Complications after surgery for colorectal cancer are common in emergency departments. Multidetector computed tomography plays a fundamental role in the follow-up of patients after surgery, because it enables the detection of relapse and complications. Radiologists need to be familiar with different surgical techniques and the normal postsurgical changes so that we can differentiate them from potential complications and relapse.
This article reviews the multidetector computed tomography findings that can be considered normal after surgical intervention for colorectal cancer as well as the most common early complications seen in postsurgical colorectal cancer patients presenting at emergency departments.
Las complicaciones posquirúrgicas del cáncer colorrectal son frecuentes en los servicios de urgencias. La tomografía computarizada multidetector tiene un papel fundamental en el seguimiento de los pacientes operados, ya que hace posible el reconocimiento de complicaciones y recidiva. Es importante que el radiólogo esté familiarizado con las diferentes técnicas quirúrgicas y los cambios postoperatorios normales, con objeto de diferenciarlas de potenciales complicaciones y recidivas.
El objetivo de este trabajo es revisar los hallazgos en tomografía computarizada multidetector que pueden considerarse normales tras la intervención quirúrgica, y hacer una revisión de las complicaciones tempranas que con mayor frecuencia encontramos en los servicios de urgencias.
Colorectal cancer (CRC) is the most common cancer in Spain when both sexes are considered together. In men, it ranks third after prostate and lung cancer. In women, it is the second most common form after breast cancer.1 The only treatment with curative intent in CRC is surgery, which depends on the tumour site, the extent of local invasion and the existence of distant metastasis.2
This work will review the most common surgical procedures, including: segmental colon resections, Hartmann's operation, total colectomy, lower abdominal resection (LAS) and abdominoperineal resection (APR). Postsurgical anatomical changes in multidetector computed tomography (MDCT) will also be described as well as findings that may be considered normal following a surgical intervention, relating them to the time of onset and their clinical assessment. The most common early complications will also be reviewed and the optimal protocol for performing MDCT will be established according to the type of complication suspected.
Technical considerationsThe protocols used at our centre are as follows:
- •
The standard study is performed from the base of the thorax to the pubic symphysis, with non-iodinated intravenous (i.v.) contrast (100–120ml), in portal venous phase. Oral and rectal contrast media are not typically used.
- •
However, in select cases, the administration of a rectal contrast medium may be indicated to reveal small anastomotic leaks. A 2% Gastrografin dilution may be used or an iso-osmolar contrast diluted to 5%.
- •
Although they may provide important information such as the presence of pneumoperitoneum, haemoperitoneum, haematomas and dilated loops, studies without i.v. contrast are not usually performed. These are always indicated prior to the performance of a computed tomography (CT) scan with enteral contrast or a CT angiogram, as they allow sutures, clips and surgical staples to be distinguished as well as possible contrast extravasation outside the bowel lumen or active bleeding.
- •
Late-phase CT (15–20min) is indicated when postsurgical urinary tract involvement is suspected, to detect possible contrast extravasation.
- •
If active bleeding is suspected, a CT angiogram will be performed: without contrast and with i.v. contrast in the arterial and venous phases.
Segmental resections refer to the removal of the focal lesion and part of the adjacent healthy bowel in order to provide large surgical margins. In neoplastic lesions, removal of the mesentery related to the affected intestinal segment and lymphadenectomy are performed.3
Based on the location of the tumour, segmental colon resection includes3,4:
- •
Ileocaecal resection (resection of the caecum and terminal ileum with ileocolic anastomosis).
- •
Right hemicolectomy (resection of the terminal ileum, caecum, ascending colon and proximal portion of the transverse colon, with anastomosis between the ileum and transverse colon).
- •
Left hemicolectomy (resection of the left flexure, caecum, descending colon and proximal portion of the sigmoid colon, with anastomosis between the transverse and distal sigmoid colon). Sigmoidectomy is a modification of this technique, which is performed on small sigmoid colon tumours involving their resection and anastomosis between the descending colon and rectosigmoid junction.
Total colectomy (complete removal of the colon with ileorectal anastomosis) is not routine in colon cancer, but may be necessary in the event of complications such as severe colonic ischaemia or in case of synchronous tumours.
Anatomical changes will depend on the surgical intervention performed. The absence of the resected bowel segment, with anastomotic surgical clips and displacement of the adjacent viscera into the resected space will be observed in imaging studies.5
Hartmann's operation6,7 is typically performed in emergency surgery when there is a high risk of the anastomosis leaking. In neoplastic lesions, generally when they present with obstruction or perforation, the affected segment is resected and a colostomy is formed in the left iliac fossa. In a second procedure, it is then anastomosed with the rectal stump, thereby re-establishing intestinal transit continuity.
In rectal cancer, APR is indicated when the lesion is more than 8cm above the pectineal line or 5cm above the anal margin. The distal portion of the descending colon, sigmoid colon and distal rectum are resected with low rectal anastomosis, preserving intestinal continuity.3,4
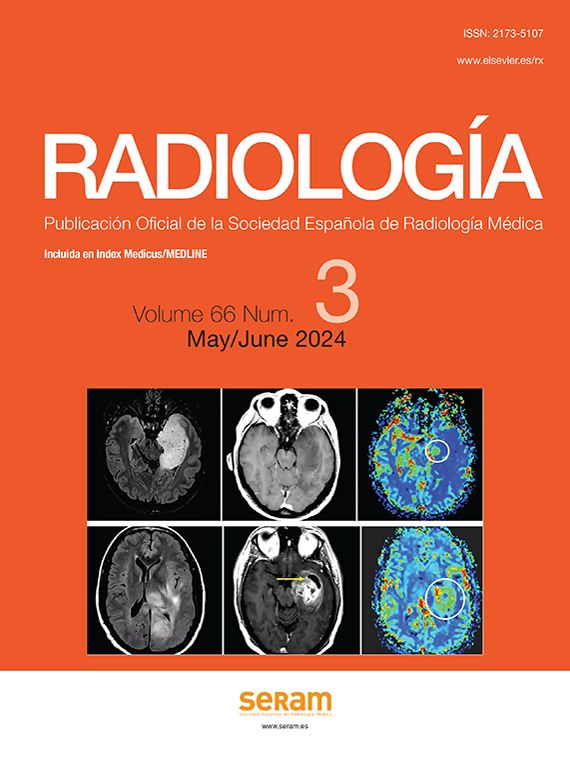
Findings in APR include colorectal anastomosis with surgical staples and increased presacral space, with the rectum separated from the sacrum by around 2cm.
