Acute appendicitis is the most common indication for emergency abdominal surgery throughout the world and a common reason for consultation in emergency departments.
In recent decades, diagnostic imaging has played a fundamental role in identifying acute appendicitis, helping to reduce the rate of blind laparotomies and hospital costs.
Given the results of clinical trials supporting the use of antibiotic therapy over surgical treatment, radiologists need to know the diagnostic criteria for complicated acute appendicitis to be able to recommend the best treatment option.
This review aims not only to define the diagnostic criteria for appendicitis in different imaging modalities (ultrasonography, computed tomography, and magnetic resonance imaging), but also to explain the diagnostic protocols, atypical presentations, and other conditions that can mimic appendicitis.
La apendicitis aguda es la urgencia quirúrgica abdominal más frecuente en el mundo y un motivo de consulta habitual en nuestros servicios de Urgencia.
En las últimas décadas, la imagen diagnóstica ha desempeñado un papel fundamental, lo que ha contribuido a disminuir tanto el índice de laparotomías en blanco, como los costes hospitalarios.
Debido a los ensayos clínicos que defienden el uso de antibioterapia frente al tratamiento quirúrgico convencional, debemos conocer los criterios diagnósticos de la apendicitis aguda complicada de cara a ofrecer la mejor opción terapéutica.
Esta revisión tiene como objetivo no solo definir los criterios diagnósticos de la apendicitis en las diferentes modalidades de imagen, ecografía, tomografía computarizada y resonancia magnética, sino también abordar los protocolos diagnósticos, las presentaciones atípicas y la enfermedad apendicular simuladora.
Acute appendicitis (AA) is the most common indication for emergency abdominal surgery throughout the world, with an annual incidence of 96.5 to 100 cases per 100,000 adults1. Imaging has become fundamental to the diagnosis of AA2. Appendectomy, initially open, but laparoscopic since the 1980s, is the standard treatment for AA, as it is associated with less postoperative pain and a faster recovery1. However, recent clinical trials suggest that in 60% of patients, uncomplicated AA can be treated with antibiotics only, with diagnostic imaging being central to identifying which patients can be treated without surgery3.
Pathophysiology of acute appendicitisThe increased incidence of Crohn's disease, ulcerative colitis and Clostridium difficile infection after appendectomy suggests that the appendiceal microbiota play an important immunological role4. Classically, it was considered that the obstruction of the lumen was the fundamental trigger of AA, along with lymphoid hyperplasia in young patients, but recent theories consider genetic, environmental or infectious factors in the origin of this process5. When the appendix is obstructed it continues to secrete mucus and so becomes dilated. The dilation causes an increase in endoluminal pressure and if this exceeds the capillary wall pressure, it can lead to ischaemia and mural necrosis. Parallel to this process, endoluminal bacteria proliferate and when the mucosal barrier is disrupted, a transmural and ultimately periappendiceal infection occurs1,5.
Diagnosis of acute appendicitis. Clinical scalesThe diagnosis of AA is based on medical history, physical examination and laboratory and imaging tests5. Clinical prediction scales make it possible to stratify the risk of AA, identifying patients with intermediate risk who need an imaging test to reach a diagnosis. The scales found to be most useful are the Adult Appendicitis Score, the Appendicitis Inflammatory Response score in adults and the RIPASA score, all three of which, in terms of diagnostic accuracy, are superior to the more well-known Modified Alvarado Score6. However, the utility of these scales is subject to debate. In the World Society of Emergency Surgery (WSES) Jerusalem 2020 guidelines, there was no consensus among the advocates of these scales and those who advocated always performing imaging tests7. In fact, a recent study points to the loss of diagnostic accuracy after the use of these scoring systems in triaging patients for computed tomography (CT), again raising questions about their utility8.
Diagnosis of acute appendicitis. Imaging techniquesOver the last twenty to thirty years, the use of imaging has grown exponentially, contributing to a decrease in negative appendectomies and hospital costs9. A recent retrospective study from our institution compared imaging tests and negative appendectomies in two periods (2007 and 2015), confirming that the rate of negative appendectomies had fallen from 9.6% in 2007 to 5% in 201510. The use of imaging tests had increased significantly; in 2007, 40.1% of the cases did not have an imaging test, while in 2015 only 13.2% of patients did not have one10.
UltrasoundGraded compression ultrasound is an excellent initial imaging technique used in many hospitals (Fig. 1)10,11. In addition to the lack of radiation, it is accessible, does not require contrast and its high resolution allows assessment of the layers of the appendix12,13. According to a meta-analysis of 14 studies, the sensitivity (S) and specificity (Sp) of ultrasound is 86% and 81%, respectively14, with less diagnostic accuracy than CT (S=91% and Sp=90%)15. The lower diagnostic accuracy of ultrasound may well be influenced by the fact that it is an operator-dependent examination. When ultrasound is inconclusive, a recent meta-analysis shows that a CT, magnetic resonance imaging (MRI) or even repeat ultrasound can be performed without significant differences between the three techniques16. A second ultrasound after clinical observation would be based on the assumption that the ultrasound signs of AA become more evident with time and that it could be performed by a more experienced radiologist.
