The brainstem, situated in the posterior fossa, connects the brain to the spinal cord. Owing to its location, the nerves of the brainstem are closely related with vascular structures.
ObjectivesTo correlate the finding of vascular loops in the cerebellopontine angle on imaging with symptoms indicative of vestibulocochlear involvement.
Materials and methodsThis retrospective descriptive study included all patients evaluated between 2011 and 2017 with findings suggestive of vascular loops in the cerebellopontine angle for whom the clinical history and imaging studies were available.
ResultsA total of 102 patients (63 women and 39 men) had vestibulocochlear involvement. The most common clinical indication was dizziness (41.18%). A unilateral vascular loop was found in 43 patients (right: 21.57%, left: 20.59%) and bilateral loops were found in 59 (57.84%) patients. The most common type of vascular loop was type II (right: 69.14%; left: 58.75%). The most common origin of vascular loops was the anterior inferior cerebellar artery (right: 66.67%, left: 65.00%). No associations were observed between vascular loops and sensorineural hearing, nystagmus, or vertigo. There was an association with tinnitus.
Conclusions and significanceThe presence of vascular loops is not associated with most auditory symptoms. Nevertheless, all findings on imaging studies must be reported. The interpretation of the findings of imaging studies must be correlated with the clinical symptoms after other more common causes that can explain the symptoms have been ruled out.
El tronco encefálico, situado en la fosa posterior, conecta el cerebro con la médula espinal. Debido a su ubicación, sus componentes nerviosos guardan una estrecha relación con estructuras vasculares.
ObjetivosDescribir una relación clínico-radiológica del asa vascular del ángulo pontocerebeloso en pacientes con síntomas indicativos de afectación vestibulococlear mediante evaluación por neuroimagen.
Materiales y métodosSe realizó un estudio retrospectivo y descriptivo. Se incluyeron todos los pacientes evaluados entre 2011 y 2017 con indicios de asa vascular del ángulo pontocerebeloso e historial clínico y estudios de diagnóstico por imagen disponibles.
Resultados102 pacientes (63 mujeres y 39 hombres) presentaban afectación vestibulococlear. La indicación clínica más frecuente fue mareos (41,18%). Se halló asa vascular unilateral en 43 pacientes (derecho: 21,57%, izquierdo: 20,59%) y bilateral en 59 pacientes (57,84%). El tipo de asa vascular más frecuente fue el tipo II (derecho: 69,14%; izquierdo: 58,75%). El origen más frecuente fue la arteria cerebelosa anteroinferior (ACAI) (derecha: 66,67%, izquierda: 65,00%). No se observó ninguna asociación entre asas vasculares y pérdida de audición neurosensitiva, nistagmo o vértigo. Se halló una asociación con acúfenos.
Conclusiones y significaciónLa presencia de asas vasculares no se asocia a la mayoría de los síntomas auditivos. No obstante, deben notificarse todos los hallazgos de los estudios por imagen. La interpretación de los hallazgos de los estudios por imagen debe correlacionarse con los síntomas clínicos después de excluir otras causas más frecuentes que puedan explicar la sintomatología.
