To compare the usefulness of CT angiography against the gold standard, digital subtraction angiography (DSA), in the characterisation of cerebral arteriovenous malformations (AVM) that present with bleeding.
Material and methodsWe retrospectively analysed patients with intracranial bleeding due to an AVM who were included in a prospective database in the period comprising January 2007 through December 2012. We reviewed radiologic variables such as the characteristics of the AVM (size, location, presence of deep venous drainage), involvement of eloquent areas, and the presence of associated aneurysms. Two neuroradiologists blinded to clinical and radiological information analysed the CT and DSA in consensus.
ResultsA total of 22 patients were included in the study. CT angiography correctly classified 15 of the 16 cases of AVM measuring less than 3cm (93.75% sensitivity). All cases of deep venous drainage and all those located in eloquent areas were correctly detected (100% sensitivity). The presence of any type of aneurysm related with the AVM was detected in 13 of 15 cases (86.6% sensitivity); 7 of 9 of the intranidal aneurysms were detected (77.78% sensitivity), as were 6 of the 9 flow aneurysms (66.67% sensitivity).
ConclusionCT angiography is highly sensitive in the characterisation of cerebral AVMs measuring less than 3cm, of those located in eloquent areas, and of those with deep venous drainage; it is also highly sensitive in detecting aneurysms related with AVMs. However, CT angiography is less sensitive in detecting intranidal and flow aneurysms related with AVMs.
El objetivo es determinar la utilidad de la angio-TC cerebral en la caracterización de las malformaciones arteriovenosas (MAV) cerebrales con presentación hemorrágica comparada con la angiografía por sustracción digital (DSA) como patrón de referencia.
Material y métodosSe realizó un análisis retrospectivo de una base de datos prospectiva de pacientes con sangrado intracraneal debido a una MAV cerebral desde enero de 2007 hasta diciembre de 2012. Se revisaron variables radiológicas, como las características de la malformación (tamaño, localización, presencia de drenaje venoso profundo), afectación de un área elocuente y presencia de aneurismas relacionados. Dos neurorradiólogos ciegos a cualquier información clínico-radiológica analizaron por consenso las imágenes de tomografía computarizada y DSA.
ResultadosVeintidós pacientes fueron incluidos en el estudio. La angio-TC clasificó correctamente 15 de los 16 casos de MAV menores de 3cm, con una sensibilidad del 93,75%. Todos los casos con drenaje venoso profundo y localizados en un área elocuente fueron correctamente detectados (sensibilidad 100%). La presencia de cualquier tipo de aneurisma relacionado con la MAV fue detectada en 13 de 15 pacientes (sensibilidad 86,6%); 7 de 9 en los intranidales (sensibilidad 77,78%) y 6 de 9 de los aneurismas de flujo (sensibilidad 66,67%).
ConclusiónLa angio-TC tiene una alta sensibilidad en la caracterización de MAV cerebrales en cuanto al tamaño menor de 3cm, localización en área elocuente, presencia de drenaje venoso profundo y la detección de cualquier aneurisma relacionado con la MAV. Sin embargo, la angio-TC tiene una menor sensibilidad en la detección de aneurismas intranidales y de flujo relacionados con la MAV.
Cerebral arteriovenous malformations (AVMs) are developmental disorders consisting of direct intracerebral arteriovenous shunts (connections), without the normal presence of a capillary bed.1 These vascular malformations affect 0.01–0.5% of the population,2 present in more than half of cases as an intracranial haemorrhage, have an annual risk of bleeding of 1.5–3% and have a risk of death on first bleeding of 10%; this risk increases with each new episode.3,4
The diagnosis and treatment of these entities requires a detailed characterisation of their angioarchitecture and haemodynamics (location, size of the nidus, arterial inflows and venous drainage). It is of particular importance to, on the one hand, determine the existence of intranidal aneurysms, since they would correspond to a greater risk of rebleeding,1 and, on the other hand, determine the risk to the patient in both surgical treatment (Spetzler-Martin grading system)5 and endovascular treatment (eloquent area and single deep venous drainage).6
Digital subtraction angiography (DSA) of the brain is the standard of care for the diagnosis and follow-up of cerebral AVMs.7 However, the test is bloody, not risk-free and of limited availability.8,9 Some studies have shown a good correlation between magnetic resonance imaging (MRI) and DSA in characterising AVMs with different presentations10–12; however, the latter is usually relatively unavailable and the process of image acquisition is slower than in computed tomography (CT) angiography. The objective of this study is to determine the usefulness of cerebral CT angiography in characterising cerebral AVMs with a haemorrhagic presentation compared to DSA as the standard of care (Figs. 1–4).