The existence of a presacral, midline fluid collection or small soft tissue mass is common, with average diameters of 1×5.3cm in the anteroposterior and longitudinal directions (Fig. 1). In 70% of cases, this disappears altogether or is minimal after a few months, and in the other 30% it persists, though it may reduce or stabilise over time.4
A rectal displacement greater than 3.5cm or an increase in the amount of soft tissues should prompt suspicion of an anastomotic leak or tumour recurrence, depending on how early or late it occurs.5
APR is performed on neoplastic lesions in the lower rectum, at least 8cm from the ileopectineal line or 5cm from the anal verge. This technique includes resection of the sigmoid colon, rectum and anus with perineal resection. A permanent colostomy is also put in place.3,5
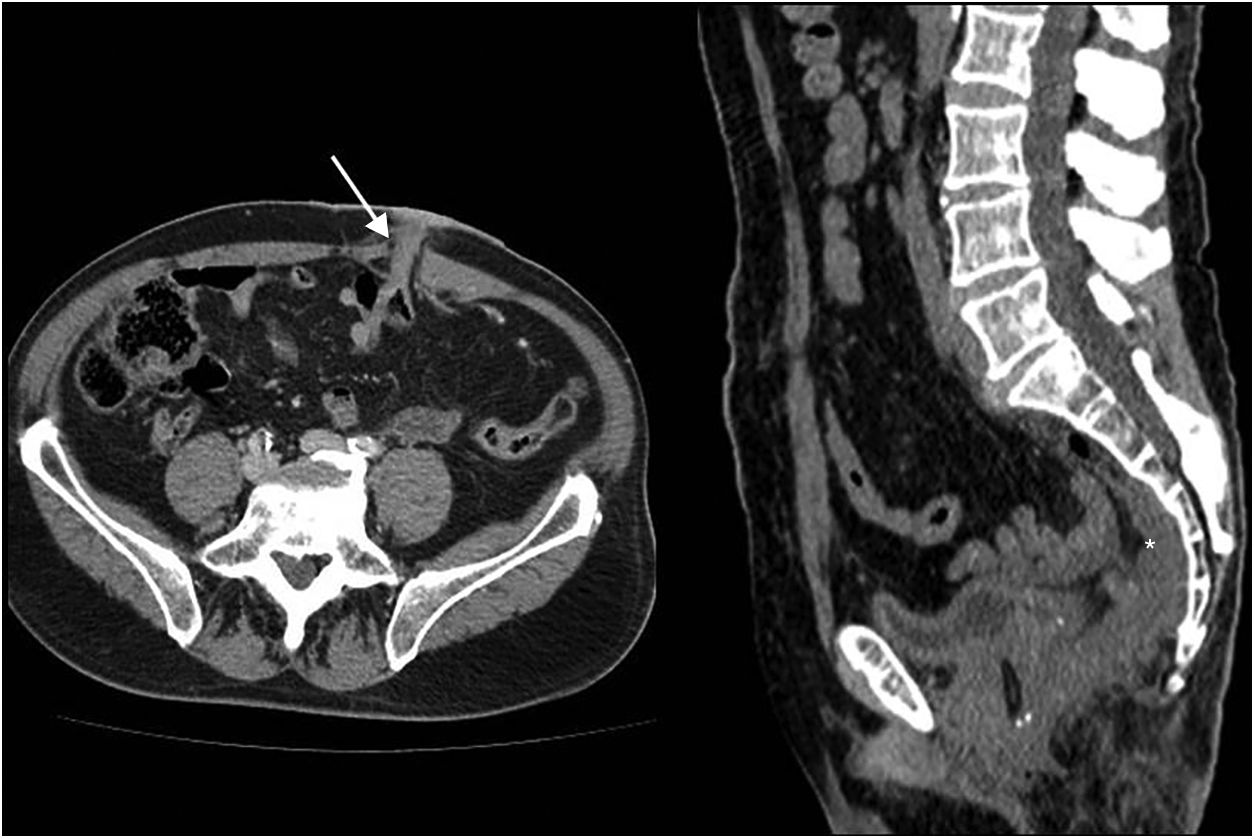
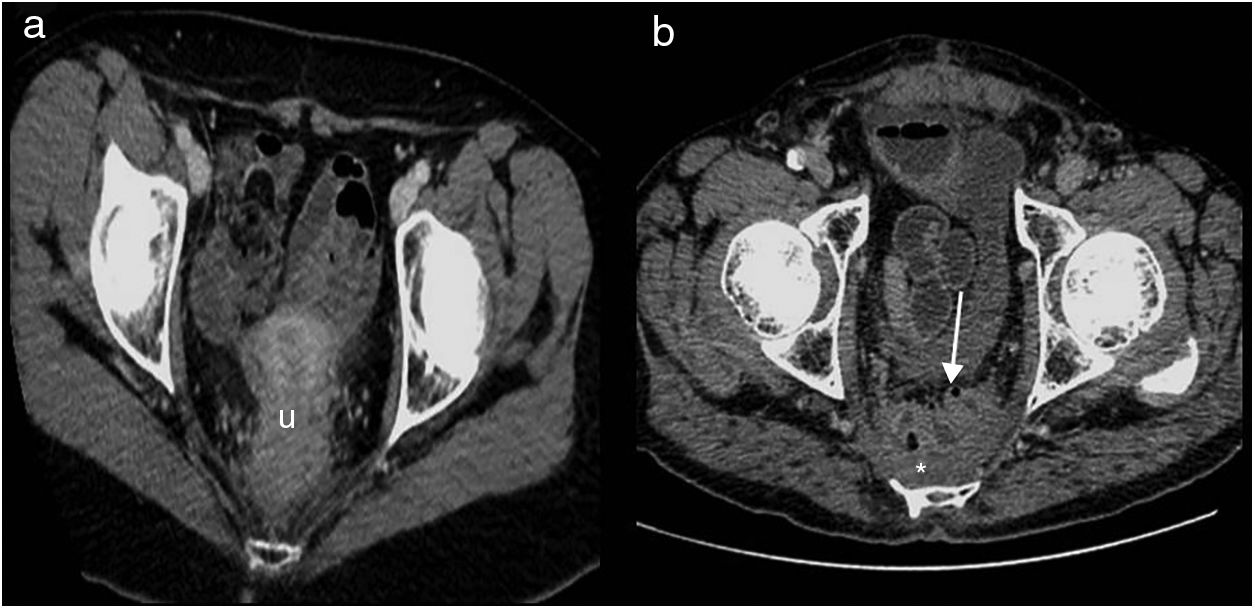
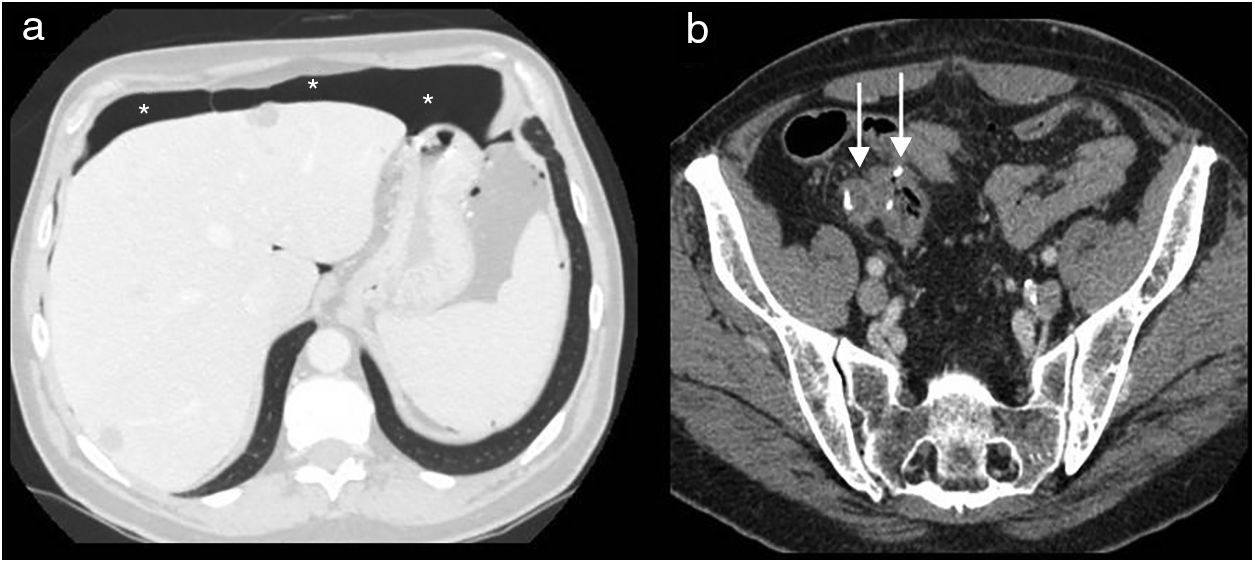
In CT, the permanent colostomy is seen in the abdominal wall (Fig. 2a), along with posterior displacement of the pelvic urogenital structures (bladder, uterus and prostate) and small bowel loops into a precoccygeal location (Fig. 3a). In this location, the uterus may be mistaken for postsurgical changes or local recurrence (Fig. 3b).