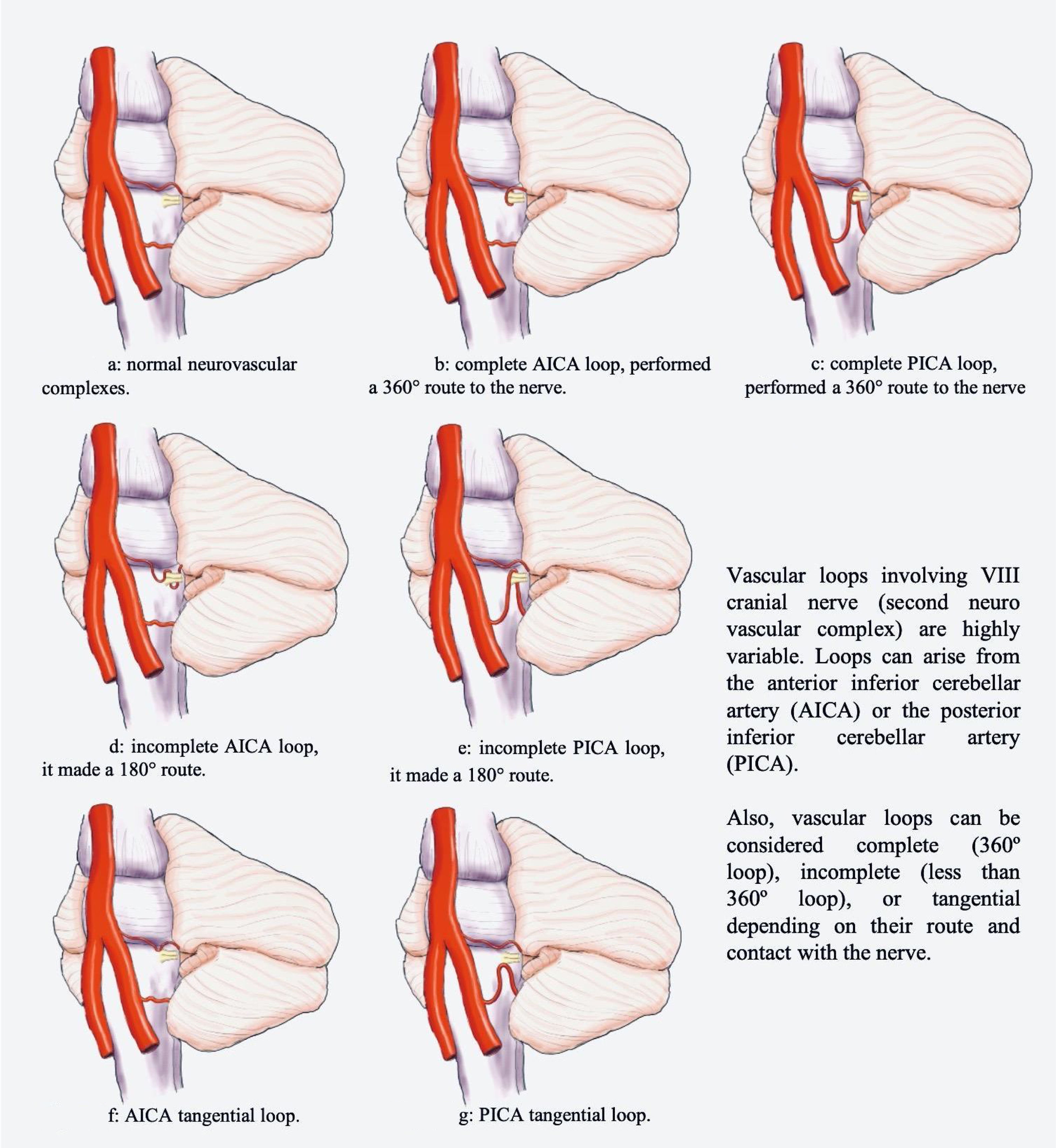
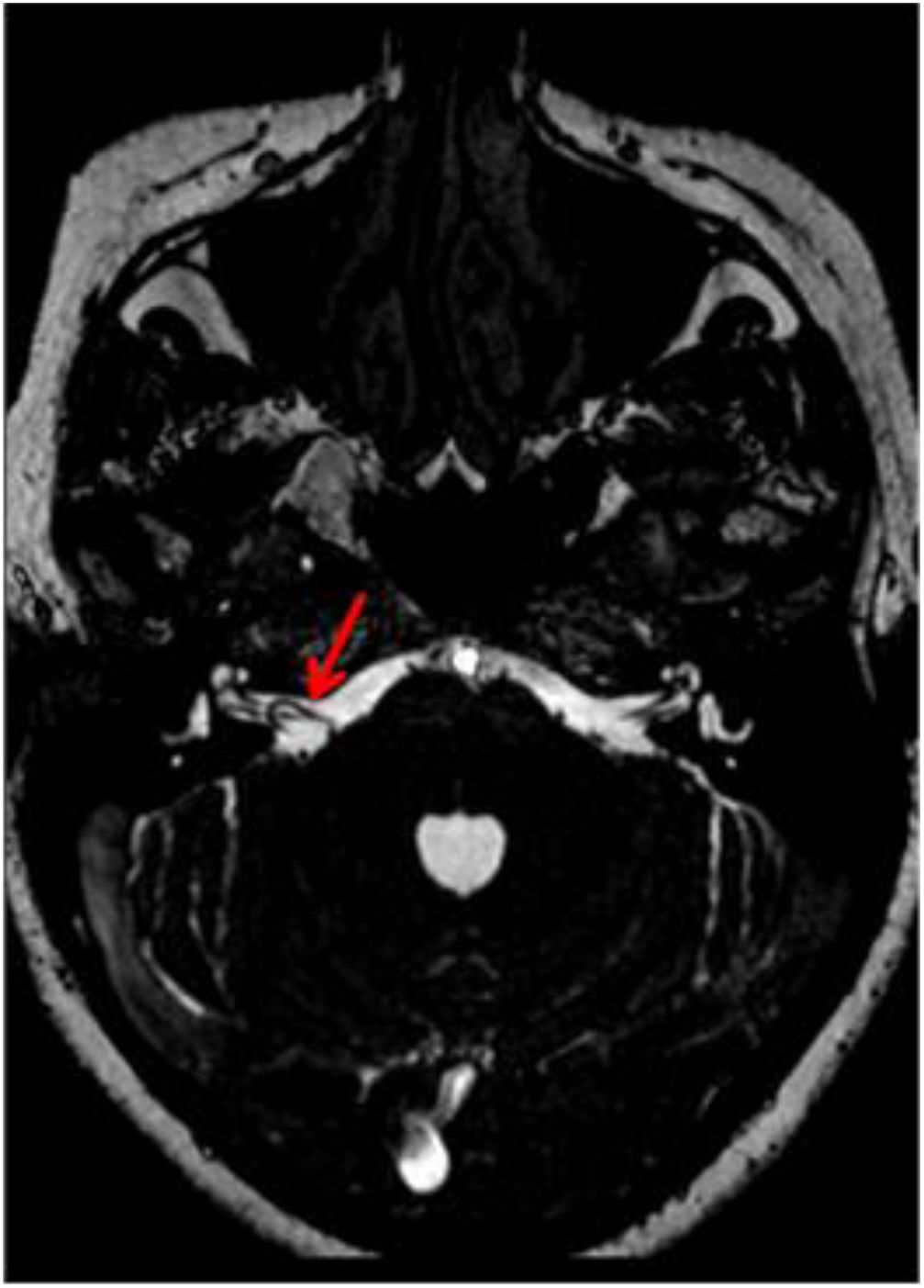
Located in the posterior fossa of the skull, the brainstem connects the brain and the spinal cord. Due to its location, its nervous components (tracts and cranial nerves) are closely related to vascular structures. Classically, three neurovascular complexes located along the brainstem have been described. The first is located in the mesencephalon related to the superior cerebellar artery (SCA), the second in the pons related to the anterior inferior cerebellar artery (AICA), and the third in the spinal bulb related to the posterior inferior cerebellar artery (PICA) and vertebral artery (Fig. 1a).1,2
From the embryological point of view, while the structures of the posterior fossa develop, the transverse arteries of the longitudinal canal widen to meet the demand of blood of the cerebellum and spinal cord. In the upper part, the dominant transverse arteries are transformed into the superior cerebellar artery (ACS), and the middle and lower segments of it give rise to the anterior inferior cerebellar artery (AICA). Depending on the recruitment of the transverse arteries, anatomical variants of the ACS, AICA, and PICA can be developed.3 The microanatomy of the circulatory system is a random process, the genome can not code the complicated network of capillaries in each individual in the same way and therefore each individual will have a similar but different circulatory system.4
Currently, in Latin America, there is no data about incidence or prevalence, and the existing information about clinical features associated with the radiological findings of the neurovascular complexes (NVC) of the brainstem is low. Regarding the NVC, one of the most frequent findings are the vascular compression syndromes. In these cases, the arteries that accompany the cranial nerves (eg. facial) usually come into contact with them in their intracranial course.5
The most studied vascular compression syndrome is the hemifacial spasm. In this case, cranial nerve VII is in contact with vascular branches of the SCA.6 Based on clinical and pathological findings, only a few theories have been described to explain the neurological manifestations associated with vascular loop compression.7 There are no conclusive findings that associate a single pathological pathway to the appearance of vascular loop syndrome, however, the possible etiologies include:
- ●
Direct compression theory: Nervous hyperfunction and demyelination derived from the mechanical stimulation of the vessels.7