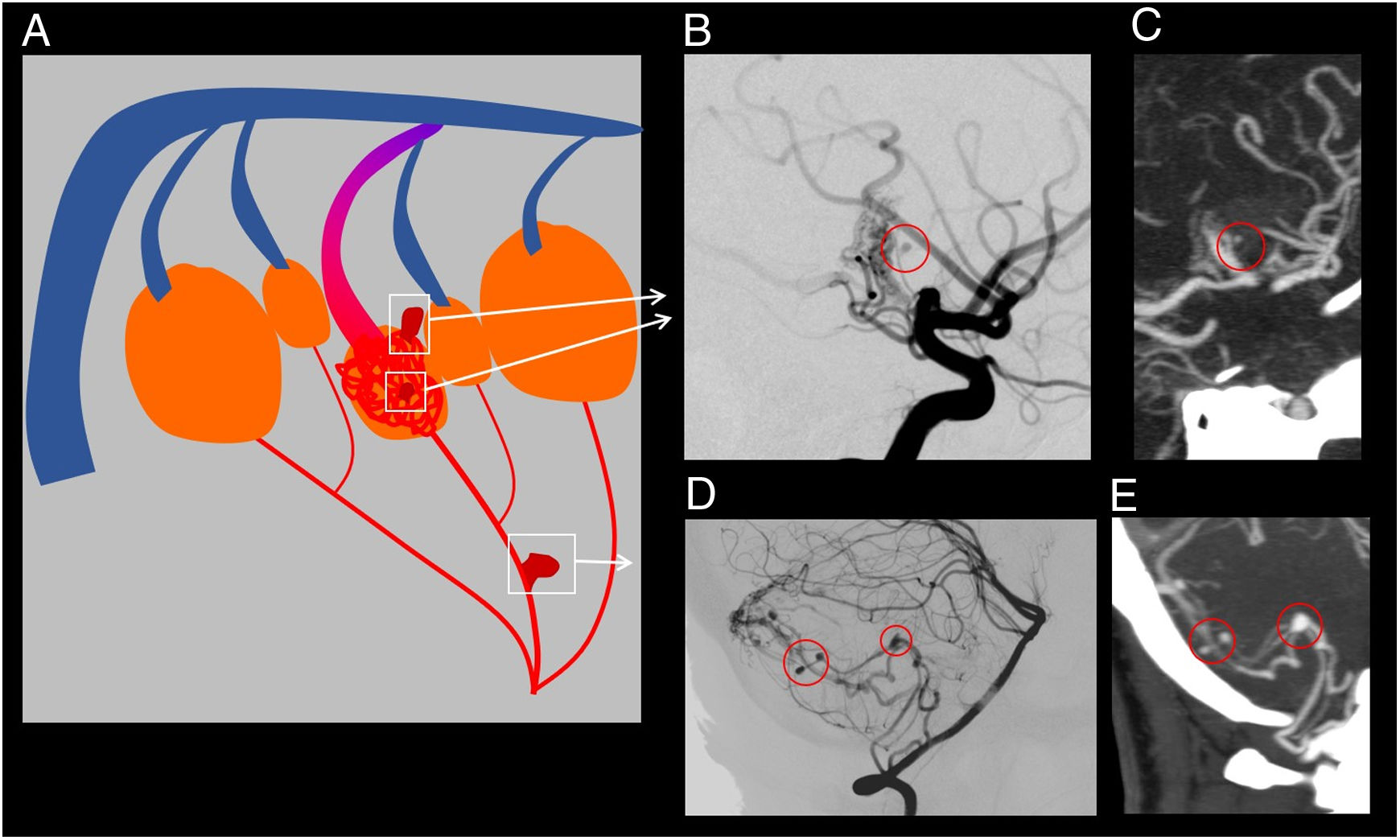
(A) Explanatory diagram on intranidal aneurysms and flow-related aneurysms. (B) and (C) Cerebral angiography and CT angiography for the same patient showing the presence of an intranidal aneurysm. (D) and (E) Cerebral angiography and CT angiography for the same patient showing the presence of various flow-related aneurysms.