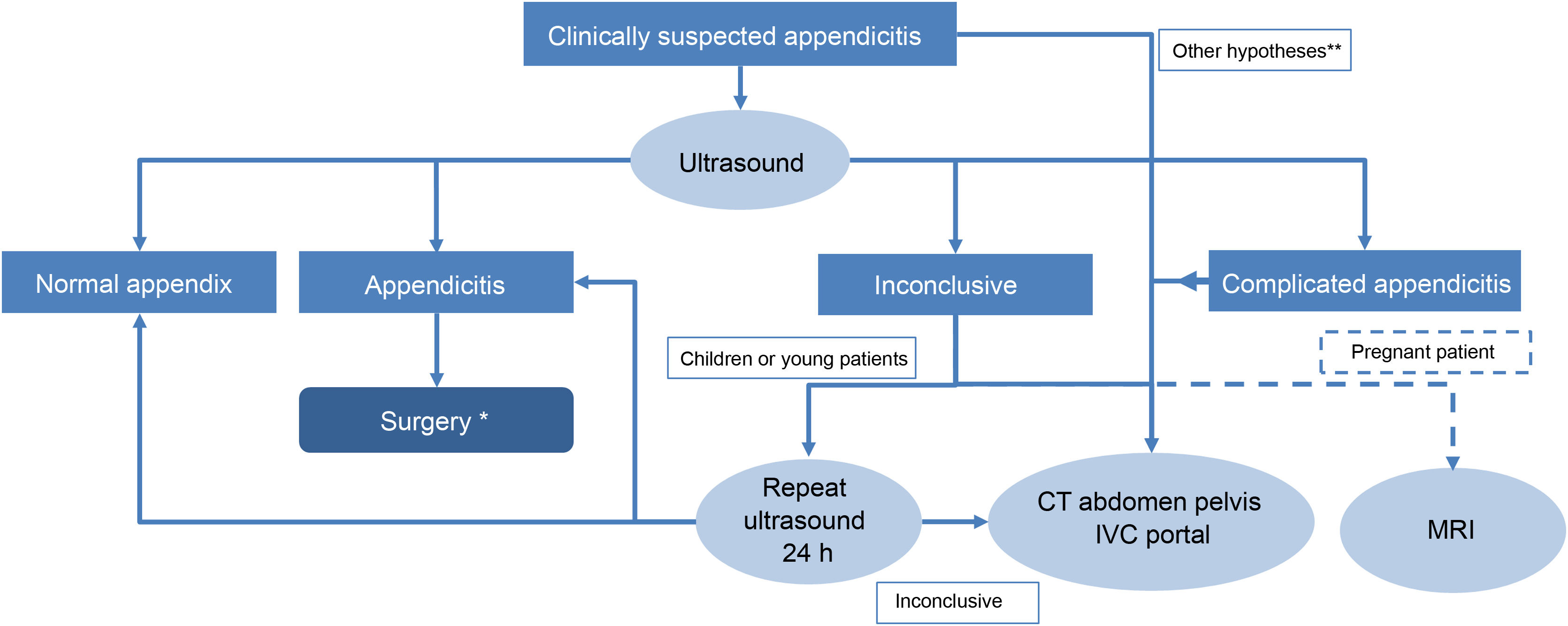
Diagnostic algorithm for acute appendicitis proposed at our centre.
* According to the 2020 WSES Jerusalem guidelines, in cases of uncomplicated acute appendicitis, medical treatment can be opted for instead of urgent surgery.
** Patients with obesity, older adult patients with other associated diseases that may complicate the diagnosis of acute appendicitis, and patients with peritonism or suspicion of complicated acute appendicitis may require a CT as the first diagnostic test.
CT is an outstanding tool in the diagnosis of typical, atypical and complicated AA17,18; the American College of Radiology (ACR) continues to recommend CT with intravenous contrast as the technique of choice in the diagnosis of AA in adults, due to its high sensitivity and specificity19. However, exposure to ionising radiation is no trivial issue. A CT scan can reach up to 10 mSv and some authors estimate this could induce cancer in one patient for every 2000 scans20. Recent studies show that the radiation dose can be reduced to 2 mSv without affecting clinical outcomes, and potentially prevent the future development of radiation-associated malignancies21. To avoid exposure to ionising radiation, in 2020 the WSES recommended performing an ultrasound first in young patients with suspected AA and only resorting to CT if the ultrasound proved negative or inconclusive7. Dual-energy CT has been found to improve the differentiation of simple and gangrenous appendicitis compared to conventional CT22. The CT protocol has been the subject of debate as to whether or not to use oral or intravenous contrast, whether to study the entire abdomen or only the pelvis, and whether to use conventional CT or CT with low-dose radiation18,23–26. There is consensus on scanning the entire abdomen to include atypically located appendices and other causes of abdominal pain. Intravenous contrast improves sensitivity, but low-dose CT in selected cases has been shown to be equivalent to standard-dose CT21,27,28. For oral or rectal contrast, although it can be useful in thin patients, it is not free of complications and the ACR considers it optional, at the discretion of each institution19,29. At our centre, we perform CT of the entire abdomen in the portal phase except where contraindicated, without oral contrast, and low-dose scans in young patients. We only perform CT after an inconclusive ultrasound or in exceptional circumstances as the first line of diagnosis in obese patients30, the very old or patients suspected of having complicated appendicitis and peritonism making ultrasound assessment difficult (Fig. 1).
Magnetic resonance imagingThe long scanning time and its limited accessibility make MRI far from the first line of diagnosis in the accident and emergency department, but the ACR recommends it in children and pregnant women to avoid exposure to radiation19, and it is indicated after an inconclusive ultrasound. Its diagnostic accuracy is similar to that of CT30. The protocol includes apnoea sequences with fat suppression on T1 and T2 (axial and coronal planes), diffusion in case of suspected complication15,18,31, and gadolinium is not administered.
Diagnostic imaging criteria in acute appendicitisThe normal appendix is an aperistaltic tubular structure with a blind end. Its wall is less than 3mm thick and its maximum transverse diameter is less than 6mm. Its average length is 8−10cm, but there are long forms that measure up to 35cm20,32. The different imaging criteria are summarised in Table 1.