- ●
Endogenous inflammatory theory: The hemifacial spasm occurs as a consequence of intrinsic neuronal activity derived from the repetitive stimulation, which could constitute the base of successful management with anticonvulsants and modulators of the excitatory response such as benzodiazepines.8
- ●
Multifactorial theory: There is a basal inflammatory process which causes nerve damage that makes the nerve susceptible to being stimulated by movement which change the central connectivity of the VIII nuclei causing the symptoms.7
In 1975, Janetta introduced the concept of redundant arterial loops in the cerebellopontine angle as a direct etiology of audio-vestibular symptoms.9 Nevertheless, finding a causal link between the radiological and clinical findings has been difficult. It has been reported that around 95% of the world population experience tinnitus at some point in their lives, and up to 15% present this symptom with sufficient frequency and intensity to be clinically significant for consulting the hospital care services.7 The main clinical manifestations of the vestibular syndrome are tinnitus, hearing loss, and dizziness.. Abnormal spontaneous or positional nystagmus rarely occurs. Also, patients present a unilateral neurosensory hearing loss of variable pattern.10,11
The most frequent artery associated with the vascular loop of the eighth cranial nerve is the AICA, which in 72% of cases it arises as a simple trunk from the basilar artery, while between 25% and 50% of cases the AICA courses inferior to the vestibulocochlear nerve to the internal auditory meatus. The vascular loops can be located prominently in any segment of the AICA, however, there is an important association between the presence of arterial redundancies and the transition sheath between the nerve and the meninges known as the Obersteiner-Redlich area.6,7,12
Given the anatomical evidence and the acceptance of various theories, the radiological characterization of the findings in the cerebellopontine angle has become increasingly important. It has been found that up to 50% of patients may have studies suggesting a vascular loop, but only in 50% of cases, it is in contact with the nerve.7 The incursion of new technologies has allowed the improvement of MRI protocols including multiple sequences such as the constructive interference in steady state (CISS) or fast imaging employing steady-state acquisition (FIESTA), that provide enough anatomical detail to classify even the extension of the vascular loop in the internal auditory canal.12,13