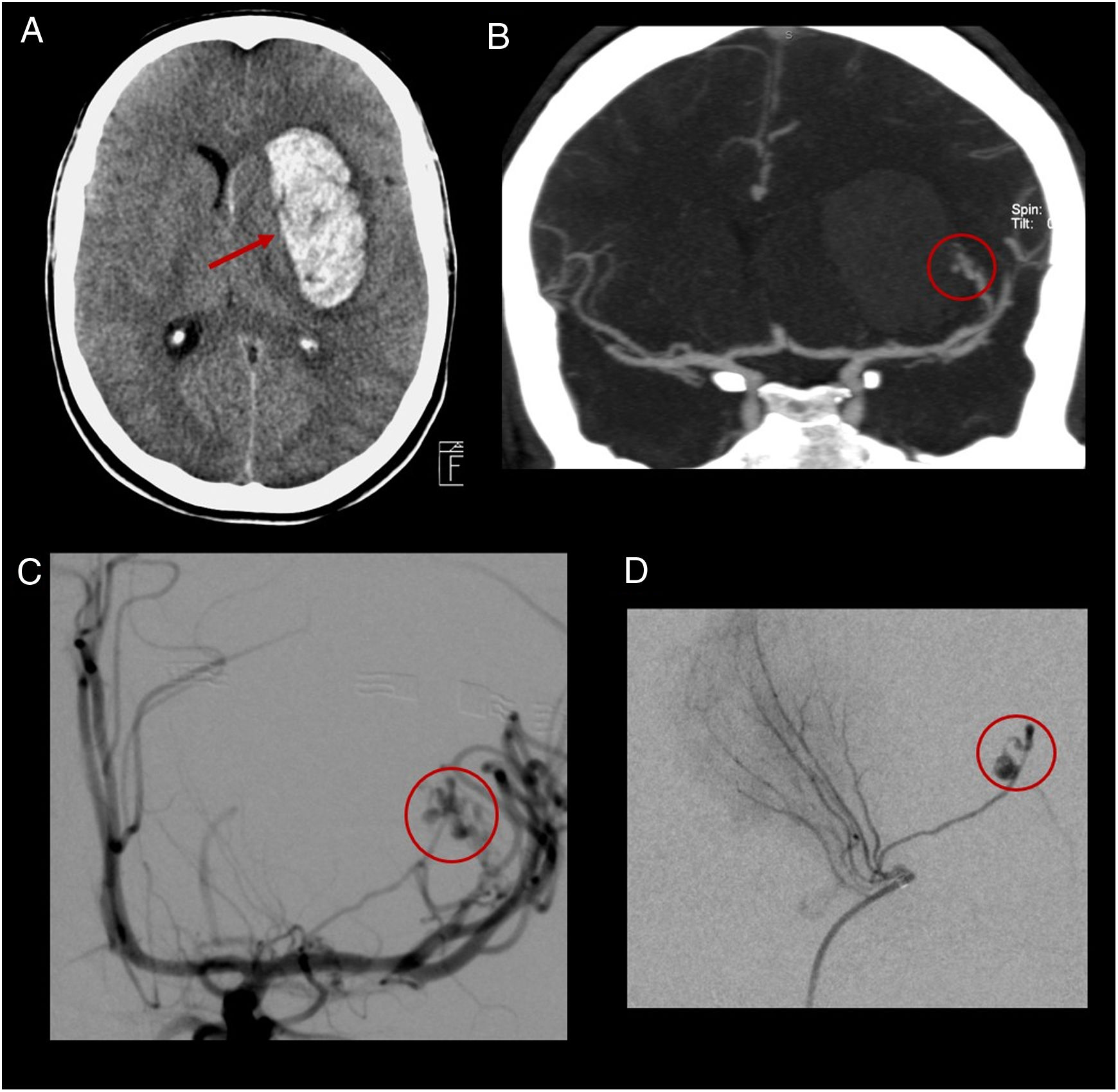
(A) Computed tomography without contrast showing a left intraparenchymal haematoma of the basal ganglia (arrow). (B) Coronal CT scan showing an arteriovenous malformation (AVM) dependent on the M2 segment of the left middle cerebral artery with the presence of intranidal aneurysms (circle). (C) and (D) Non-selective cerebral angiography (C) and selective cerebral angiography (D) confirming the presence of the AVM and the presence of intranidal aneurysms (circles).