Abdominoperineal resection. Multidetector computed tomography of the abdomen with intravenous contrast, transverse planes. (a) Posterior displacement of the uterus seen in the presacral region (u). (b) Small fluid collection (asterisk) and small bowel loops (arrow) in the presacral region.
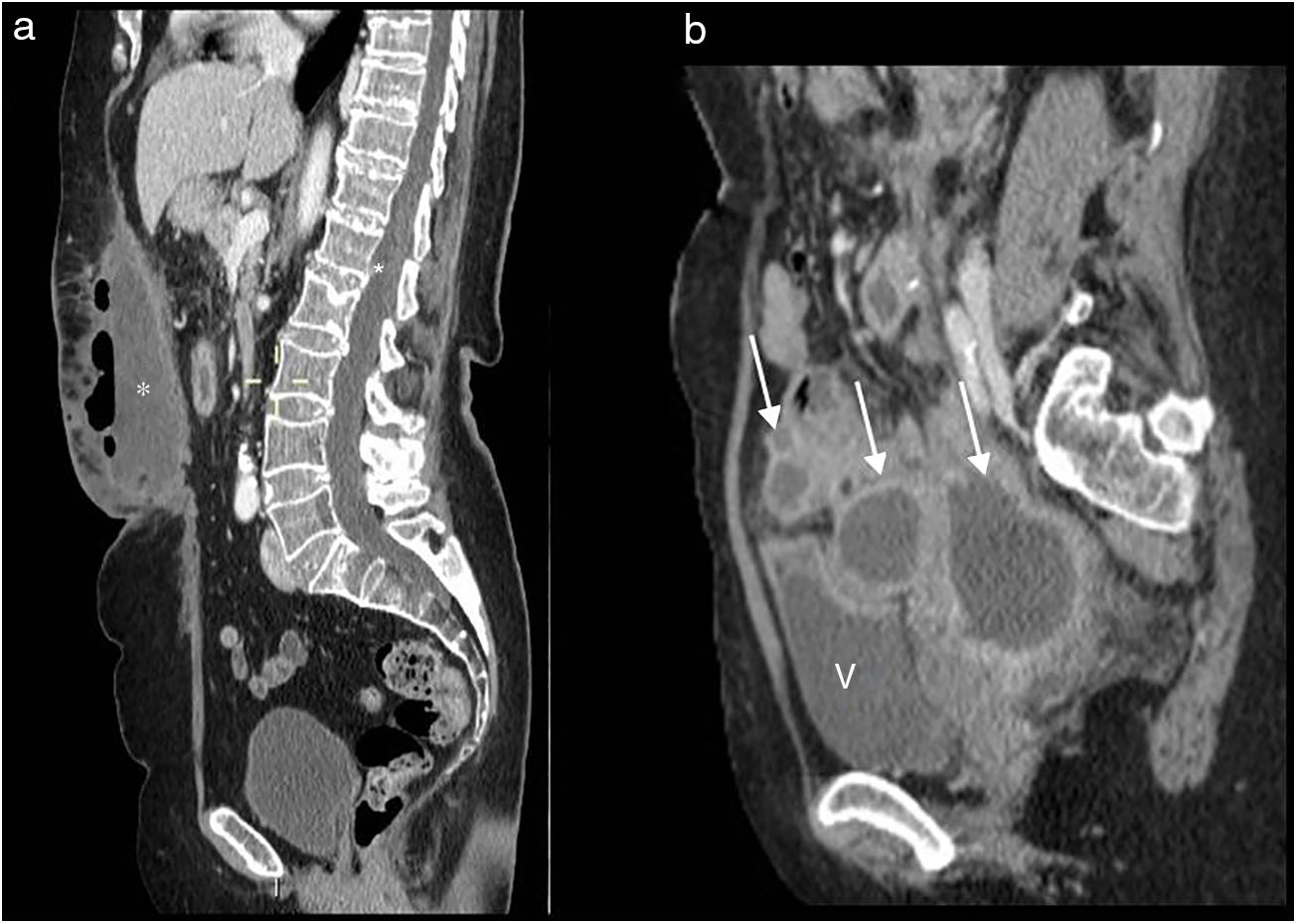
Most patients who undergo APR present a presacral mass in the midline with a maximum diameter of approximately 3–5cm at the initial assessment8 (Fig. 2b). This mass represents granulation tissue, postsurgical fibrosis. It may not be possible to distinguish such masses from a tumour recurrence, with changes at subsequent checkups being what helps to differentiate them. Therefore, performing a reference study is indicated 2–4 months after surgery.
In general, the postsurgical mass will have reduced in size and its borders will be better defined at follow-up, although it may remain stable and persist for over two years, or even permanently. An increase in size or changes in shape would indicate local recurrence. Positron-emission tomography-computed tomography (PET-CT) is the study of choice in case of suspected recurrence, with 98% sensitivity and 96% specificity.9,10
There are three types of anastomosis:
- 1.
End-to-end anastomosis. Indicated when both ends have a similar diameter. These are usually colocolonic or colorectal anastomoses.
- 2.
End-to-side or side-to-end anastomosis. Used when the intestinal ends have different diameters. These are generally utilised in ileocolic or ileorectal anastomoses.
- 3.
Side-to-side anastomosis. Both antimesenteric edges of the bowel are joined. This has the advantage of better revascularisation and is frequently used in ileocolic and small bowel anastomoses.5,11
- 1.
In the immediate postoperative period, fat density changes and small, non-encapsulated fluid collections are frequently observed, both in the surgical bed and in normal anatomical spaces.4,12
- 2.
Anterior abdominal wall changes: the surgical incision is identified as a band of increased attenuation that extends from the peritoneal planes to the skin and is associated with small areas of subcutaneous fat changes.12
- 3.
