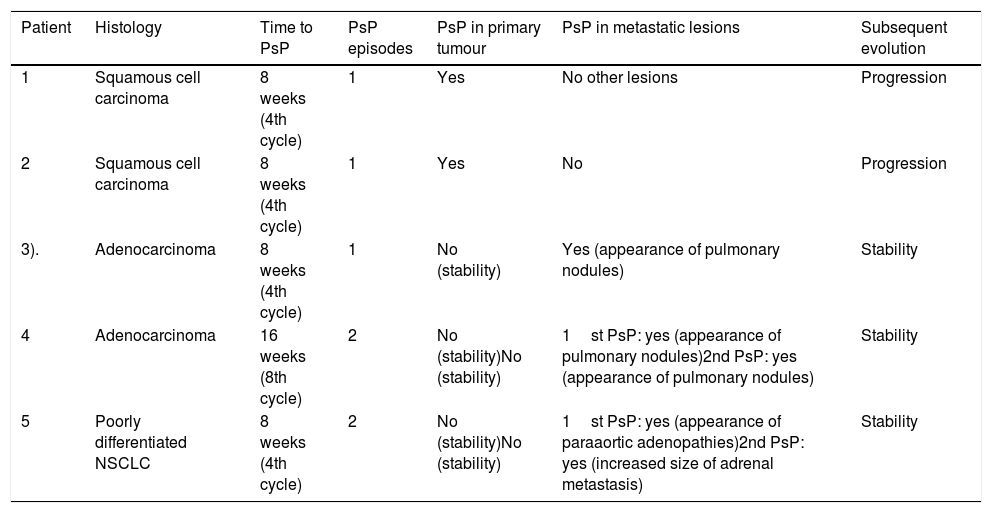
PsP was observed in 12.2% (5/41) of patients (Fig. 2–4); PsP was early in 80% (4/5) of cases (in the fourth treatment cycle). Slightly less than half (40% [2/5]) of patients with PsP presented a second episode of PsP, which occurred late (Fig. 4). Table 2 shows the lesions presented by the five patients in whom PsP was observed. In two patients, PsP was observed in the primary lung tumour (both squamous cell carcinoma), and in the remaining three (two adenocarcinomas and another poorly differentiated NSCLC), PsP was observed due to the increase in size of metastatic lesions or the unequivocal appearance of lesions suspicious of malignancy; in these three patients, the primary lung tumour was also observed to have stabilised.
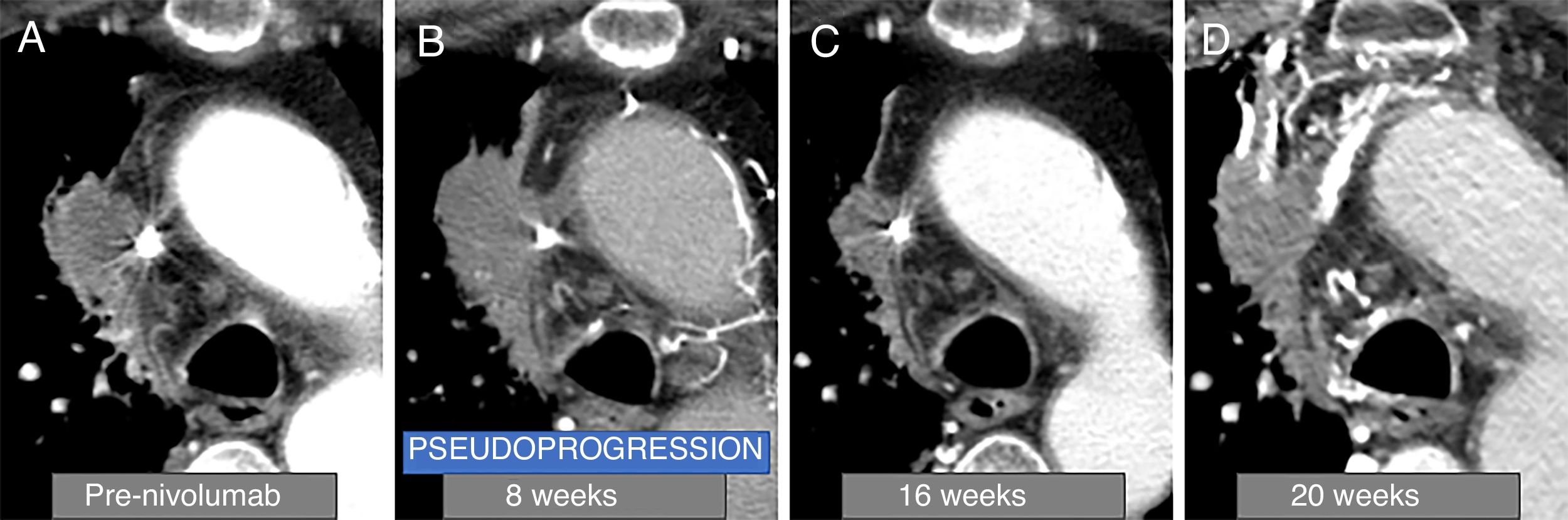