Despite the apparent physiopathological connection and the imaging findings, the epidemiological strength of the relationship between the presence of neurovascular alterations, the symptoms, and their severity is still widely debated, underestimating the radiological findings. Furthermore, given the success rate of microvascular decompression surgery from 40% to 70% of the vestibulocochlear complex, the importance of neurovascular alterations in the cerebellopontine angle in patients with vestibulocochlear symptoms should not be ignored.14 For this reason, we consider important to carry out this study to clarify the epidemiological behavior of the findings trying to guide the clinicians to consider the performance of additional interventions (medical or surgical) for the management of patients with vestibular symptoms associated with vascular loops.
Materials and methodsSubjectsA retrospective and descriptive study was carried out. All brain magnetic resonance images interpreted by an experienced neuroradiologist (18 years) between 2011 and 2017 were reviewed in the study. First, a systematic review of the Picture Archiving and Communication System of the institution was carried out, the studies where radiological reports contained “vascular loop” as keyword were selected. Then, a radiology resident reviewed each report, and all radiology records that contained “vascular loop of the vestibulocochlear complex”, “vascular loop of the cerebellopontine angle” or “vascular loop surrounding the eighth cranial nerve” were selected. Since a patient could have more than one image study within the selected time period, all duplicate records were excluded.
Once the previously mentioned criteria were applied, all clinical and sociodemographic variables from the institutional electronic record system were collected. All the images went to a second review by the expert neuroradiologist. The vascular origin of the loop was determined (AICA, PICA or SCA). The classification proposed by Chavda and Mcdermott was used to define the extension of the loop. This system classifies vascular loop in three categories: type I is a vascular loop within the cerebellopontine angle but outside the internal auditory canal (IAC); type II is a vascular loop extending into less than the 50% of the length of the canal; type 3 is an IAC loop with more than 50% extension into the canal. Also, loops were considered complete if it performed a 360° route to the nerve (Fig. 1b and c), incomplete if it made a 180° route (Fig. 1d and e) or tangential (Fig. 1f and g).