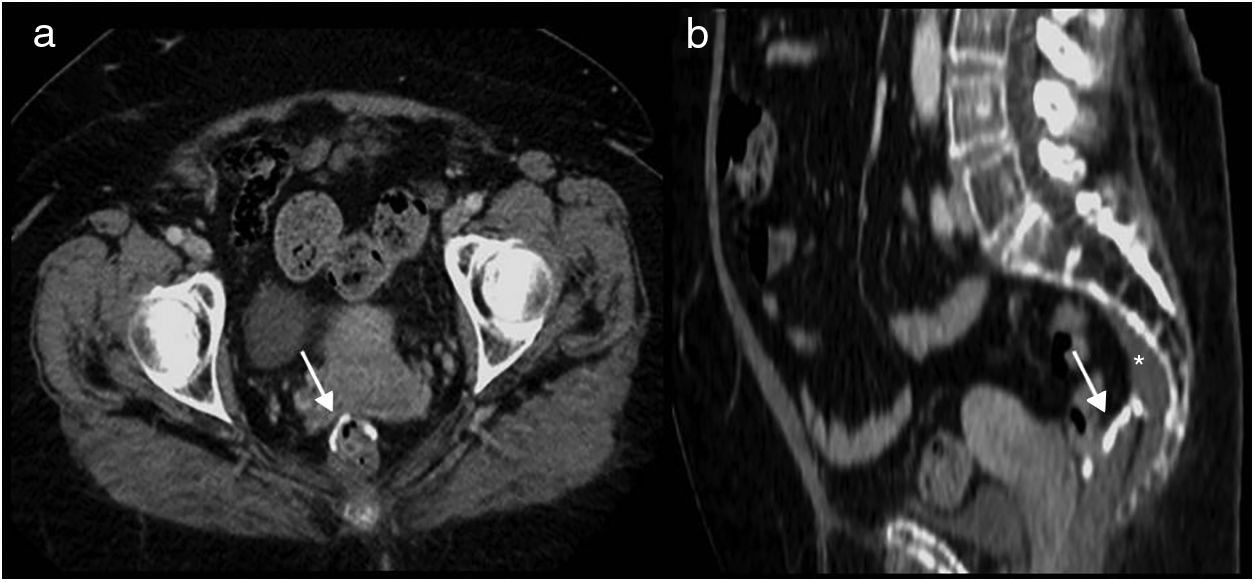
Pneumoperitoneum. Free air after abdominal surgery may simply represent residual air, which is generally well tolerated, is reabsorbed over time and does not require any form of intervention. Alternatively, it may also indicate the existence of a complication in the form of an anastomosis leak or a perforated hollow viscus, both of which require emergency surgery.
Postsurgical pneumoperitoneum is observed in 60% of open surgeries and 25% of laparoscopies. They resolve spontaneously in a period dependent on the patient's characteristics and the imaging technique used. In chest or abdominal X-rays, postsurgical pneumoperitoneum resolves within two days in 67% of patients and within 5 days in 97%. In CT, it is identified after three days in 85% of cases, and can be seen in 50% of patients six days post-surgery, but may persist for 10–24 days.13,14
The management of postsurgical pneumoperitoneum will depend on the patient's medical history, physical examination and laboratory test results. Clinical findings suggesting peritonitis, haemodynamic instability or sepsis are indications for emergency surgery. Pneumoperitoneum alone does not require an intervention, only monitoring (Fig. 4). Nevertheless, the existence of associated free fluid should prompt us to suspect a surgical complication.15
- 4.
Postsurgical ileus: postsurgical ileum is considered a normal phenomenon in the first three or four days after abdominal surgery (up to five days in colon surgery), as the body's physiological response to external aggression.16
- 5.
Subcutaneous emphysema: this is more common in laparoscopic surgery where prolonged CO2 insufflation in the abdominal wall can lead to a subcutaneous emphysema, which may become widespread. This “crackling” usually resolves after the deflation of the abdomen, leaves minimal clinical sequelae and disappears within seven days of the procedure. It is important to recognise this finding and not to confuse it with necrotising fasciitis, which constitutes a surgical emergency. The incidence of necrotising fasciitis after laparoscopic surgery is very rare, usually manifests later (approximately 10 days after the intervention) and is typically associated with peri-incisional erythema, foul-smelling drainage from the wound, fever and pain.17
Postsurgical pneumoperitoneum on day 8 following a total colectomy. Multidetector computed tomography of the abdomen with intravenous contrast, transverse planes. (a) Lung window: anterior perihepatic pneumoperitoneum chamber seen (asterisks). (b) Ileorectal anastomosis (arrows) with no signs of complication. No clinical signs of peritonitis. Spontaneous resolution.
The most common early postsurgical complications include: surgical wound complications, anastomotic leak, abscesses, paralytic ileus/bowel obstruction, abdominal bleeding and injury to the adjacent organs.4,5,16
Abdominal wall complicationsInfectionIncisions become infected in approximately 5–10% of patients who undergo abdominal surgery.12 Despite adequate bowel preparation and prophylactic antibiotics, the risk of infection remains high and is often due to contamination anaerobic bacteria. This can result in inflammation, infection and necrosis of the surgical wound.5
Infection of the wall manifests clinically with signs of wound inflammation, low-grade fever and leukocytosis, usually occurring in the second to third postoperative week.4
In MDCT, cellulitis is observed with thickening and septation of the skin and subcutaneous fat and thickening of the adjacent superficial fossa. Abscesses present as low-density collections with peripheral enhancement, with or without air inside them, and inflammatory changes, of the adjacent subcutaneous tissue18,19 (Fig. 5a).
Multidetector computed tomography of the abdomen with intravenous contrast, sagittal reconstructions. (a) Abdominal wall abscess: fluid collection, with air contents forming a gas-fluid level and increased density and spiculations in the adjacent subcutaneous tissue (asterisk). (b) Intraabdominal abscesses: hypodense collections, with peripheral contrast-enhanced capsules (arrows). V: bladder.
These collections must be drained and broad-spectrum antibiotics administered to avoid both local (dehiscence and incisional hernia) and systemic (peritonitis or systemic sepsis) complications.12
The differential diagnosis usually includes seromas, which are fluid collections that resolve gradually during the first and second postoperative weeks.12
HaematomaA small haematoma at the incision site is not uncommon in the immediate postoperative period. However, this may develop into a significant rectus sheath haematoma as a result of coagulation issues or due to injury to the epigastric vessels during the abdominal incision.12
The most characteristic signs are abdominal pain, a palpable mass and decreased haemoglobin.
If the haematomas are supraumbilical, they may be confined to the thickness of the muscle or limited by the sheath. As for infraumbilical haematomas, where there is no posterior rectus sheath, they can extend to the supravesical extraperitoneal space and then to the peritoneal cavity, causing a haemoperitoneum.20
Their appearance in CT varies according to how developed they are. Initially they may appear as a highly-attenuated fusiform mass, which may show a blood-fluid level. In the non-contrast-enhanced study, the haemorrhage has a density of more than 60 Hounsfield units (HU) in the acute period. Subsequently, the contents turn to liquid.