Summary of radiological findings in acute appendicitis by different techniques.
| Diagnostic criteria | Ultrasound | CT | MRI |
|---|---|---|---|
| Increased appendix diametera | ≥7mm with graded compression techniqueNon-compressible appendix | ≥10mm as without compression | |
| Thickening and stratification of the wall | Loss of wall stratification with a predominance of the submucosal layer | >3mm | |
| Inflammation and vascularisation of the wall | Increased Doppler signal due to hyperaemia. Absent in gangrenous cases | Wall enhancement with IVCGreater sensitivity in dual-energy CT | T2 signal hyperintensity, restricted diffusion |
| Inflammation of the periappendiceal fat | Hyperechoic fat | Increased attenuationThickening of fascial planes | T2 signal hyperintensity |
| Appendix content | Gas: in the lumen without other signs of AA indicates patency and normal appendix | ||
| Associated with other signs of AA indicates probable gangrenous AA | |||
| Appendicolith: not synonymous with AA | |||
| Supports the diagnosis and associates more complications | |||
| Thickening of the caecum | It can be diffuse or focal coinciding with the opening to the appendix (more specific) | ||
| Lymphadenopathy | In the ileocaecal region and smaller than 1cm in size; if larger or necrotic centre, consider other diseases | ||
The diameter has classically been considered the most important criterion, but it is a mistake to consider it in isolation. Faecal impaction or gas increase the diameter of the appendix without AA, and associated signs should always be assessed. On ultrasound, the appendix is measured along its axial axis from serosa to serosa and is considered abnormal if it is greater than 7mm33. This cut-off has a better positive predictive value than the 6-mm criterion originally suggested by Jeffrey et al.34 Even so, an appendix of 6−8mm should be considered suspect according to some authors