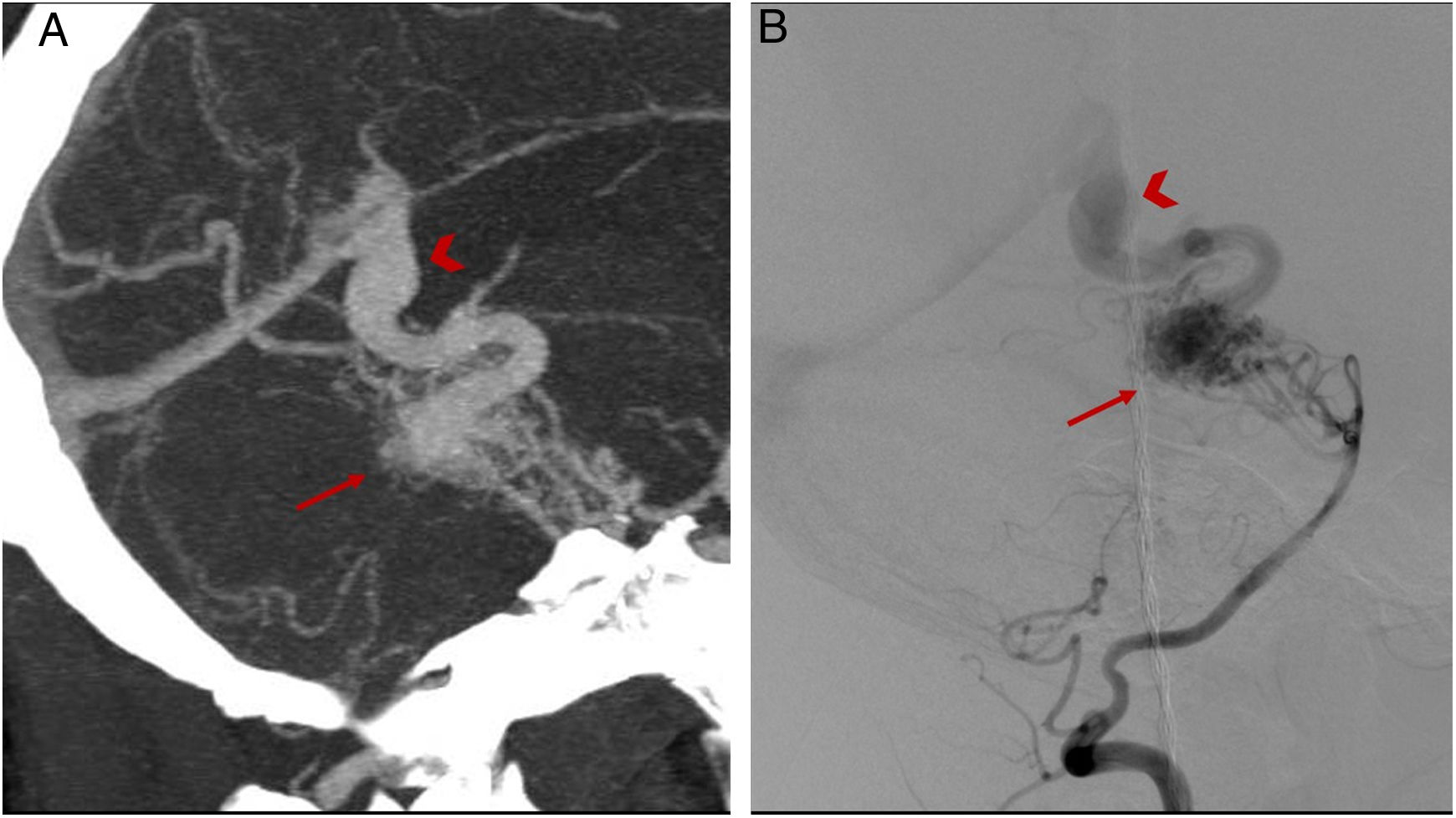
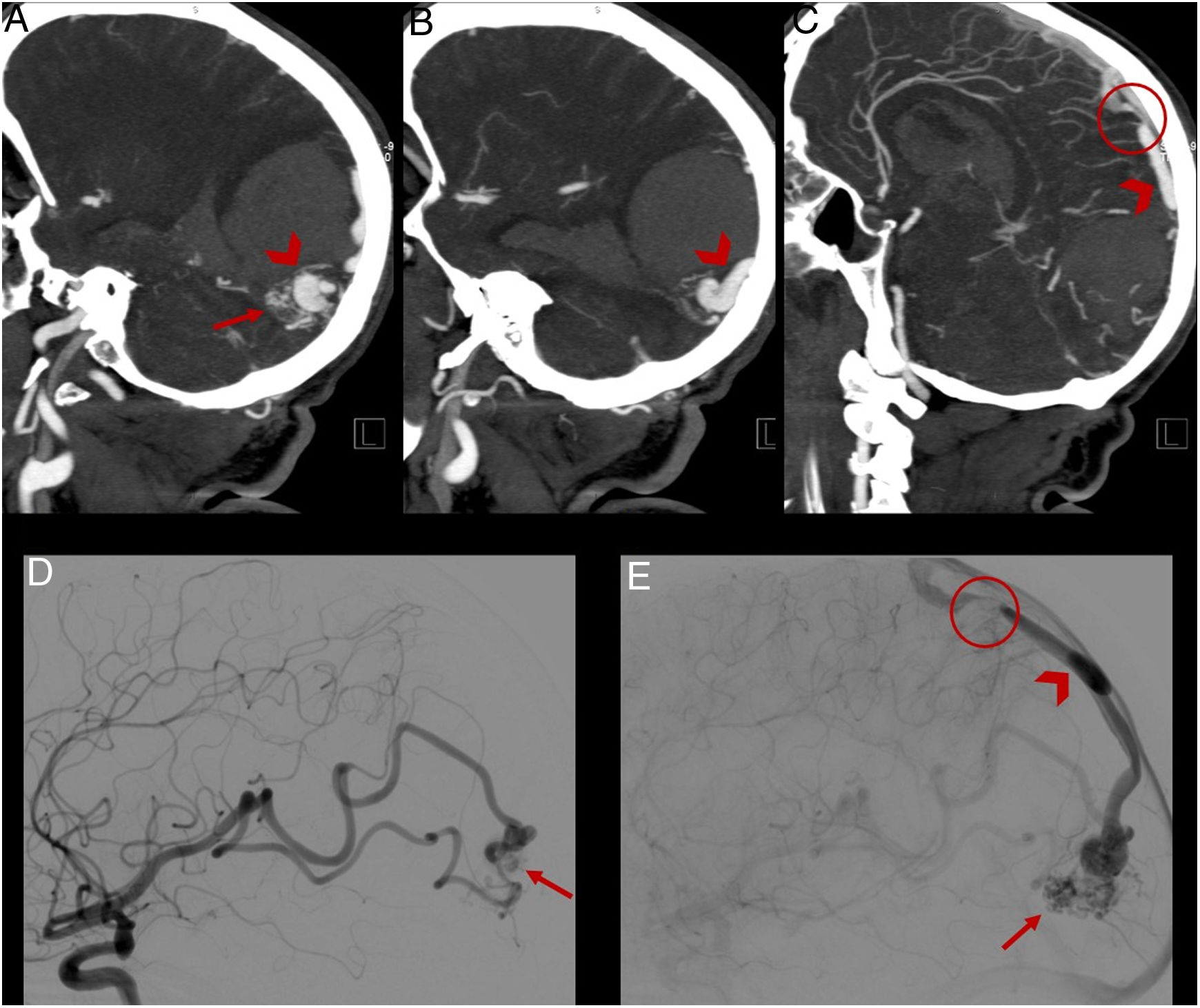
(A–C) CT angiography showing an arteriovenous malformation located in the occipital lobe (arrow), with a drainage vein towards the superior sagittal sinus (arrowhead) presenting stenosis in its distal segment (circle). (D) and (E) Digital subtraction angiography confirming the findings reported.
A retrospective analysis was conducted based on a prospective database from our centre of patients admitted with cerebral haemorrhage secondary to an AVM between January 2007 and December 2012. Only patients who had undergone intracranial CT angiography and complete cerebral angiography with a lapse of less than 72h between the two tests and who showed no clinical changes suggestive of rebleeding were enrolled. The patients or their family members signed the informed consent form for cerebral angiography to be performed. Patients whose CT angiography was of limited quality and therefore not valid for determining the radiological variables being studied were excluded. As this was a retrospective analysis, it was not necessary to obtain independent ethics committee approval.