CT angiography is indicated in large or haemodynamically unstable haematomas. This technique is able to identify the active bleeding site as a hyperdense area, similar to contrast-enhanced CT, of 85–300 HU.21
Treatment is usually conservative and involves interrupting anticoagulation therapy, correcting haemostasis and blood transfusion. In cases of active bleeding, embolisation is also indicated and, if this is not possible, a surgical intervention.22
Dehiscence/incisional herniaDehiscence or evisceration of the abdominal wall is defined as the separation of the abdominal fascia in the early postoperative period. There is no peritoneal lining, so the bowel loops are not contained in a peritoneal sac. It may be partial or complete, depending on whether there is skin dehiscence.23,24
Surgical wound dehiscence occurs around postoperative day 9.23
It is usually associated with postsurgical complications, with the most common being surgical wound infection and paralytic ileus. The main clinical manifestations are: the leakage of serosanguineous fluid (blood-tinged exudate), seeping through the dressing, adynamic ileus and swelling of the surgical wound. Treatment involves emergency surgery with closure of the wall.25
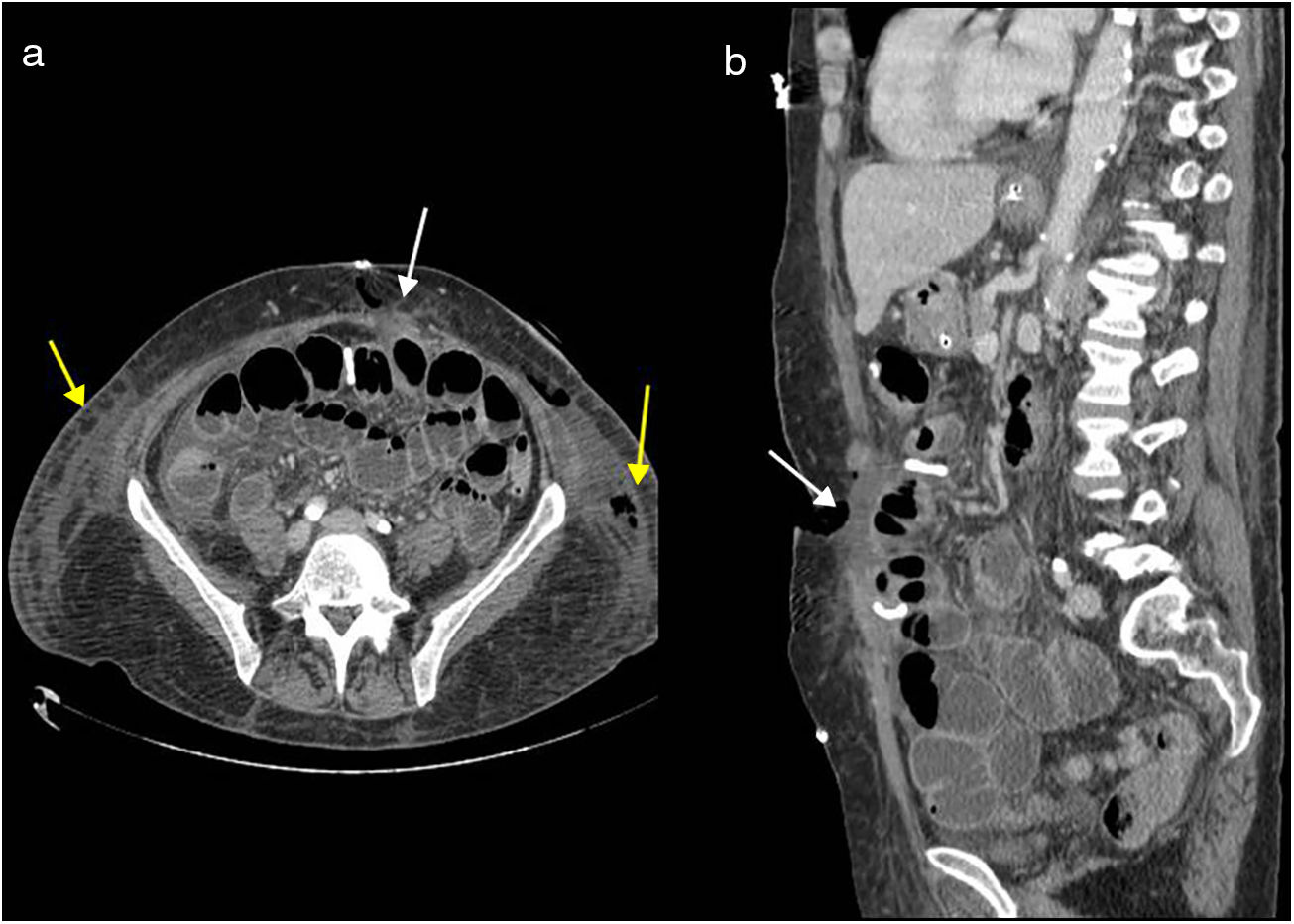
Early CT findings of wall dehiscence include the accumulation of fluid and air within the wall and adjacent tissues and separation of the different wall layers5 (Figs. 6a and b). Complete dehiscence is a surgical emergency associated with a high morbidity and mortality of between 15 and 35%.23
Abdominal wall dehiscence. Evisceration caused by left rectal muscle ischaemia and major infection with slough. Multidetector computed tomography of the abdomen with intravenous contrast, transverse plane (a) and sagittal reconstruction (b). Complete disruption of abdominal wall seen with fluid content between the edges (white arrows) and fluid collections in both flanks (yellow arrows), with air content in the left hand side.
Incisional hernia is an abnormal protrusion from the peritoneum through the pathological surgical wound scar, involving the aponeurotic-fascial-muscle planes.24 It is a late-onset complication of abdominal surgery and typically develops within the first four months of the intervention. The progressive expansion of these hernias will usually manifest with signs and symptoms within the first year.26
CT is the method of choice for defining the wall defect and the composition of the hernia sac, and particularly for assessing the associated complications, such as bowel obstruction.
A stomal or parastomal hernia is a common form of incisional hernia where there is an abnormal protrusion of the abdominal cavity contents at the site of or adjacent to the stoma. An urgent consultation is not usually required unless complications such as bowel obstruction, perforation or incarceration of the herniated loop are suspected.5
Intraabdominal complicationsAbdominal cavity infection: abscess/peritonitisThese essentially occur for two reasons: due to massive microbial contamination during the surgical intervention or due to wound dehiscence. They may also be secondary to an abdominal wall infection, fistulae and perforation. Contamination of the peritoneum occurs in all of the above scenarios, giving rise localised abscesses or generalised peritonitis.27
Clinically, it presents around postoperative day 5 or 6, with abdominal pain accompanied by a raised temperature (>38°C) and a systemic inflammatory response which includes fever, tachycardia and tachypnoea. Rigidity with abdominal guarding suggests the presence of peritonitis.27,28
CT is the technique of choice for diagnosis. In CT images, the most characteristic signs of abscess are: hypodense collection (10–30 HU), with peripheral contrast enhancement, and the possibility of septations, gas or gas-fluid levels in said collection5,12,29 (Fig. 5b).