and the diagnosis supported by associated signs35. In CT and MRI, the cut-off should be higher as it is being measured without appendiceal compression, and considered abnormal when greater than 10mm. A lumen of 7−10mm is suspect, and associated signs should be sought to support the diagnosis, such as thickening and enhancement of the appendix wall or periappendiceal fat involvement2,24.
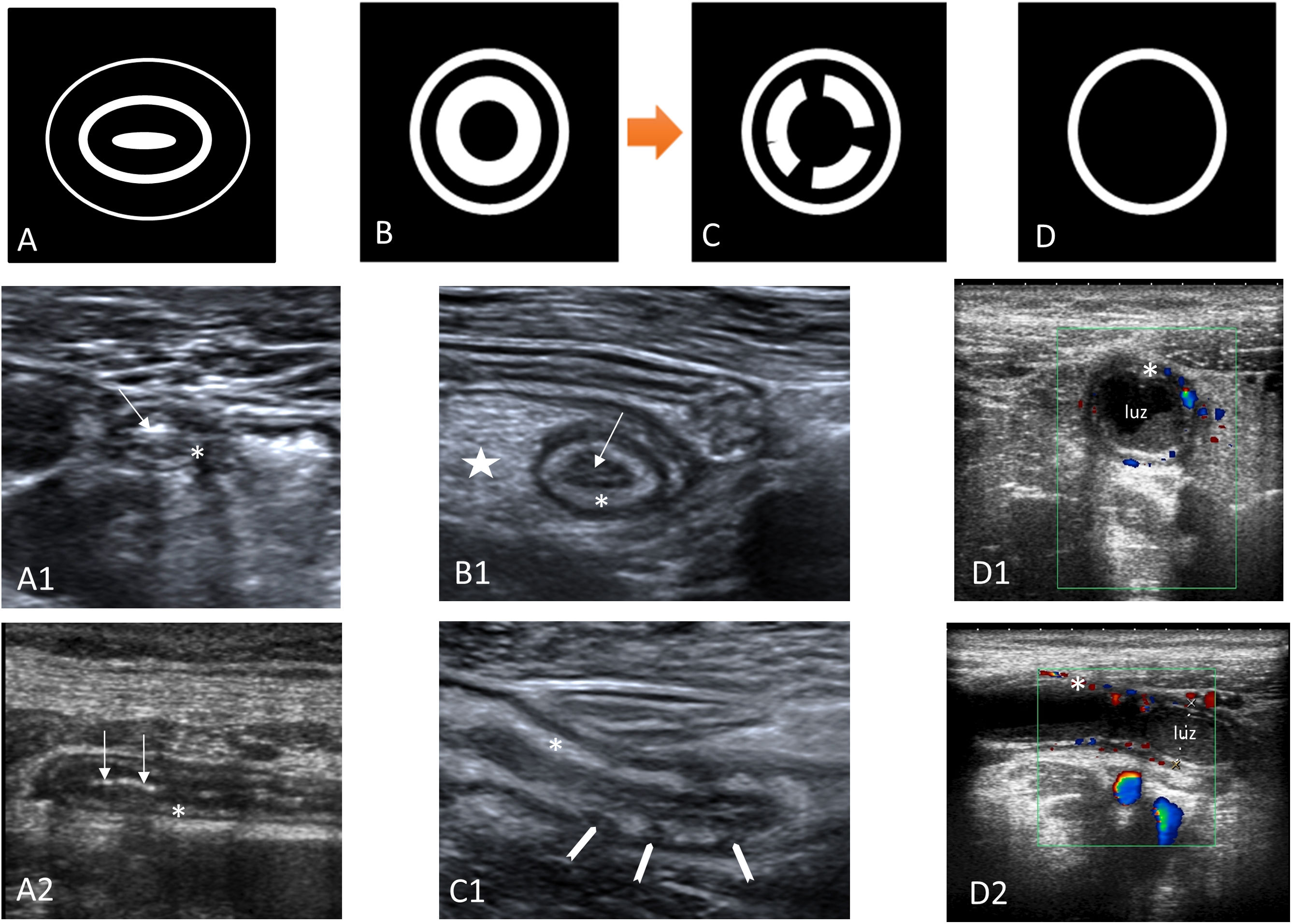
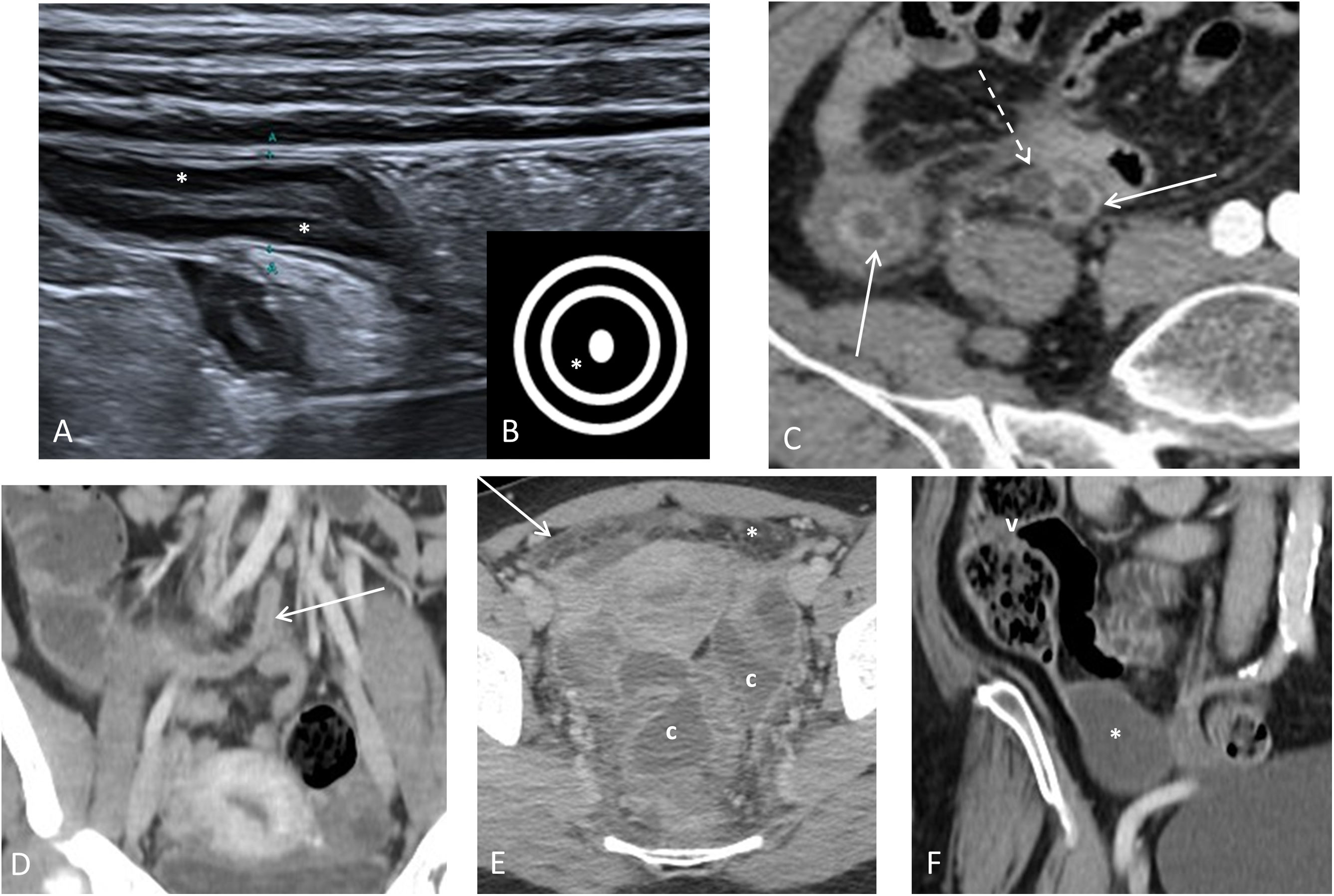
Thickening and stratification of the wallIn all imaging techniques, the thickness of the appendix wall is considered to be increased when it measures more than 3mm. On CT and MRI, measurement is sometimes difficult due to the lack of thickness, and in ultrasound it has to be measured from the mucosal surface to the serosa, excluding the endoluminal content. On ultrasound, assessment of the layers of the appendix is more useful than wall thickness, as they are an important marker of an inflamed appendix (Fig. 2). Like the rest of the gastrointestinal tract, the appendix has five layers with alternating echogenicity, three hyperechoic, which are (from the inside out) the mucosal surface, the submucosa and the serosa, and between them two hypoechoic layers, the muscularis mucosa and muscularis propria, which have muscle fibre12,13,36,37. With inflammation of the appendix there is a loss of layer structure beginning with the disappearance of the central layer (mucosal surface), followed by an increase in the hyperechogenic middle layer (submucosa). As the inflammation progresses, there is discontinuity and eventual disappearance of the submucosal layer, with this being a sign indicative of transmural inflammation and complicated AA32,38. There are other ultrasound patterns of involvement in AA in which the endoluminal content of the appendix predominates, leaving the layers of the appendix pushed out towards the periphery36.