Clinical and radiological variablesBoth sociodemographic variables (age and sex) and radiological variables (baseline CT scan, CT angiography and DSA) were collected. Baseline brain CT images were acquired with a sequential acquisition protocol in a multi-slice CT scanner (Siemens Somatom Definition Flash or Siemens Sensation 64, Erlangen, Germany), using 140kV, 230mA, with a slice thickness of 5mm from the base to the vertex of the skull, with continuous axial slices parallel to the orbitomeatal line.
CT angiography images were acquired with a helical technique. Images were obtained following administration of a bolus of 50ml of intravenous contrast (Ultravist 300–370mg/ml, Bayer Hispania, Barcelona, Spain) at a rate of 5ml/s. Acquisition was performed using CARE Dose 4D automated exposure control technology by positioning a circular area of interest on the ascending aorta. The slice thickness was 0.6mm. Reconstructions were performed with a thickness of 5mm on the axial plane at half-slice intervals. Complete cerebral angiography was performed which included a study of six vessels (both internal carotid arteries, external carotid arteries and vertebral arteries) within 24h of patient admission.
Radiological variables from the baseline CT scan considered included: type of haemorrhage (intraparenchymal haematoma [IPH], subarachnoid haemorrhage [SAH] or intraventricular haemorrhage [IVH]), specific location (lobe, basal ganglia or cerebellum) and greatest diameter of the haematoma (expressed in millimetres).