Peritonitis usually manifests with ascites, increased mesenteric fat attenuation and focal or diffuse thickening of the peritoneum, which shows increased contrast uptake. Associated collections and paralytic ileus reactive to neighbouring inflammatory changes may also be identified, as well as pneumoperitoneum in cases of perforation or wound dehiscence.30
Antibiotics and drainage are indicated for intraabdominal abscesses when there are no signs of generalised peritonitis. In cases of peritonitis, the indicated treatment is surgery and the primary objective is to remove the focus, closing and controlling the source of contamination.27–29
Anastomotic leakThis is defined as the leakage of intraluminal contents from the surgical join between two hollow viscera. Some authors classify leaks as subclinical or clinical.31
It is a serious and potentially fatal complication, although some cases may be managed conservatively. The majority require emergency surgery with a marked increase in patient morbidity and mortality.32,33
In general, leaks occur within the first two postoperative weeks, and are most common between days 5 and 7.5,33
The clinical presentation is not always specific, usually manifesting with fever, leukocytosis, raised C-reactive protein, abdominal pain and paralytic ileus. Other more specific signs would be the presence of purulent matter or faecal drainage.
In cases of clinically visible leaks or those with signs of sepsis or peritonitis, an urgent relaparotomy is indicated. The diagnostic challenge lies in identifying the anastomotic leak in the early postoperative period and in cases with mild or nonspecific symptoms.33–35
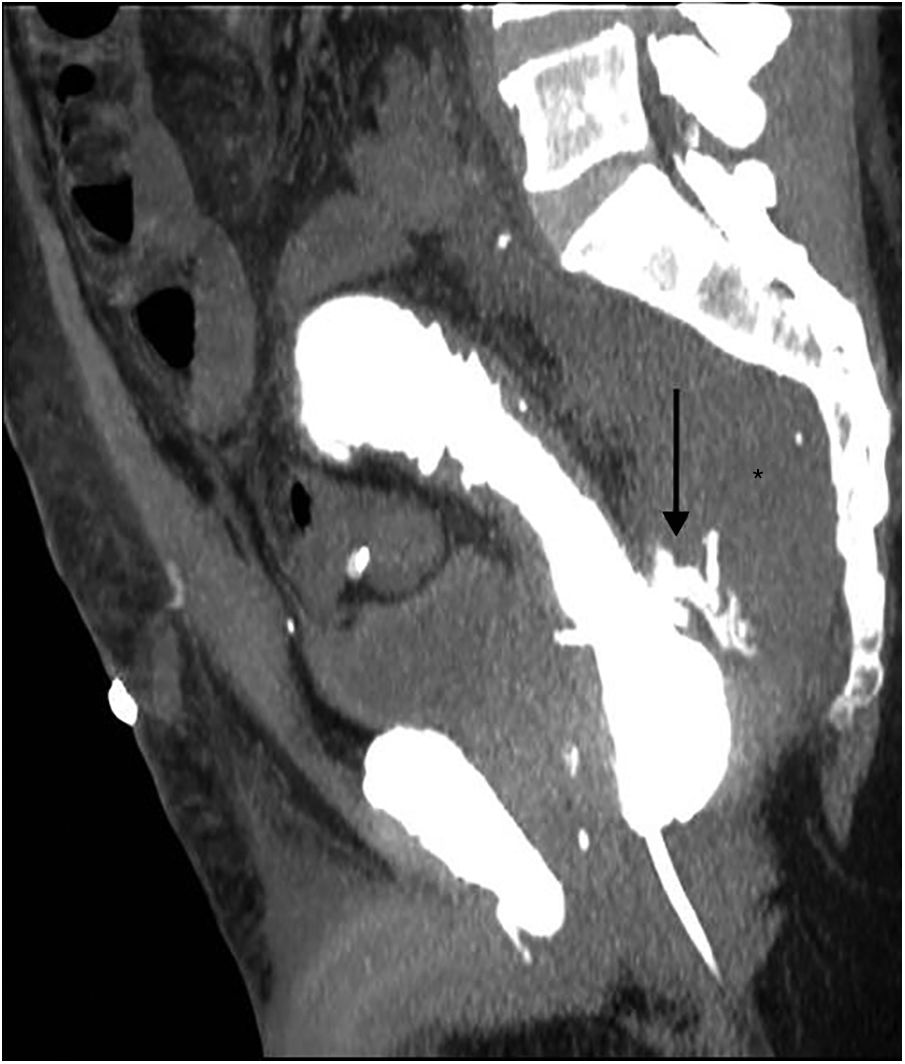
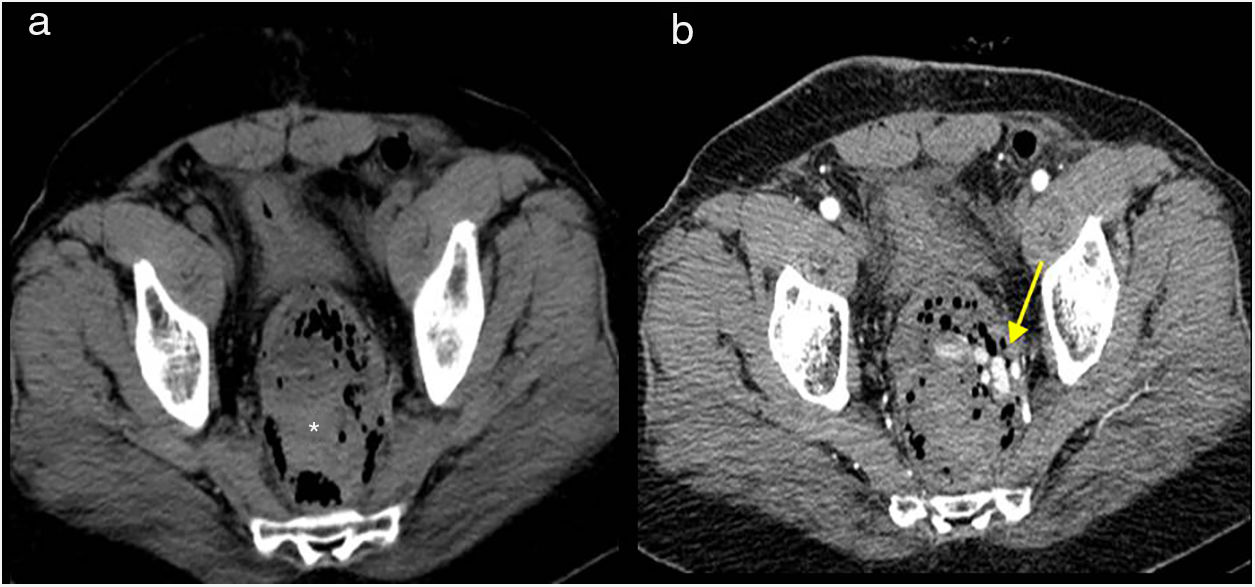
Intravenous contrast-enhanced CT is the technique of choice. The extravasation of rectal contrast, when used, is the most specific sign of an anastomotic leak (Fig. 7). Rectally, when there is frank extravasation, the double rectum sign may be observed.5,32 False negatives may occur with very minor anastomotic defects or in the most proximal anastomoses, where the contrast is diluted and there may not be sufficient pressure to cause a leak.35
Other signs of leak are: large air and fluid collections (hydropneumoperitoneum), surgical bed abscess, and perianastomotic collections with liquid and/or air, which is the most common finding. Anterior displacement of the rectum (separated from the sacrum by more than 5cm) may also be seen when the leak is rectal32,33,35,36 (Figs. 8 and 9).
