To review the different types of urinary diversion surgeries (UDS) in order to recognize the expected findings in a postoperative study, using different imaging techniques. To recognize the main postoperative complications, both early and late.
ConclusionUDS are surgical procedures whose purpose is to redirect urine flow after cystectomy, generally in an oncologic context. The imaging evaluation of urological surgeries is often a radiological challenge, with CT being the most commonly used image modality. Therefore, it is essential to know the main surgical techniques, the expected postoperative findings and the optimization of imaging techniques for early diagnosis and correct evaluation of postoperative complications.
Revisar los diferentes tipos de cirugías derivativas urinarias (CDU) para reconocer los hallazgos esperables en un estudio postoperatorio mediante distintas pruebas de imagen. Revisar las principales complicaciones postquirúrgicas, tanto precoces como tardías.
ConclusiónLas CDU son procedimientos quirúrgicos cuya finalidad es redirigir el flujo de orina tras la realización de una cistectomía, generalmente en contexto oncológico. La evaluación en imagen de las cirugías urológicas supone, a menudo, un desafío radiológico, siendo la TC la modalidad de imagen más utilizada. Para ello, es fundamental el conocimiento de las principales técnicas quirúrgicas, de los hallazgos postoperatorios esperables y la optimización de las técnicas de imagen de cara al diagnóstico precoz y correcta evaluación de las complicaciones postoperatorias.
Radical cystectomy is a surgical procedure that is used in the treatment of both non-oncological causes (complex fistulas) and oncological causes (urothelial, rectal, cervical, sigmoid, prostate cancer, etc.).1–3 Its implementation involves the reconstruction of urinary transit through different types of diversion surgeries.
In postoperative evaluation, radiology plays a fundamental role, both in the assessment of the complex postsurgical anatomy and in the detection of early and late complications. Thorough knowledge of the expected postsurgical and pathological findings can contribute to reducing the morbidity and mortality of these patients.2,4
This work aims to review the different types of urinary diversion surgeries (UDS) in order to train the radiologist to detect the main postoperative complications.
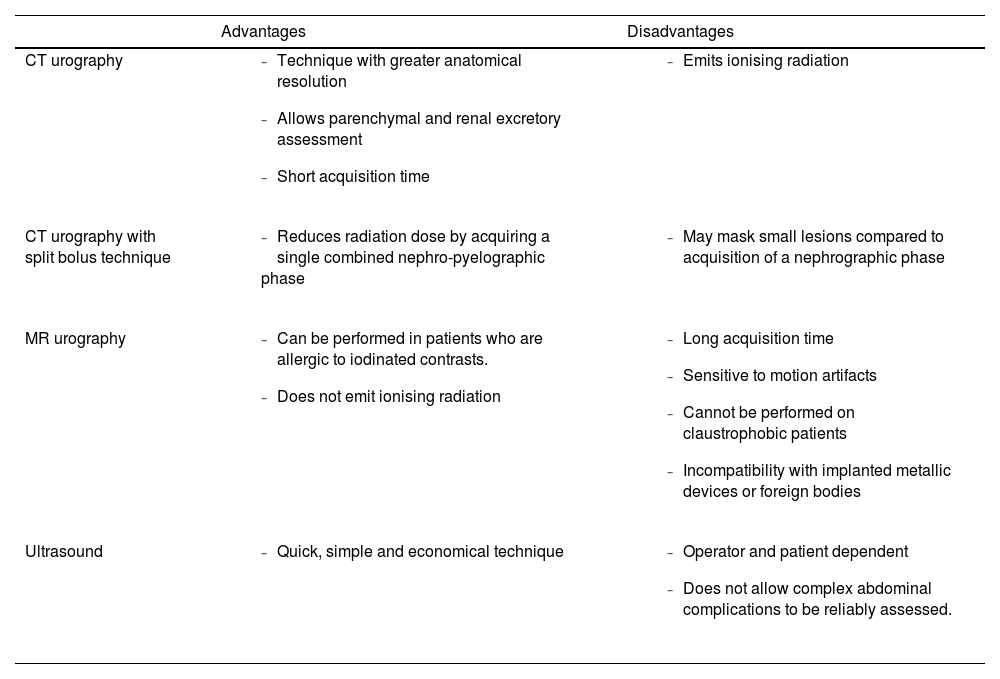
Radiological protocol for postoperative evaluationMultiple imaging techniques can be used for the post-surgical evaluation of UDS patients, with CT urography being the most widely used.5 These are carried out both in the periodic follow-up of cancer patients and in the emergency context when a complication is suspected. The main advantages and disadvantages of these techniques are listed in Table 1.