The following variables from CT angiography and DSA corresponding to particular characteristics of AVMs were assessed: variables included in the Spetzler-Martin grading system5 (AVM size expressed as <3cm, 3–6cm or >6cm; deep venous drainage and involvement of an eloquent area); grade according to the Spetzler-Martin grading scale (grade 1–5) and specific location of the AVM (frontal lobe, temporal lobe, parietal or occipital lobe, basal ganglia, midbrain, pons, medulla oblongata, cerebellum, or mixed). Finally, the following variables related to the angioarchitecture of the AVMs studied were considered: presence of any AVM-related aneurysm, presence of flow-related aneurysms, presence of intranidal aneurysms, presence of supply from a perforating artery, presence of a venous pouch, presence of venous stenosis and presence of a single drainage vein bottom, taking into account the main variables reported in the literature as potential risk factors for rebleeding.13–25
Two neuroradiologists (one with more than 10 years’ experience and the other with four years’ experience in neuroimaging) performed a consensus reading of the CT scan without contrast to determine the patient's type of haemorrhage, following an independent reading of the intracranial CT scan performed, which was blind to any clinical or angiographic data corresponding to the patient. In addition, two neuroradiologists (one with more than 10 years’ experience and the other with five years’ experience in neuroimaging) performed a consensus reading of the patients’ DSA.
Statistical analysisA diagnostic yield study was conducted taking cerebral angiography as the standard of care. The following statistical parameters were calculated for each variable reported evaluated on CT angiography and angiography: sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), diagnostic precision and consistency between readers. These parameters were evaluated with the SPSS version 20.0 software programme (SPSS, Chicago, Illinois).
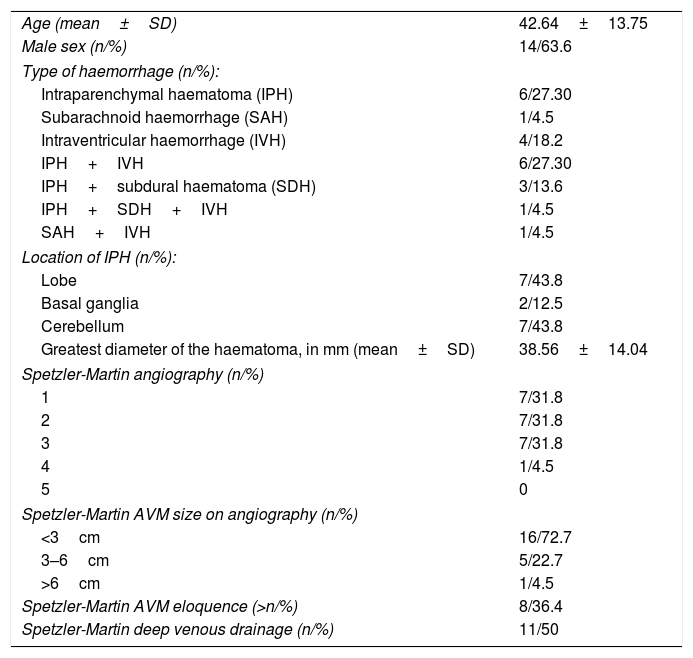
ResultsSample characteristicsTwenty-two patients met the inclusion criteria and did not meet the exclusion criteria. Table 1 summarises the main descriptive variables from the baseline CT scan and the DSA for the sample. The most common types of haemorrhage were IPH and IPH+IVH, both with six patients (27.3% each). Regarding the location of the IPHs, lobar and cerebellar IPHs were the most common, with seven patients each (43.8%).
Descriptive variables for baseline computed tomography and angiography.
| Age (mean±SD) | 42.64±13.75 |
| Male sex (n/%) | 14/63.6 |
| Type of haemorrhage (n/%): | |
| Intraparenchymal haematoma (IPH) | 6/27.30 |
| Subarachnoid haemorrhage (SAH) | 1/4.5 |
| Intraventricular haemorrhage (IVH) | 4/18.2 |
| IPH+IVH | 6/27.30 |
| IPH+subdural haematoma (SDH) | 3/13.6 |
| IPH+SDH+IVH | 1/4.5 |
| SAH+IVH | 1/4.5 |
| Location of IPH (n/%): | |
| Lobe | 7/43.8 |
| Basal ganglia | 2/12.5 |
| Cerebellum | 7/43.8 |
| Greatest diameter of the haematoma, in mm (mean±SD) | 38.56±14.04 |
| Spetzler-Martin angiography (n/%) | |
| 1 | 7/31.8 |
| 2 | 7/31.8 |
| 3 | 7/31.8 |
| 4 | 1/4.5 |
| 5 | 0 |
| Spetzler-Martin AVM size on angiography (n/%) | |
| <3cm | 16/72.7 |
| 3–6cm | 5/22.7 |
| >6cm | 1/4.5 |
| Spetzler-Martin AVM eloquence (>n/%) | 8/36.4 |
| Spetzler-Martin deep venous drainage (n/%) | 11/50 |
AVM: arteriovenous malformation.