Outline of the layers of the appendix on an ultrasound scan. Diagram of the layers of the appendix on an ultrasound scan. A) Diagram of a normal appendix. The muscle fibre layers (muscularis mucosa and muscularis propria) are hypoechoic, and the mucosa, submucosa and serosa are hyperechoic. The normal appendix is usually oval-shaped in axial slices. A1–A2) Ultrasound images in axial and longitudinal slices of the normal appendix. The mucosa shows gas suggestive of patency (arrow). The submucosal layer is thin (asterisk). B–B1) Diagram and axial image of acute appendicitis. Thickening of the submucosal layer (asterisk), loss of the mucosal layer and collapse of the appendix lumen (arrow), and hyperechogenicity of mesoappendix fat (star). C-C1) Diagram and sagittal image of acute appendicitis with discontinuity of the submucosal layer (arrowheads), indicating probable gangrenous appendicitis. D) Diagram of acute appendicitis where the endoluminal content predominates. D1-D2) Axial and sagittal images with colour Doppler mode. Appendix in which the endoluminal content predominates. Hyperaemic submucosa (asterisk) pushed out towards the periphery.
On ultrasound this appears as an increase in Doppler flow in the wall of the appendix39. This is a specific sign, but with limited sensitivity, absent in gangrenous appendicitis, and which can be detected with high-end equipment without the need for the appendix to be inflamed (Fig. 2). On CT there is enhancement of the wall of the appendix. This is best seen with dual-energy CT scanners, which detect acute inflammation better with single-energy images at 50kV40. On MRI, hyperintensity is seen on T2-weighted images with diffusion restriction15.
Non-compressible appendixPain coinciding with the pressure of the transducer on the appendix (positive McBurney sign) or the presence of an appendix that cannot be compressed are signs of AA on ultrasound36,37.
Inflammation of the periappendiceal fatThis is the best diagnostic criterion for AA. On ultrasound it is seen as an increase in the echogenicity of the periappendiceal fat37,41,42 (Figs. 2–4), on CT as an increase in attenuation, and on MRI as hyperintensity in T2-weighted images, especially fat suppression sequences.
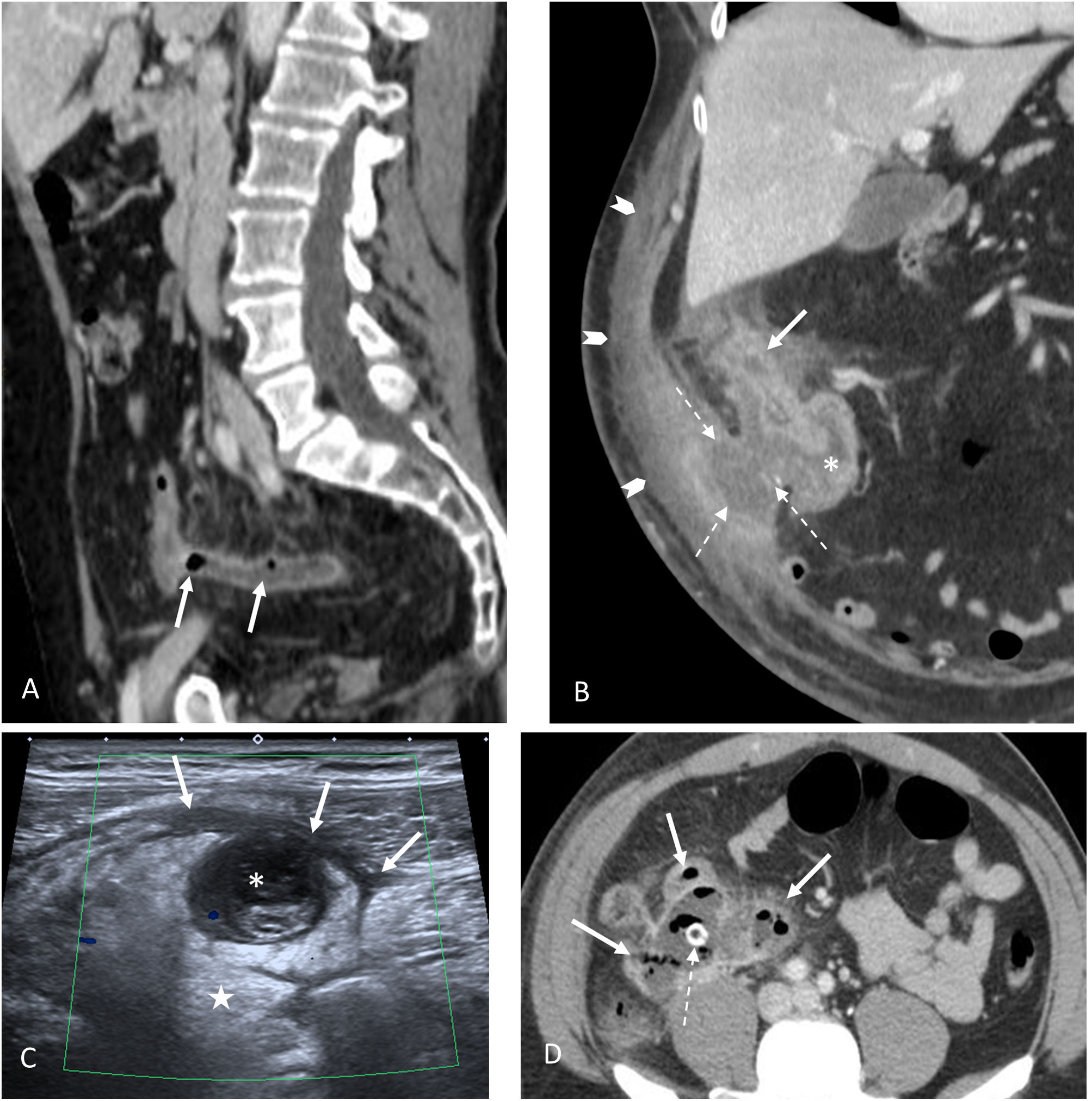
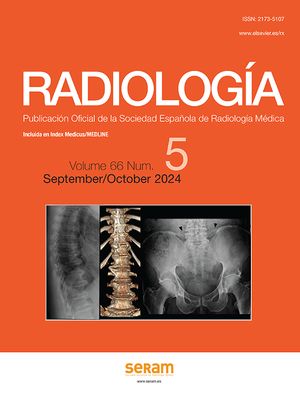
Complicated acute appendicitis. A) Gangrenous appendicitis. Sagittal CT image with IVC. The presence of gas (arrows) within an inflamed appendix is highly indicative of gangrenous appendicitis. B) Complicated appendicitis with fistula to the abdominal wall. Coronal CT image with IVC. Disrupted appendix in subhepatic location (continuous arrow) with marked inflammatory changes in the caecum (asterisk). There is an associated fistula towards the anterior abdominal wall and an abscess (dashed arrows). Extensive cellulitis of the anterior abdominal wall (arrowheads). C) Perforated appendicitis. Abdominal ultrasound. Complete loss of the various appendix layers. Lumen occupied by echogenic content (asterisk) in continuity with sheets of free fluid (arrows) and marked inflammatory changes in the periappendiceal fat (star). D) Abscess of appendiceal origin. Large abdominal abscess with air bubbles inside (solid arrows). Although the appendix is not distinguishable, the presence of an appendicolith inside it (broken arrow) reveals its appendiceal origin.