Example of tumour pseudoprogression. A 78-year-old man with squamous lung cancer in the right paramediastinal region (A), who started rescue therapy with nivolumab. In computed tomography (CT) performed at 8 weeks after the start of treatment (B), the primary tumour is seen to increase in size, and subsequently decrease in the follow-up CT scan performed at 16 weeks (C), so the patient presented an episode of pseudoprogression in the 8-week CT scan (4th cycle). The patient presented progressive clinical worsening, so a CT scan was performed at 20 weeks (D), in which disease progression that led to the death of the patient was observed.
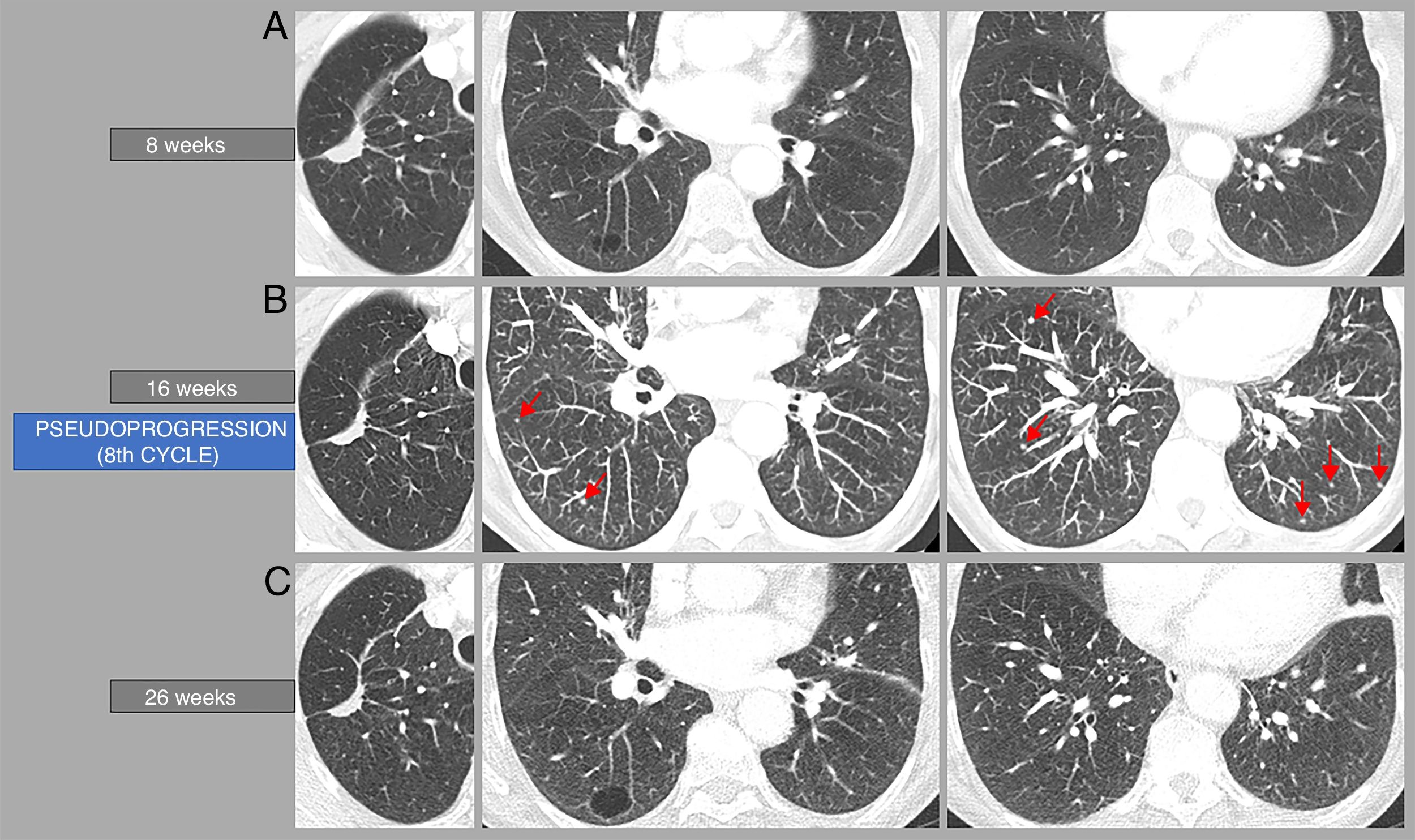
Example of tumour pseudoprogression. A 70-year-old woman with pulmonary adenocarcinoma in the upper right lobe, who began rescue therapy with nivolumab. Computed tomography (CT) performed 16 weeks after the start of treatment (B) shows the appearance of several small (millimetre) lung lesions suspicious of metastasis, not present in the CT scan performed at 8 weeks (A), which disappeared in the CT performed 10 weeks later (C). Therefore, there was an episode of tumour pseudoprogression on the CT performed 16 weeks after the start of treatment (8th cycle). This CT (B) also shows a decrease in size in the primary lung tumour compared to the CT performed at 8 weeks (A), but according to the iRECIST criteria this tumour has remained stable.
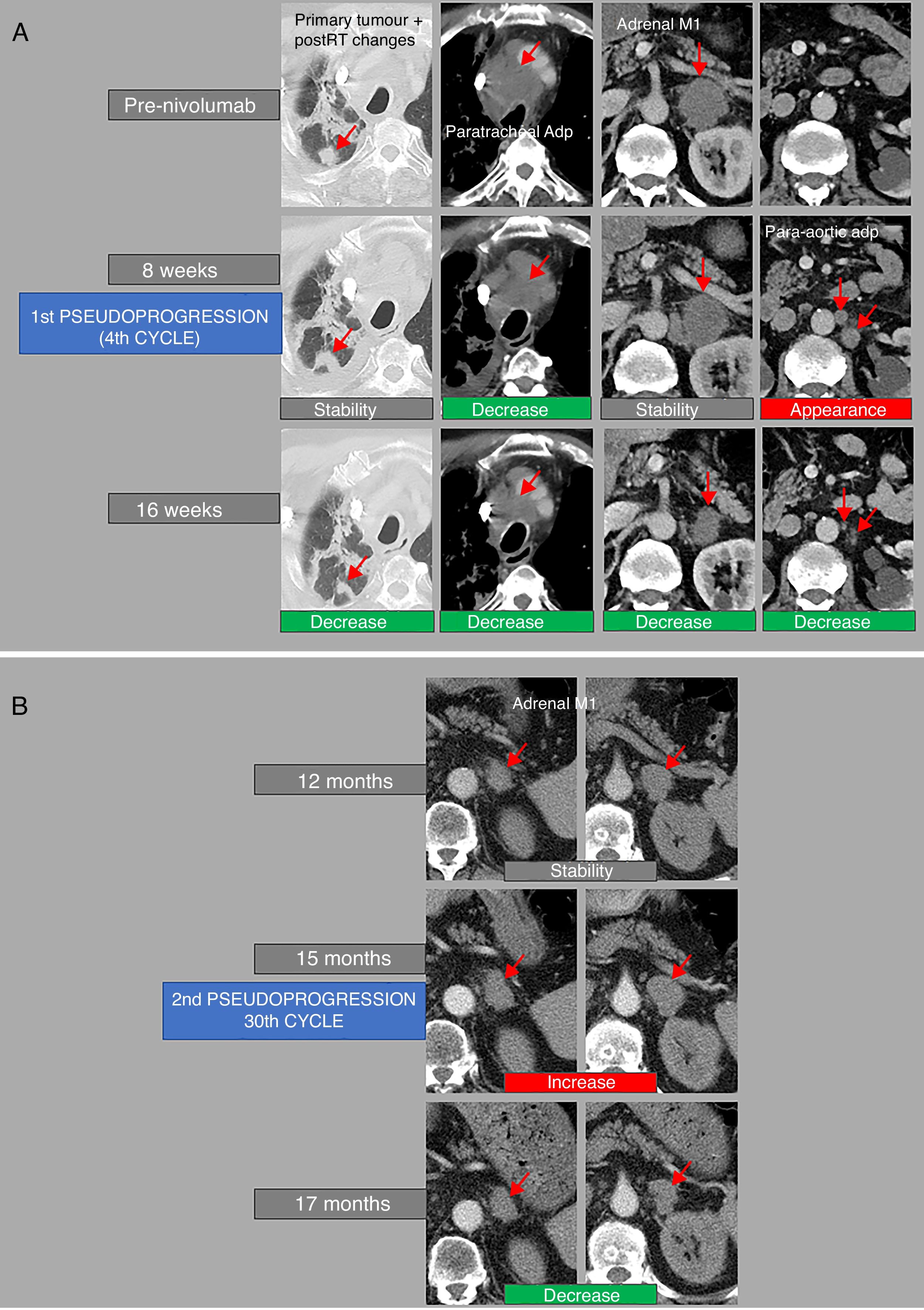
Example of repeated tumour pseudoprogression. A 71-year-old man with poorly differentiated non-small cell lung cancer under rescue therapy with nivolumab. (A) The computed tomography (CT) performed 8 weeks after the start of treatment shows stability of the primary pulmonary tumour and post-radiotherapy (post-RT) changes, decreased size of a paratracheal adenopathy (Paratracheal Adp), discrete increase in the size of an adrenal metastasis (adrenal M1), though this is stable according to RECIST criteria, and the appearance of paraaortic adenopathies (paraaortic Adp), the largest being located in the infrarenal region. In the follow-up CT performed at 16 weeks from the start of treatment, all lesions decreased in size; therefore, in the CT scan performed at 8 weeks there was an episode of tumour pseudoprogression (4th cycle). (B) One year after the start of treatment, the patient remained clinically and radiologically stable. However, a new increase in size in the left adrenal metastasis was observed in the CT scan performed at 15 months, which subsequently decreased in the follow-up CT scan performed 8 weeks later (17 months). This patient, therefore, presented a second episode of tumour pseudoprogression (in the 30th cycle).