Regarding the characteristics of the AVMs according to the Spetzler-Martin grading system, most measured less than 3cm (72.7%), only eight were found in an eloquent area (36.4%) and 11 had deep venous drainage. Regarding grades on the same scale, grades 1, 2 and 3 were the most common, with seven patients each (31.8%).
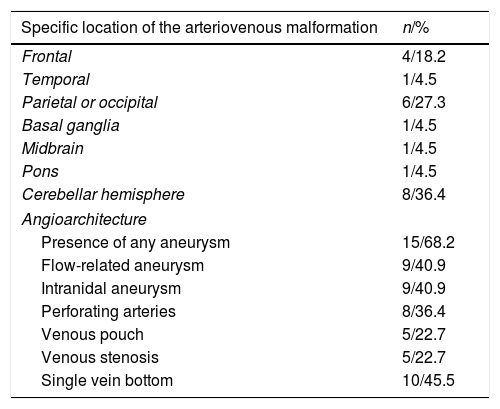
The most common locations of the AVMs were the cerebellar hemispheres with eight AVMs (36.4%), followed by the parietal or occipital lobe with six AVMs (27.3%) and the frontal lobe with four AVMs (18.2%) (Table 2). Finally, regarding the characteristics of the AVMs in terms of angioarchitecture, 15 (68.2%) patients presented at least one AVM-related aneurysm; in turn, nine (40.9%) presented at least one flow-related aneurysm and nine (40.9%) presented at least one intranidal aneurysm. In addition, eight cases (36.4%) presented arterial supply by perforating arteries, five (22.7%) presented a venous pouch, five (22.7%) presented venous stenosis and 10 (45.5%) presented a single drainage vein bottom.
Cerebral angiography. Angioarchitecture of arteriovenous malformations.
| Specific location of the arteriovenous malformation | n/% |
|---|---|
| Frontal | 4/18.2 |
| Temporal | 1/4.5 |
| Parietal or occipital | 6/27.3 |
| Basal ganglia | 1/4.5 |
| Midbrain | 1/4.5 |
| Pons | 1/4.5 |
| Cerebellar hemisphere | 8/36.4 |
| Angioarchitecture | |
| Presence of any aneurysm | 15/68.2 |
| Flow-related aneurysm | 9/40.9 |
| Intranidal aneurysm | 9/40.9 |
| Perforating arteries | 8/36.4 |
| Venous pouch | 5/22.7 |
| Venous stenosis | 5/22.7 |
| Single vein bottom | 10/45.5 |
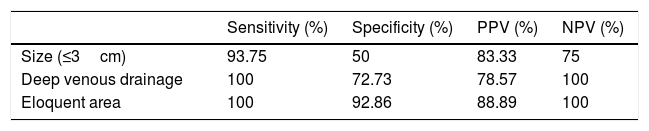
The results of the study of the diagnostic yield of CT angiography compared to cerebral angiography with regard to the AVM characteristics that figure in the Spetzler-Martin grading system are summarised in Table 3. Regarding AVM size, dichotomised at 3cm, CT angiography yielded just one false negative (FN); in that case, CT angiography erroneously classified an AVM as more than 3cm in size. In three patients, CT angiography classified the AVM as more than 3cm, when in reality it was less than 3cm (false positive [FP]). Regarding deep venous drainage and eloquent area, CT angiography did not yield any FNs. The former yielded three FPs and the latter yielded one FP, in which it was indicated that patients had deep drainage or involvement of an eloquent area, respectively, when in reality they did not.
Diagnostic yield of computed tomography angiography compared to digital subtraction angiography with regard to the characteristics of arteriovenous malformations included in the Spetzler-Martin grading system.
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|
| Size (≤3cm) | 93.75 | 50 | 83.33 | 75 |
| Deep venous drainage | 100 | 72.73 | 78.57 | 100 |
| Eloquent area | 100 | 92.86 | 88.89 | 100 |
NPV: negative predictive value; PPV: positive predictive value.
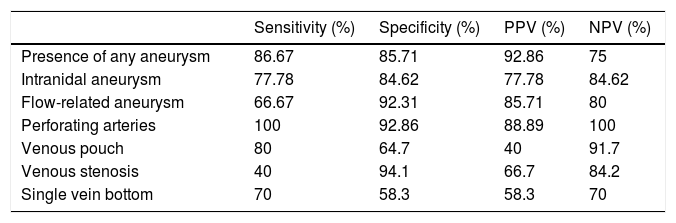
The diagnostic yield of CT angiography compared to cerebral angiography with regard to AVM angioarchitecture is summarised in Table 4. Regarding the presence of any aneurysm, CT angiography yielded two FNs in which it failed to detect aneurysms that in reality were present. When flow-related aneurysms and intranidal aneurysms were analysed separately, CT angiography was seen to yield three FNs and two FNs, respectively. Regarding the presence of perforating arteries, CT angiography did not yield any FNs. Finally, CT angiography presented one FN in the evaluation of the presence of a venous pouch, whereas it presented three FNs in the evaluation of venous stenosis and a single drainage vein bottom.