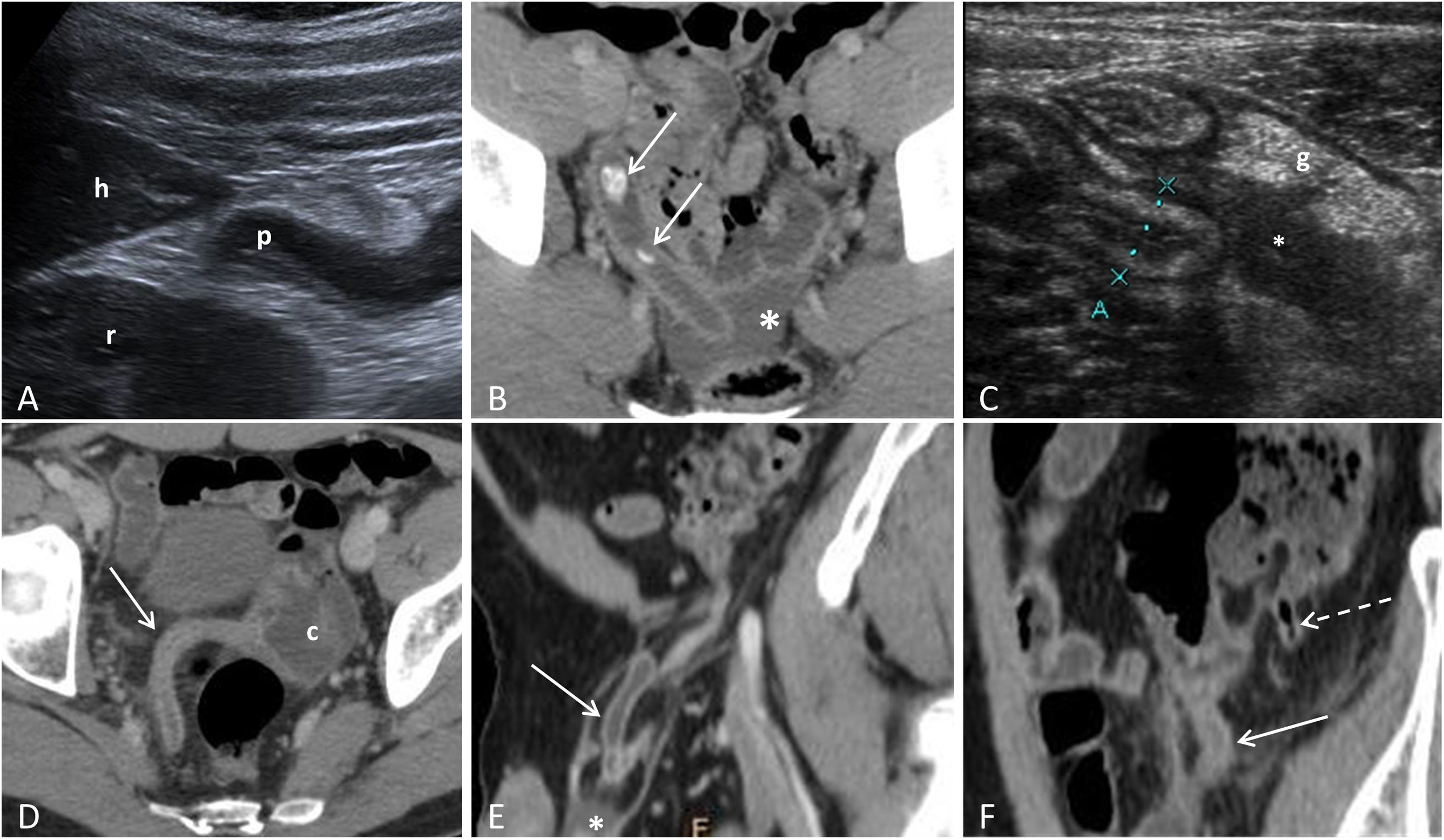
Atypical presentations of acute appendicitis. A) Subhepatic appendicitis: sagittal ultrasound image identifying the tip of the appendix (p) adjacent to the lower border of the liver (h) and the right kidney (r). B) Acute pelvic appendicitis: CT with IVC, showing the appendix with tip in a presacral location with adjacent free fluid (asterisk) and two appendicoliths (arrows) inside. C) Stump appendicitis: sagittal ultrasound section showing the stump or residual appendix (marked by the cursors) with parietal thickening with a predominance of the submucosa. There is associated free fluid (*) and hyperechogenicity of the periappendiceal fat (g). D) Acute appendicitis in a patient with intestinal malrotation: CT with IVC, showing the caecum (c) in the left half of the pelvis and the inflamed caecal appendix (arrow). E) Acute appendicitis in an Amyand's hernia: CT with IVC showing the inflamed appendix within a right inguinal hernia (arrow), as well as a small amount of free fluid (*). F) Acute appendicitis in a patient with appendiceal duplication: CT with IVC showing two vermiform appendices, one with signs of acute appendicitis (continuous arrow) and the other normal (broken arrow).