Lesions in which tumour pseudoprogression was observed (n=5).
| Patient | Histology | Time to PsP | PsP episodes | PsP in primary tumour | PsP in metastatic lesions | Subsequent evolution |
|---|---|---|---|---|---|---|
| 1 | Squamous cell carcinoma | 8 weeks (4th cycle) | 1 | Yes | No other lesions | Progression |
| 2 | Squamous cell carcinoma | 8 weeks (4th cycle) | 1 | Yes | No | Progression |
| 3). | Adenocarcinoma | 8 weeks (4th cycle) | 1 | No (stability) | Yes (appearance of pulmonary nodules) | Stability |
| 4 | Adenocarcinoma | 16 weeks (8th cycle) | 2 | No (stability)No (stability) | 1st PsP: yes (appearance of pulmonary nodules)2nd PsP: yes (appearance of pulmonary nodules) | Stability |
| 5 | Poorly differentiated NSCLC | 8 weeks (4th cycle) | 2 | No (stability)No (stability) | 1st PsP: yes (appearance of paraaortic adenopathies)2nd PsP: yes (increased size of adrenal metastasis) | Stability |
NSCLC: non-small cell lung cancer; PsP: pseudoprogression.
In 40% (2/5) of patients, disease progression was noted after the appearance of PsP. The time from PsP to true disease progression in these two patients was 7 and 25 weeks, respectively, with a total time from the start of treatment to true progression of 15 and 33 weeks, respectively. Of the rest of the patients presenting with PsP, disease stability was observed in 60% (3/5) during the follow-up period.
In the remaining subjects analysed, 68.3% (28/41) had disease progression during follow-up, most (85.7% [24/28]) within 12 weeks of the start of immunotherapy. Of this group of patients, 78.6% (22/28) had died by the end of follow-up. A total of 14.6% (6/41) of patients responded to treatment, and in 4.9% (2/41) the disease had stabilised.
DiscussionCytotoxic chemotherapy drugs have been used for decades to treat cancer due to their ability to directly destroy tumour cells. A greater understanding of the pathophysiological mechanism behind the appearance of malignancies and the role of the immune system as a natural destroyer of cancer cells has led to the development of immunotherapy. However, the atypical response patterns of immunotherapy drugs can lead to misinterpretation of imaging studies, resulting in inappropriate clinical decisions.
We included 56 patients in this study, although only 41 were included in the final analysis, and PsP was observed in 12.2% of cases. This proportion is higher than that described in the studies published in the scientific literature, in which a percentage of PsP in NSCLC of 0%–5% has been documented.3,16–18
The first guidelines designed specifically for immunomodulatory drugs, irRC and irRECIST, define four categories of tumour response (as occurs in RECIST): (1) complete response, (2) partial response, (3) stability and (4) progressive disease,3,4 but include modifications to the RECIST rules, such as the need to confirm response, and that new lesions do not imply progression. The iRECIST guidelines divide the “progressive disease” category into two new categories: “unconfirmed progressive disease” and “confirmed progressive disease”.15 According to iRECIST, if an increase of over 20% in a target lesion or the appearance of new lesions is observed, the study should be classified as “unconfirmed progression” and a follow-up should be performed after four to eight weeks. If an increase in tumour size of 5mm or more, an increase in size of non-target lesions, or the appearance of new target or non-target lesions is observed in the follow-up CT, the findings are classified as “confirmed progressive disease”. The term PsP has been coined in the scientific literature to describe cases in which disease stability or partial or complete tumour response is observed after an episode of “unconfirmed progression”. If, after an “unconfirmed progression”, the criteria for progression or response are not met, the patient can continue in the category of “unconfirmed progression”. In our study, following the RECIST criteria, PsP was defined as a patient who presented an increase in size or the appearance of new tumours that subsequently decreased in size or disappeared in a follow-up CT. The main reason for the higher proportion of PsP observed in our studies compared to others is probably related to the limited number of patients analysed; this proportion could vary in further studies performed in larger samples. Nevertheless, although we observed a higher proportion of PsP in our series, it is important to mention that this correlated with the patient’s condition in all cases, and we did not observe clinical worsening that suggested disease progression.