Diagnostic yield of computed tomography angiography compared to digital subtraction angiography with regard to the angioarchitecture of cerebral arteriovenous malformations.
| Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|
| Presence of any aneurysm | 86.67 | 85.71 | 92.86 | 75 |
| Intranidal aneurysm | 77.78 | 84.62 | 77.78 | 84.62 |
| Flow-related aneurysm | 66.67 | 92.31 | 85.71 | 80 |
| Perforating arteries | 100 | 92.86 | 88.89 | 100 |
| Venous pouch | 80 | 64.7 | 40 | 91.7 |
| Venous stenosis | 40 | 94.1 | 66.7 | 84.2 |
| Single vein bottom | 70 | 58.3 | 58.3 | 70 |
NPV: negative predictive value; PPV: positive predictive value.
Although some prior studies have examined the use of CT angiography to detect AVMs,26,27 ours is the first to examine the yield of CT angiography compared to cerebral angiography in characterising cerebral AVMs with a haemorrhagic presentation.
Regarding the analysis of the yield of CT angiography compared to DSA with regard to the Spetzler-Martin grading system variables (Table 3), it was seen that, although it underestimated AVM size, it showed an acceptable level of sensitivity (93.75%) for estimating size and an excellent level of sensitivity for estimating the presence of deep venous drainage and AVM location in an eloquent area (100%, at the expense of not yielding any FNs). These results are clinically significant, since they suggest that CT angiography is a useful tool in determining surgical risk.
Regarding the diagnostic yield of CT angiography for the angioarchitecture variables with an impact on endovascular treatment risk (presence of perforating arteries, a venous pouch, venous stenosis or a single drainage vein bottom) described in Table 4, only the presence of perforating arteries showed good sensitivity and specificity (100% and 92.86%, respectively), whereas, for all other variables, yields were suboptimal as they showed relatively low sensitivity. This means that CT angiography underestimates the risk of this type of treatment; this could lead to improper patient management. Thus, our results suggest that CT angiography could not replace cerebral angiography as a tool for decision-making with regard to endovascular treatment.
It is also important to take AVM-related aneurysms into account as risk factors for early rebleeding. CT angiography for this variable presents a sensitivity and specificity of 86.67% and 85.71%, respectively. When analysed separately, its sensitivity was found to be even lower as it was only 77.78% for flow-related aneurysms and 66.67% for intranidal aneurysms. This low sensitivity is clinically significant, since both the aneurysms and their location influence decision-making around the patient's treatment. Therefore, CT angiography, as it underestimates the presence of aneurysms related to cerebral AVMs, could lead to treatment delays in patients who would benefit from early treatment.
The main limitation of this study was its limited sample size, which, although sufficient for conducting a pilot study, would not be sufficient for generalising the results. We recommend conducting a study of diagnostic yield with a larger sample size and a larger number of readers with different levels of experience; this would confer greater external validity on the results.
In conclusion, cerebral CT angiography for the study of AVMs provides valuable and reliable information on surgical risk when a haemorrhage occurs, but its sensitivity and specificity are suboptimal for determining angioarchitecture, and, therefore, treatment timing and endovascular treatment risk. This means that using CT angiography instead of cerebral angiography to determine how to proceed in managing AVMs with a haemorrhagic presentation cannot be recommended.
Authorship- 1.
Responsible for study integrity: ALR and DC.
- 2.
Study concept: ALR, DC, JB, LSR and JM.
- 3.
Study design: ALR, DC, JB, LSR and JM.
- 4.
Data acquisition: CZ, ALR, DC, SR, JB, LSR and JM.
- 5.
Data analysis and interpretation: CZ, ALR, DC, SR, JB, LSR and JM.
- 6.
Statistical processing: ALR, SR and LSR.
- 7.
Literature search: CZ, ALR, DC, SR, JB, LSR and JM.
- 8.
Drafting of the article: CZ, ALR and SR.
- 9.
Critical review of the manuscript with intellectually significant contributions: CZ, ALR, DC, SR, JB, LSR and JM.
- 10.
Approval of the final version: CZ, ALR, DC, SR, JB, LSR and JM.
The authors declare that they have no conflicts of interest.
Please cite this article as: Zwanzger C, López-Rueda A, Campodónico D, Rosati S, Blasco J, San Román L, et al. Utilidad de la angio-TC para la caracterización de malformaciones arteriovenosas cerebrales con presentación hemorrágica comparada con la angiografía por sustracción digital. Radiología. 2020;62:392–399.