Classically, gas has been considered an exclusion criterion for AA, being synonymous with patency. However, gas associated with other signs of AA is a strong indicator of gangrenous appendicitis (Fig. 3)43. Appendicoliths by themselves are not synonymous with appendicitis. In a recent study, they were detected in 4% of asymptomatic patients and in 40% of patients with AA, being associated with a higher rate of complications (Figs. 3 and 4)44.
Thickening of the caecumThis may be diffuse or focal. If the thickening coincides with the opening of the appendix, it is more specific to AA. Thickening of the terminal ileum in AA is rare and is usually mild, with little hyperaemia. If involvement of the ileum predominates, the possibility of Crohn's disease has to be assessed45.
Reactive lymph nodesLymph nodes in the ileocaecal region are normally less than 5mm in size. If they are larger than 1cm or have a necrotic centre, other disease processes should be considered46.
Atypical presentations of acute appendicitisAtypical locationsThe appendix is a free intraperitoneal organ, only attached to the adjacent colon by the mesoappendix. This means that its location in relation to the caecum can vary: ascending retrocaecal (65%); pelvic (31%); transverse retrocaecal (2.5%); preileal ascending paracaecal (1%); and postileal ascending paracaecal (0.5%)2. It can also be located in atypical places such as the subhepatic or pelvic space, within an inguinal hernia (Amyand's hernia), within a femoral hernia (De Garengeot's hernia) or even in the left lower quadrant, in rare cases of malrotation (Fig. 4)2. The variability of its location explains why it is more difficult to identify with ultrasound47. The diagnostic key to locating the appendix in the different imaging modalities is to identify the ileum/ileocaecal valve complex, as the opening to the appendix is constant and is normally located 3 or 4cm caudal to the ileocaecal valve, on the same side as the terminal ileum.
Tip appendicitisAppendicitis can start with obstruction of the lumen at a distance from the opening, so the inflammation can be limited to only the tip of the appendix, with the base and body being normal. It is essential to examine the entire appendix on ultrasound and consider a CT scan when the full length of the appendix cannot be seen in patients strongly suspected of having appendicitis48.
Appendiceal duplicationAlthough rare, appendiceal duplication is one of the most common congenital abnormalities of the appendix. The modified Cave-Wallbridge classification is the most widely used (Fig. 4)49. In these cases, appendectomy of both appendices is recommended, even if only one has appendicitis.
Stump appendicitisThis is inflammation of the residual appendix tissue after an incomplete appendectomy (Fig. 4). It can occur anywhere from two weeks to several decades after surgery. An appendix stump greater than 5mm is considered a risk factor. The symptoms are the same as for AA, but the diagnosis and treatment are delayed in view of the history of previous appendectomy, so complications are more common50,51.
Disease of the appendix mimicking appendicitisIncrease in the appendix lumen without appendicitisAny content within the appendix, such as gas or faecal content, can increase its diameter and it is important to assess for other associated signs of AA. Appendiceal mucocele is a distension of the appendix caused by mucus secondary to a chronic obstruction, mucosal hyperplasia or underlying cancer. It is usually asymptomatic and there is no involvement of the periappendiceal fat (Fig. 5)52. Lymphoid hyperplasia, common in children and adolescents, typically associated with inflammatory processes, can cause a non-compressible appendix with a slight increase in diameter. On ultrasound there is no disruption of the layers and there is a relative predominance of the hypoechoic central layer corresponding to the lamina propria or muscularis mucosa, instead of the submucosa being thickened, which is what happens in AA53.
Disease of the appendix mimicking appendicitis. A) Lymphoid hyperplasia: ultrasound image of the appendix in its longitudinal axis, with a slight increase in diameter due to thickening of the muscularis mucosa layer, which is hypoechoic (*). B) Diagram showing a cross-sectional view of follicular hyperplasia of the appendix with thickening of the muscularis mucosa (*). C) Appendiceal diverticulitis: CT with IVC showing wall thickening of the appendix with hyperenhancement (solid arrows); the diverticulum can be seen in the distal portion (broken arrow). D) and E) Pelvic inflammatory disease (PID) with inflammatory involvement of the appendix due to contiguity: CT with IVC in a coronal slice (D). The appendix appears slightly thickened with hyper-uptake; axial slice (E) in the pelvis of the same patient, showing signs of PID: hyperdensity and trabeculation of pelvic fat (*), fine enhancement of the peritoneum (arrow) and collections with peripheral enhancement (c). F) Appendiceal mucocele: CT with IVC. Cystic dilation of the appendix (*) consistent with a mucocele, without periappendiceal inflammatory changes. Ileocaecal valve (v).