Acquisition protocolThe studies were carried out in a 1.5T MRI scanner (Avanto, SIEMENS; Erlangen, Germany). The patients were placed in the supine position with immobilization of the head in the antenna using pads. Within the conventional MRI protocol for brain studies, a CISS sequence was performed (TR=5.42ms, TE=2.42ms, Slice thickness=0.70mm), and when the MR angiography protocol was used, a Time-Of-Flight (TOF) sequence was included (TR=26.00ms, TE=7.15ms, Slice thickness=0.5mm).
Statistical analysisThe information was collected using a database in a spreadsheet. Categorical variables were reported as frequencies and percentages. Continuous variables were reported as the mean and standard deviation if they were normally distributed or as the median and interquartile range if they were not normally distributed. For the final analysis, all inner ears were considered individually. Chi-square test was used to compare categorical variables between groups. All statistical analysis was performed using STATA 13.0.
EthicsThis study was approved by the institutional review board the Fundación Valle del Lili. As in other retrospective studies, informed consent was not administered to the patients.
ResultsSociodemographic characteristics of the sampleBetween 2011 and 2017, 5543 brain MRI studies were read by the expert neuroradiologist. From these, 252 reports include “vascular loop” as a keyword. Fifteen duplicated registers were excluded; thus, 237 radiological records were reviewed. Finally, 102 patients met all the inclusion criteria, 39 were male (38.24%) and 63 female (61.76%), with a median age of 52 years (IQR 42–68). Of the 102 patients, 41.18% reported dizziness, 25.49% tinnitus, 14.71% hearing loss, 5.88% had evident nystagmus on physical examination, and 38 patients (37.25%) did not present any symptoms suggestive of vestibulocochlear involvement.