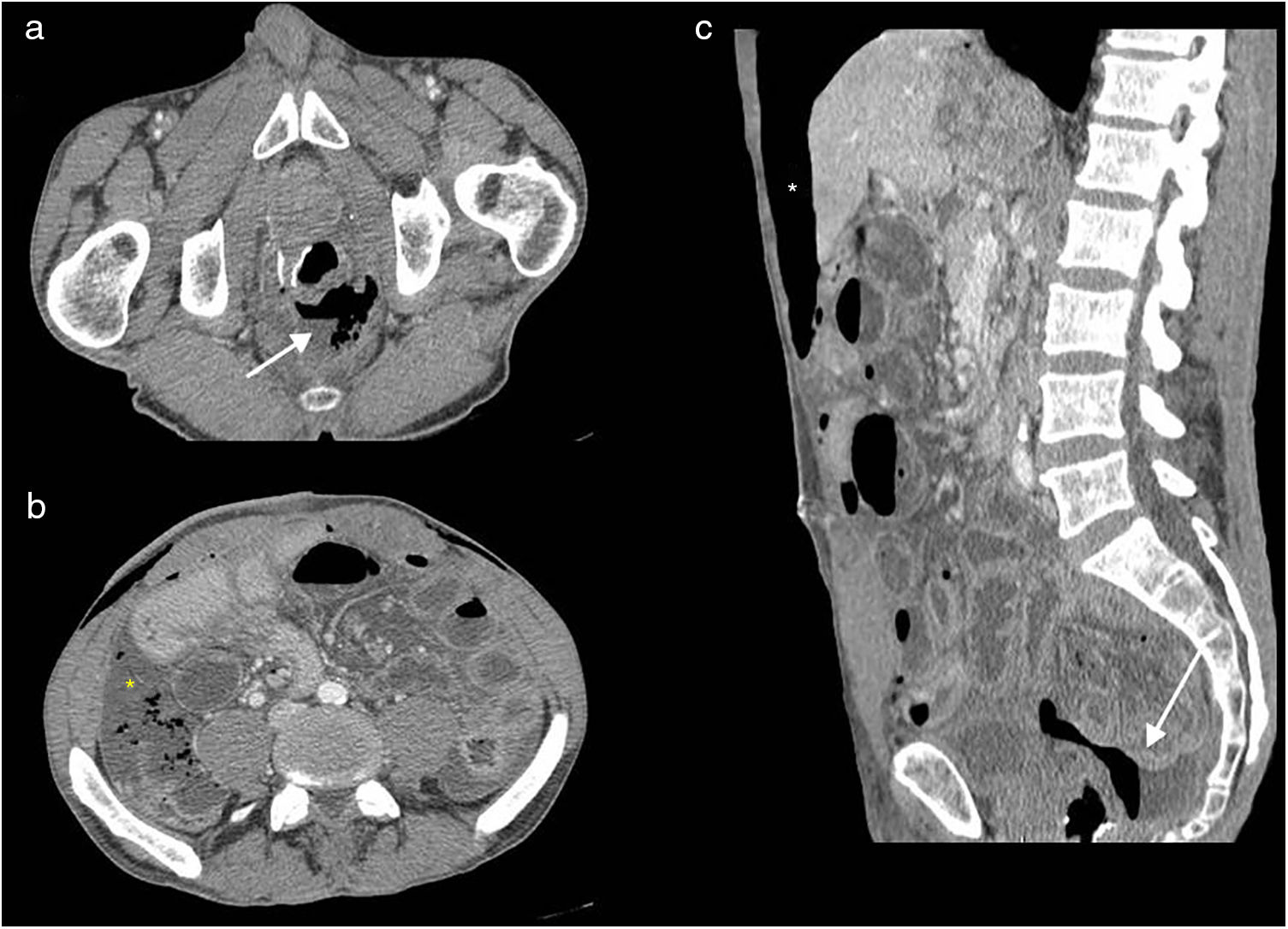
Lower abdominal resection. Wound dehiscence. Multidetector computed tomography of the abdomen with intravenous contrast, transverse (a and b) and longitudinal (c) planes. Air and fluid collection adjacent to the anastomosis (white arrows), anterior perihepatic pneumoperitoneum chamber (white asterisk) and free fluid with air bubbles mainly in the right paracolic gutter (yellow asterisk). Mind wall thickening of small bowel loops, with fluid content in the wall related to reactive inflammatory changes (secondary ileus).
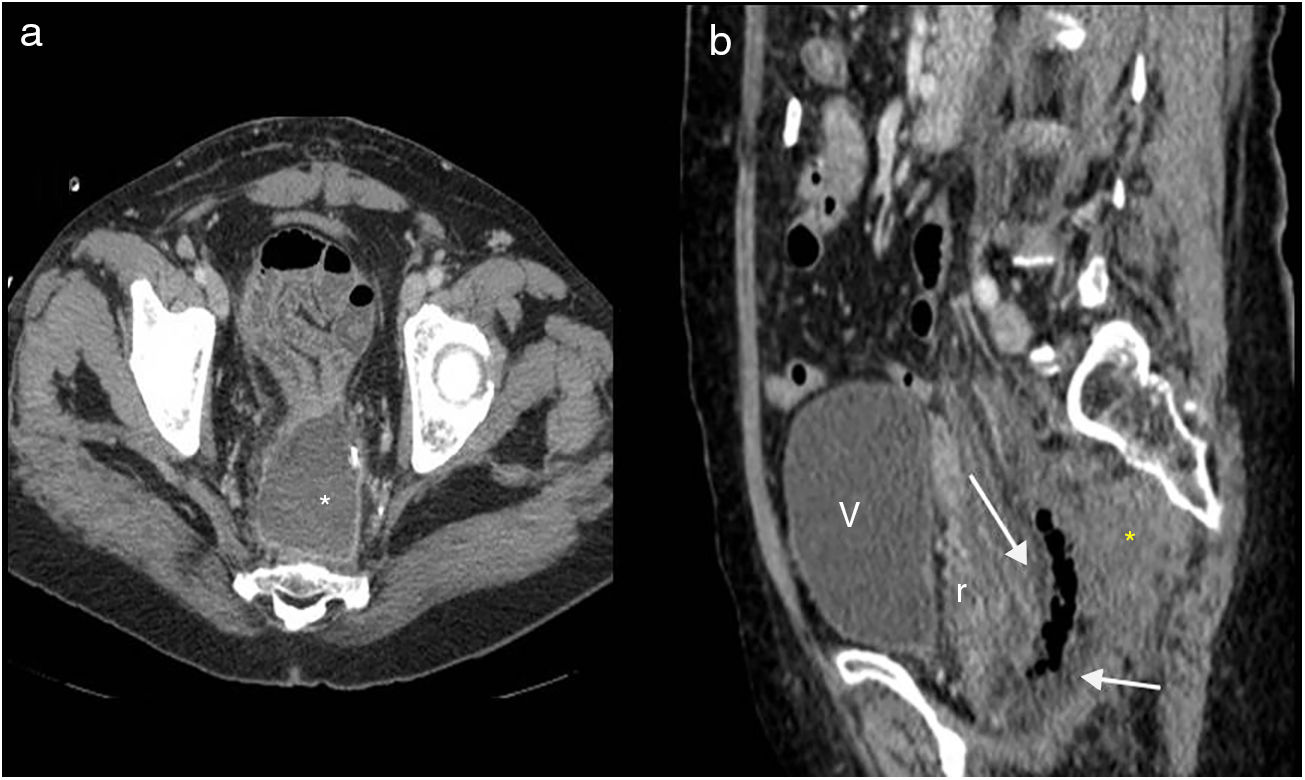
Lower abdominal resection. Wound dehiscence. Multidetector computed tomography of the abdomen with intravenous contrast, transverse plane (a) and sagittal reconstruction (b). (a) Pelvic abscess in the surgical bed (asterisk). (b) Soft tissue mass anterior to the sacrum (asterisk) causing anterior displacement of the rectum (r), small fluid collection with abundant air in the surgical bed. V: bladder.