The presence of diverticula in the caecal appendix is rare (<3%) and generally acquired. It is asymptomatic unless inflammation/perforation of one of these diverticula occurs (Fig. 5). It usually affects older individuals (over 30 years of age) and the duration of symptoms is longer (1–13 days). Perforation is four times more common than in AA54.
Appendiceal endometriosisAppendiceal endometriosis affects 0.4–1 % of women, while its prevalence among patients with endometriosis is 4–22 %. The signs and symptoms are nonspecific, with chronic cyclical pelvic pain being the most typical. On ultrasound it is seen as a hypoechoic nodular thickening of the body or tip of the appendix originating from the serosa and invading the muscle layer without altering the intestinal layer structure55. The suspected diagnosis can also be assessed with MRI, which shows a hyperintense appendix on T1-weighted and T1-weighted fat-suppressed sequences, compatible with haemorrhagic signal intensity. On CT, it can be seen as an enhanced nodule in the wall without any specific features56.
Tumours of the appendixAppendiceal tumours are rare, approximately 0.5–1% of appendectomy specimens51, with the most common (32–57%) being carcinoid52. Most are found in the distal third of the appendix, as a small focal nodule or thickening on CT. In most cases they are an incidental finding in the appendectomy specimen. Other types of tumour include epithelial cancer (adenoma, appendiceal mucinous neoplasms), the main manifestation of which is mucocele (Fig. 5); this should be suspected in the presence of linear calcifications or when the appendix diameter is greater than 15mm57,58.
Reactive appendicitisThis is secondary to other abdominal inflammatory processes and does not require appendectomy, as the cause of the inflammation does not originate in the appendix. It occurs in cases of disease close to the appendix (caecum and ileum), but it can be secondary to other more diffuse processes such as pelvic inflammatory disease (Fig. 5) or peritonitis. The involvement of the appendix in Crohn's disease is a major diagnostic challenge. Ripollés et al.45 state that the appendix is inflamed in 21% of patients with Crohn's disease and can even show mild hyperaemia on ultrasound. A thickening of the terminal ileal wall greater than 5mm and the presence of colour Doppler flow in the wall of the ileum support the diagnosis of Crohn's disease versus AA.
Complicated appendicitisDiagnostic imaging has contributed to a significant decrease in negative appendectomies, but there has been no decrease in complicated appendicitis. It is possible that simple and complicated appendicitis are different disease processes, with different causes and behaviours5,10.
If the AA is complicated, it is important to set this out clearly in our report because:
- -
Medical treatment can be considered as a therapeutic option in some uncomplicated appendicitis cases3.
- -
Serious complications may require open laparotomy instead of laparoscopy.
- -
Abscesses may require percutaneous drainage before surgery.
Although different studies associate ultrasound signs indicative of complicated appendicitis such as loss of normal morphology with collections and complex periappendiceal fluid (Fig. 3)59, in general, complicated appendicitis is best assessed with CT60,61. In addition to the signs of complicated AA, signs have been described that predict failure of antibiotic therapy (such as the presence of appendicoliths or a dilated appendix >13mm in size)1.
Signs of complicated appendicitis- -
Phlegmon: inflammatory soft tissue mass surrounding the appendix.
- -
Abscess (Fig. 3): an appendiceal abscess shows an enhanced peripheral wall on CT with intravenous contrast or a peripheral hyperaemic ring with colour Doppler on ultrasound. This is the most specific sign of perforation and is the most common complication of perforated appendicitis. The presence of an appendicolith within the collection is virtually pathognomonic for an appendiceal abscess.
- -
Wall enhancement defects: this sign has a diagnostic accuracy of 96%.
- -
Extraluminal air: gas bubbles outside the appendix. Very specific sign (98%), but with low sensitivity (22–35%).
- -
Extraluminal appendicolith: very specific (100%), but not very sensitive (32%) (Fig. 3).
- -
Gangrenous appendicitis: the presence of gas associated with other signs of AA is an important marker of gangrenous appendicitis (Fig. 3)43.
- -
Pylephlebitis: ascending infection through the portomesenteric venous drainage, which can cause venous thrombosis and liver abscess.
- -
Fistulas: rare complication of perforated AA and can affect the bowel, bladder, vagina and the adjacent abdominal wall (Fig. 3).
- -
Diffuse peritonitis: rare and occurs when there are no local adhesions after perforation of the appendix to contain the infection. It is more common in children and is seen as echogenic free fluid or with peritoneal enhancement on CT.
- -
Other complications: complicated AA can cause obstructive uropathy or bowel obstruction secondary to involvement of the right ureter and terminal ileum, respectively.
Imaging is fundamental to the diagnosis of AA. In children and pregnant women, ultrasound should be performed first, followed by MRI in uncertain cases. In adults, the strategy of initial ultrasound and CT only in inconclusive ultrasounds has been shown to have high diagnostic accuracy with a significant reduction in radiation. In addition to knowing the diagnostic criteria, radiologists must be able to recognise atypical presentations and AA-mimicking appendiceal disease, and include signs of complicated AA in their report. This is important not only for situations such as abscesses, which may require drainage before surgery, but also in relation to the paradigm shift posing the option of non-surgical management in uncomplicated cases.
AuthorshipAll authors declare having contributed substantially to all aspects of the preparation of the manuscript:
- 1
In the conception and design of the study, data collection and the analysis and interpretation of the data;
- 2
In the drafting of the article and the critical review of the intellectual content; and
- 3
In the final approval of the version submitted.
None.
Conflicts of interestThe authors declare that they have no conflicts of interest.