Imaging indication and neuroanatomical findingsNinety-two MRI studies and 10 MR angiographies (MRA) were performed. The most frequent clinical indication for neuroimaging was the presentation of some vestibular symptom (65.68%; n=67), followed by headache (10.78%; n=11), eight patients presented symptoms associated with the facial nerve (7.84%), three patients had trigeminal neuralgia (2.94%), and three patients had suspected acute cerebrovascular event in the brain posterior circulation (2.94%). Other clinical indications were reported in 10 patients (10.78%).
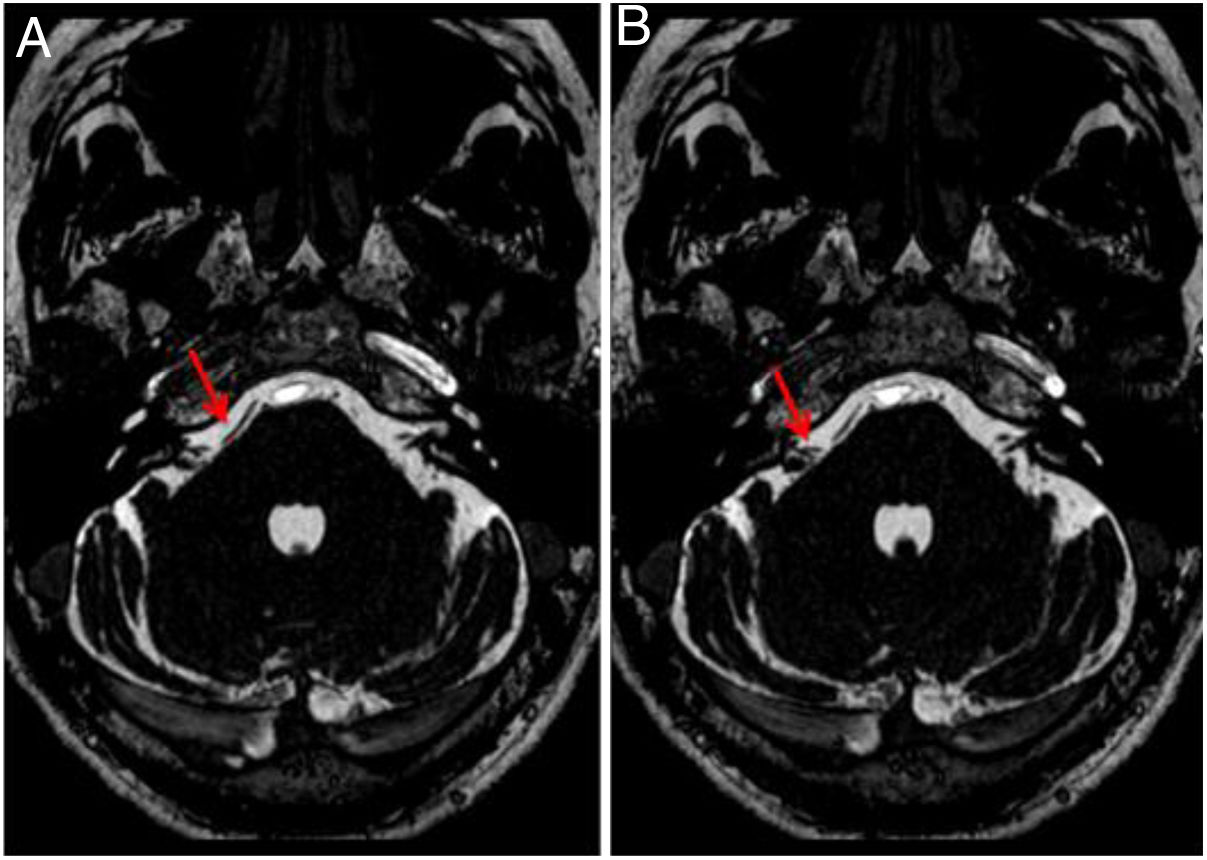
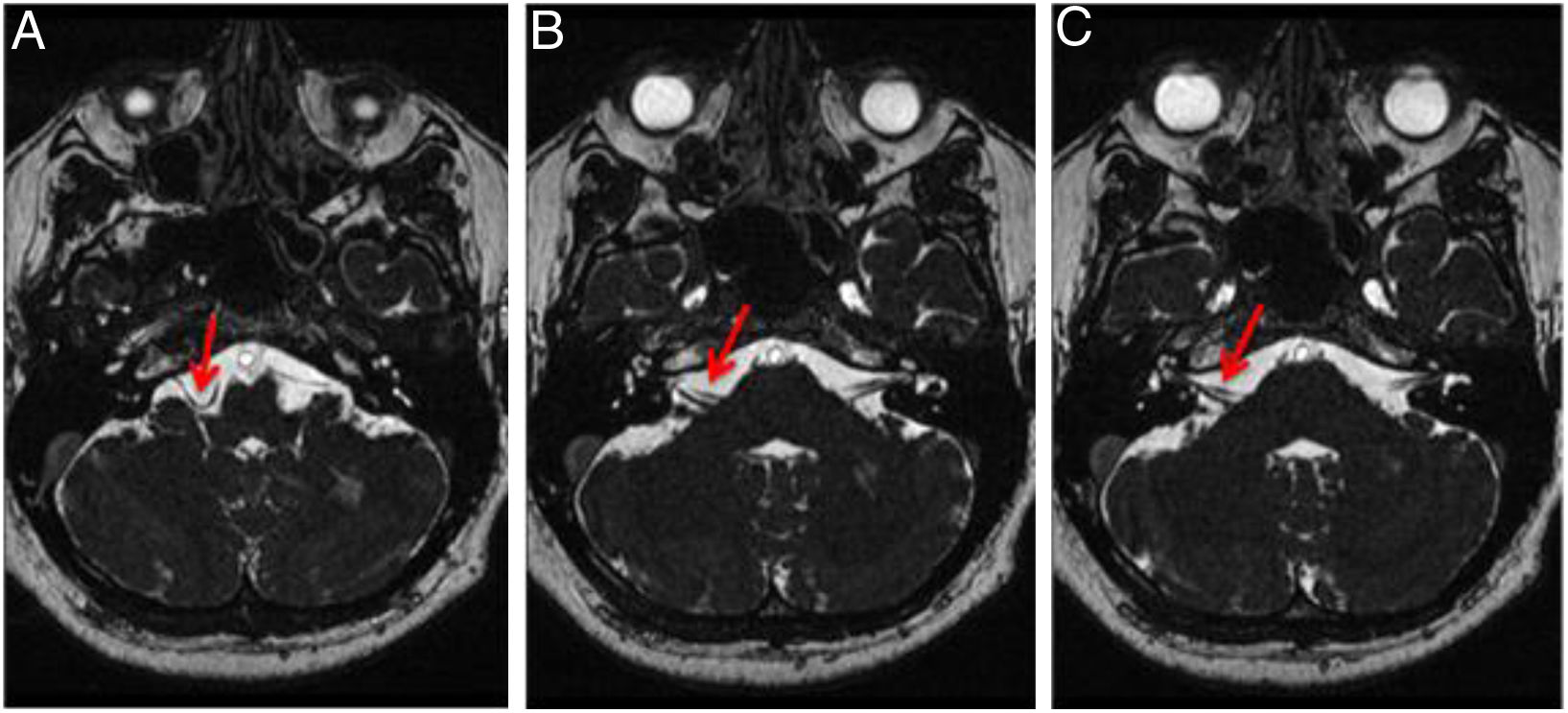
Regarding the imaging findings, the involvement was unilateral in 43 patients (right ear, 21.57%; left ear, 20.59%) and bilateral in 59 cases (57.84%). The most frequent origin was the AICA (right ear, 66.67%; left ear, 65.00%) (Figs. 2 and 3), followed by the PICA (right ear, 30.86%; left ear, 32.50%) (Fig. 4), and the SCA (right ear, 2.47%; left ear, 2.50%). According to the classification proposed by Chavda and Mcdermott, the most frequent type of loop was type II (right ear, 69.14%; left ear, 58.75%) (Fig. 7), followed by type I (right ear, 25.93%; left, 26.25%), and type III (right ear, 4.93%; left ear, 15%) (Figs. 2 and 3).
The frequency of symptoms and the image features of the vascular loops per ear are summarized in Table 1. A comparison was made between the laterality of the symptoms and the presence of the vascular loop (Table 2), for localizing symptoms significant differences for tinnitus (p: <0.01) but not for hearing loss were found. The distribution of dizziness and nystagmus was similar in both groups. No relationship was found between the loop type, the loop turn, and the number of symptoms referred by the patient (Tables 3 and 4).
Characteristics of the population.
| Patients | Patients (n:102) |
|---|---|
| Age | |