In our study, the majority of cases in which PsP was observed (4/5) occurred within 12 weeks of the start of treatment (in the 4th cycle). This is consistent with previous studies3 in which PsP was observed in the first few weeks after the start of treatment. However, we also observed three episodes of PsP occurring after 12 weeks from the start of treatment, a fact little known in NSCLC, but documented in other malignancies such as melanoma.19 It is also important to note that two of the three cases of late PsP observed in our study involved a second episode of PsP. We could find no reports of a second episode of PsP in NSCLC in the literature, and only one case of multiple PsP has been described in a melanoma patient.20 This phenomenon highlights the importance of evaluating the patient’s clinical status if existing tumours are observed to increase in size or if new lesions appear on CT. In fact, iRECIST recommends that oncologists evaluate the clinical status of patients with “unconfirmed progression” before deciding whether to continue or interrupt immunotherapy: treatment should continue if there is an increase in the size of the lesions but the patient does not present clinical deterioration.3,15 Radiologists must also be familiar with the phenomenon of multiple PsPs in order to correctly evaluate CT studies in these cases.
In our series, PsP was observed in the primary tumour in two patients, and in metastatic lesions (mostly pulmonary) in the remaining three cases. In melanoma, studies have shown that PsP occurs more frequently in metastases located in the kidneys, liver, lungs, peritoneum, adrenal glands, and the thoracic and abdominal wall than in lymph nodes.19 Nevertheless, there is little information about tumour lesions associated with PsP in NSCLC, and it is not known whether the histological type could influence the type of PsP. In our study, patients with squamous cell carcinoma (n=2) presented with PsP of the primary lung tumour, while the other lesions remained stable, and those with other histological types (n=3) presented with PsP in metastatic lesions while the primary tumour remained stable. It could be useful for radiologists to be aware of these factors in cases where an increase in size or appearance of new lesions is observed.
The main limitations of our study are its small sample size and the biases inherent to its retrospective design, which, as already mentioned, probably gave rise to the higher proportion of PsP observed in this study compared to other published articles. However, it should be noted that ours is the first study to document episodes of multiple PsP in NSCLC. Our findings should be evaluated in future studies with a larger number of patients.
ConclusionIn conclusion, immunotherapy is a new treatment for diseases such as NSCLC, and radiologists must be aware of the new patterns of tumour response, such as PsP. In this study, this phenomenon was observed in 12.2% of patients with NSCLC treated with nivolumab, a larger proportion than that described previously. In most cases, the episode of PsP occurred within 12 weeks of the start of treatment. Slightly less than half (40%) of these patients also presented with a second episode of PsP, which occurred after 12 weeks from the start of treatment.
The increase in size or the appearance of new lesions should be evaluated over time and in conjunction with the patient’s clinical condition, in order to avoid a misdiagnosis of disease progression and poor clinical decisions.
Authorship- 1
Responsible for the integrity of the study: MM, EC.
- 2
Study conception: MM, EC.
- 3
Study design: MM, EC, XG, MA, ED, YG.
- 4
Data collection: MM, EC.
- 5
Data analysis and interpretation: MM, EC, XG, MA, ED, YG.
- 6
Statistical processing: MM.
- 7
Literature search: MM, EC.
- 8
Drafting of the article: MM, EC.
- 9
Critical review of the manuscript with intellectually relevant contributions: XG, MA, ED, YG.
- 10
Approval of the final version: MM, EC, XG, MA, ED, YG.
The authors declare that they have no conflicts of interest.
The authors would like to thank Dr Eva Ballesteros Gomiz for her help and advice in the evaluation of CT images, and Joan Carles Oliva Morera and Víctor José de Pedro Ambrosio for their help in drafting this article.
Please cite this article as: Mayoral M, Castañer E, Gallardo X, Andreu M, Dalmau E, Garcia Y. Pseudoprogresión tumoral en el tratamiento inmunoterápico con nivolumab en el cáncer de pulmón. Radiología. 2019;61:497–504.