The appearance of surgical staples is not accurate for assessing anastomotic integrity.32
DuBrow et al. and Matthiessen et al. showed that patients with and without anastomotic leaks had similar findings in the early postoperative period (small reactive fluid collections and pneumoperitoneum). This indicates that the timing of the CT scan may affect diagnostic accuracy as regards anastomotic leaks.36
It is vital to compare CT findings with clinical data and laboratory results. In case of clinical suspicion where the CT scan is negative, additional tests with oral or rectal contrast may be performed or CT repeated. Matthiessen et al. showed that peri-anastomotic air and fluid is increased in patients with anastomotic leaks, in CT scans repeated seven days after surgery.36
The treatment approach is often based on clinical data. Leaks do not always require surgical management because some may be self-limiting. If the leak is contained and the patient remains stable with no signs of sepsis or peritonitis, first-line treatment may be conservative.3,31
Ileus/bowel obstructionIntestinal ileus is defined as the “the functional inhibition of propulsive bowel activity”.37 The motility of the gastrointestinal tract is altered by anaesthesia and surgery.38 The causative agents involved are multiple and interrelated.37–39
Postoperative ileus is considered a physiological response related to the surgical intervention and usually resolves within a few days, or between 24 and 48h. Although there is no consensus on the definition of a normal interval to resumption of transit, between 2 and 7 days is considered normal, according to various authors.39
When the symptoms persist over time, this is considered a paralytic ileus—also known as pathological or prolonged ileus—and it must be differentiated from secondary ileus which is linked to extrinsic causes such as abscesses or peritonitis.
It is sometimes difficult to distinguish between paralytic ileus and bowel obstruction based only on clinical data and an abdominal X-ray. In such cases, CT is highly useful. In paralytic ileus, there is moderate and generalised dilation of the colon and small bowel loops, with no transition zone. The colon appears distended with gas or fluid, unlike in bowel obstructions where it is usually collapsed.40
Bowel obstruction in the early postoperative period is that which occurs within the first 30 days following the surgical intervention, and should be differentiated from prolonged postoperative ileum.41
Bowel obstructions in this period are uncommon, with an approximate incidence of 1%. 90% of these obstructions are caused by adhesions, particularly in case of symptoms of peritonitis. They may also be linked to internal hernias due to omental or mesenteric defects resulting from the intervention, with the formation of abscesses or technical factors.41,42
Clinically, it is difficult to differentiate a bowel obstruction from postoperative ileus. When bowel function is not recovered by postoperative day 5, an underlying cause should be ruled out. Early bowel obstruction can be initially treated conservatively, for 10–14 days, after which recovery without surgical intervention is highly unlikely.42,43
CT allows us to identify whether there is an obstruction or not. The most important radiological sign for diagnosing a bowel obstruction is the sudden transition from a dilated to undilated bowel, which indicates the point of obstruction. The dilated loops have a diameter greater than 2.5cm. The faeces sign may also be seen proximal to the point of obstruction, as well as the absence of gas in the colon and rectum.
The use of undiluted oral Gastrografin (100ml) can be useful for differentiating between paralytic ileus and obstruction and, above all, for determining whether the obstruction is complete. If Gastrografin reaches the colon within 24h after its administration, the obstruction is considered to be partial and may initially be treated conservatively.44
BleedingPerioperative abdominal bleeding is an uncommon complication; management is usually conservative. Intense bleeding is rare.
Perioperative abdominal bleeding is defined by the presence of direct bleeding signs (haematochezia if the bleeding is intraluminal, or the presence of blood in the drainage if it is in the abdominal cavity) or indirect signs (anaemia, haemodynamic repercussion).45,46
If the patient is unstable, in hypovolaemic shock, emergency surgery is indicated. If he or she is stable, CT angiography is the technique of choice for diagnosis, as it allows the bleeding to be located and identifies whether it is active or not (Fig. 10).
Lower abdominal resection. Wound dehiscence with active bleeding in the surgical bed. Computed tomography of the abdomen, transverse planes. (a) Without intravenous contrast: collection in surgical bed with air bubbles and blood content in the dependent portion. (b) Arterial phase: contrast extravasation (yellow arrow) which indicates active bleeding.
In non-contrast-enhanced CT, there may be high-attenuation free fluid (>40 HU) or a haematoma, which will be seen as a hyperdense collection.
The sentinel clot sign refers to the presence of clotted blood close to the area of active bleeding. Contrast extravasation in arterial or portal phase images will indicate whether the bleeding is arterial or venous. Active bleeding measuring more than 1cm in diameter or peritoneal extension are signs of intense bleeding suggesting extensive extravasation.45
Postoperative bleeding which manifests in the form of gastrointestinal bleeding is less common, affecting 1–5.4% of cases. The average time-to-onset is usually one week after surgery.46
Generally, this bleeding is self-limiting and subsides with conservative treatment. When this is ineffective and, as an alternative to surgery, endoscopic management and angiographic embolisation may be used, which are considered less aggressive than another surgical intervention, albeit not without risks.46
ConclusionPostsurgical complications of CRC are common. It is important to be able to recognise and differentiate normal postsurgical findings from early complications in order to avoid unnecessary interventions.
Authorship- 1.
Responsible for the integrity of the study: ARD.
- 2.
Study conception: ARD.
- 3.
Study design: ARD.
- 4.
Data collection: ARD, DMR, TRG, AGO and LCA.
- 5.
Data analysis and interpretation: ARD, TRG, DMR, AGO and LCA.
- 6.
Statistical processing: N/A.
- 7.
Literature search: ARD.
- 8.
Drafting of the article: ARD, DMR, TRG.
- 9.
Critical review of the manuscript with intellectually relevant contributions: ARD, DMR, TRG, AGO and LCA.
- 10.
Approval of the final version: ARD, DMR, TRG, AGO and LCA.
The authors declare that they have no conflicts of interest.
Please cite this article as: Rivera Domínguez A, de Araujo Martins-Romeo D, Ruiz García T, García de la Oliva A, Cueto Álvarez L. Tomografía computarizada multidetector urgente de la cirugía del cáncer colorrectal: Cambios posquirúrgicos y complicaciones tempranas. Radiología. 2019;61:286–296.