| Age (Years) | 52 (IQR 42−68) |
| Gender | |
| Male | 39 (38.24%) |
| Female | 63 (61.76%) |
| Type of study | |
| MR | 92 (90.19%) |
| MRA | 10 (9.8%) |
| Characteristics of the symptoms by ear | Ears n: (204) |
| Without symptoms | 94 (46,08%) |
| With symptoms | 110 (53.92%) |
| Dizziness | 84 (41.18%) |
| Nystagmus | 12 (5.88%) |
| Tinnitus | 29 (14.22%) |
| Hearing loss | 18 (8.82%) |
| Features of vascular loops | Vascular loops n: (161) |
| Origin of vascular loop | |
| AICA | 106 (65.84%) |
| PICA | 51 (31.68%) |
| SCA | 4 (2.48%) |
| Type of vascular loop (Chavda y Mcdermott) | |
| Type I | 42 (26.09%) |
| Type II | 103 (63.98%) |
| Type III | 16 (9.94%) |
| Type of vascular loop | |
| Complete | 107 (66.64%) |
| Incomplete | 54 (33.54%) |
Characteristics of the symptoms according to the ear affected by the vascular loop.
| Ears | With vascular loop (n:161) | Without vascular loop (n:43) | p-Value |
|---|---|---|---|
| With symptoms | 86 (53.42%) | 24 (55.81%) | – |
| Dizziness | 68 (42.24%) | 16 (37.21%) | 0.35 |
| Nystagmus | 9 (5.59%) | 3 (6.98%) | 0.11 |
| Tinnitus | 23 (14.29%) | 6 (13.95%) | <0.01 |
| Hearing loss | 13 (8.07%) | 5 (11.63%) | 0.53 |
p-Value: Chi-2.
Characteristics of symptoms according to the type of loop Chavda and Mcdermott.
| Symptoms | Type I (n:42) | Type II (n:103) | Type III (n:16) |
|---|---|---|---|
| Without symptoms | 17 (40.48%) | 55 (53.04%) | 3 (18.75%) |
| 1 Symptoms | 16 (38.10%) | 34 (33.01%) | 12 (75.00%) |
| 2 Symptoms | 8 (19.05%) | 12 (11.65%) | 1 (6.25%) |
| 3 Symptoms | 1 (2.38%) | 2 (1.94%) | 0 (0%) |
In this retrospective work, we studied the association between vascular loop imaging findings and auditory symptoms. According to our results, there was no relationship between the loop characteristics (route and length) and the occurrence of vestibular symptoms (hearing loss, nystagmus, or vertigo) only with tinnitus.
Since 1930, the clinical-radiological correlation of the vascular loop in the vestibulocochlear complex has been studied,9 however, limited and uncertain information was found in the literature and there is not a clear association between the clinical symptoms and the radiological findings. Also, there is no standard assessment protocol, currently requiring more objective tools when relating the symptoms with its causality.
Jannetta et al. reported for the first time in 1975 the microvascular decompression surgery as a management of the vascular syndrome with a success between 40% and 77%, measured as a reduction of the symptoms, which reinforced the idea of a nexus between the presence of vascular loop and the symptomatology.14,15 However, there are discrepancies between studies inasmuch as none of the studies has shown a statistical relationship between those aspects, explaining this probably by an interobserver variability. Besides that, the vestibulocochlear syndrome includes a wide variety of complex symptoms and also a broad possibility of causes leading to a difficult evaluation.13
The Mcdermott Al study in 2003 associate the presence of symptoms with vascular loop and their symptoms, being this the larger study with 332 adults and 664 ears.16 Our study with 102 adults and 204 ears evaluated, is the second with the highest volume of patients. In both of them, MR CISS sequences were used, and the results were similar showing no statistical significance between the symptoms and radiologic findings.
On the other hand, a retrospective case series study reported that the symptomatology referred by the patients, comprised mainly by hearing loss and tinnitus and the findings in the audiometric studies, did not correlate with the laterality of the radiologic finding, which corresponds to the results of our study.10 Similarly, in the present study the most frequently affected artery was the AICA which correspond with the data in the universal literature; however, in this study the most prevalent type of loop according to the Chavda y Mcdermott classification was the type II, contrary to the reports in prior studies where the most frequent were the type I.13
Despite these findings, it was shown that the presence of vascular loop by image is a common finding in people with symptoms such a vertigo, tinnitus and hearing loss and also in a small proportion of asymptomatic patients, thus, some reports conclude these findings as a non-pathologic anatomic variant in a proportion of the population.13 In addition, Sabarbatti explains the need of a lesion between the glial central union and the peripheral nonglial union known as the transition zone to provoke vestibulocochlear symptoms.17
LimitationsAs in other retrospective studies, this work has some limitations. Regarding the inclusion criteria, a keyword search was carried out in an image registry, so some MRI with vascular loops may not be included in the sample if the keywords were absent in the records. On the other hand, the limited information in some clinical records does not allow us to know the characteristics of nystagmus (spontaneous, evoked, laterality). Also, not all patients had other diagnostic studies such as auditory evoked potentials, so this variable could not be taken into account. Finally, the absence of data on patients with vestibulocochlear symptoms without vascular loop is part of the limitations of this study. These limitations will be taken into account in the development of future research.
ConclusionsThere is no clear relationship between the radiological finding of a vascular loop and most of the vestibular symptoms, except for tinnitus. However, this frequent radiological finding must be reported. In case of the need for surgical management it will help to establish the anatomic relationship, the course of the vascular loop and allow a safer and effective approach.6 Finally, the radiologic findings interpretation always should be correlated with the clinical symptoms once the more frequent causes which explain the symptoms have been ruled out.
Authorship- 1.
Responsible for study integrity: VMQ.
- 2.
Study conception: JFOZ y AGS.
- 3.
Study design:
- 4.
Data acquisition: JVC y SPP.
- 5.
Data analysis and interpretation: VMQ, JVC, JFOZ y AGS.
- 6.
Statistical processing: JFOZ.
- 7.
Literature search: JVC y SPP.
- 8.
Drafting of the manuscript: VMQ, JVC, SPP, JFOZ y AGS.
- 9.
Critical review of the manuscript with intellectually sig-nificant contributions: VMQ, JVC, SPP, JFOZ y AGS.
- 10.
Approval of the final version: VMQ, JVC, SPP JFOZ y AGS.
The authors declare no conflict of interest.