Advantages and disadvantages of the main diagnostic techniques for the evaluation of urinary diversion surgeries.
| Advantages | Disadvantages | |
|---|---|---|
| CT urography |
|
|
| CT urography with split bolus technique |
|
|
| MR urography |
|
|
| Ultrasound |
|
|
CT urography is a multiphase study that includes an unenhanced acquisition (kidneys-pubis) and a subsequent acquisition of nephrographic (diaphragm-pubis) and excretory (kidneys-pubis) phases at 90−100s and 10−15min, respectively, after the administration of approximately 70−100ml of intravenous iodinated contrast (IVC).6,7 The prior administration of 1l of water favours distension of the upper urinary tract (UUT).6
We have two possible additional strategies to reduce the radiation dose to the patient:
- -
Multi-energy CT: It eliminates the need to acquire an unenhanced phase because it generates it virtually.8
- -
Split bolus protocol: It consists of an uncontrasted acquisition followed by the administration of a first bolus of 30−50ml of IVC material, with a second bolus of 80−100ml of IVC 10min after the first acquisition, achieving a single combined nephro-pyelographic phase (90−100s).5,9,10
MR urography (static) may be the most appropriate technique in selected patients with mild-moderate renal failure, given that it does not depend on renal excretory function as it does not require the administration of gadolinium.7,11 Furthermore, it has the added advantage of not emitting ionizing radiation.5,7,11 It is based on strongly T2-weighted sequences, which take advantage of the long relaxation times of urine to completely visualise the entire urinary tract.5,7
UltrasoundIt is usually used routinely for the initial evaluation of hydronephrosis, the degree of UUT dilation, the degree of renal cortical atrophy and in the initial assessment of infectious complications.12
FluoroscopyIt consists of the retrograde injection of iodinated contrast through the stoma in case of diversions directed to the abdominal wall.2
Main surgical techniques and normal postoperative findingsIncontinent diversion techniquesThese are techniques that do not allow the voluntary release of urine. Their advantage is that, as they are technically easier than a continent diversion,2–4 they can be performed in patients with associated medical comorbidities (liver failure, kidney failure, cardiovascular, respiratory or metabolic pathology), poor prognosis or those in whom continent diversion techniques are contraindicated.2,13 Among them, the Bricker procedure is the most commonly used.
Cutaneous diversion. Uretero-ureterocutaneostomyCurrently, it is only used palliatively or when it is impossible to use an intestinal segment for reconstruction.2,4,14,15 An anastomosis of both ureters is performed to the anterior abdominal wall (Fig. 1A–C).
Incontinent urinary diversion techniques. Normal findings. (A–C) 80-year-old patient with cutaneous ureteroureterostomy. A graphic representation (A), a coronal reconstruction in excretory phase (B) and a three-dimensional volumetric reconstruction (C) are shown. The ureterourethral anastomosis (circle) and ureterostomy (arrow) are highlighted as points with the highest incidence of complications. (D–F) 65-year-old patient with Bricker urinary diversion. A graphic representation (D), a coronal reconstruction in the excretory phase (E) and a three-dimensional volumetric reconstruction (F) are shown, highlighting the ureteroileal anastomosis (circle) as a point of special interest.
It is the most commonly used technique.14,15 A segment of distal ileum (15−20cm from the ileocaecal valve) is isolated for use as a conduit while preserving peristalsis. The two ureters are anastomosed to its proximal end, either separately (Bricker procedure) or together (Wallace procedure).2–4 Subsequently, the ileal conduit is anastomosed to the abdominal wall in the form of a stoma (right flank). Finally, the transit is reconstructed with an ileoileal anastomosis2–4 (Fig. 1D–F).
The ileal conduit is identified on the image as an intestinal loop filled with fluid (mucous secretion). It must be remembered that the excretory phase can be helpful in differentiating it from an abscess, since the ileal loop will be filled with contrast while the abscess will present peripheral uptake.14,16
Continent diversion techniquesThese are those in which voluntary continence is preserved, preserving the patient's body image with the construction of a neobladder. This requires a long segment of intestine, a functional urethra with negative tumour margins, and the conscious participation of the patient for its optimal functioning.2,4,17,18
Continent diversion with cutaneous stoma: Indiana pouchThis technique is not widely used today. A 20−25cm segment of ascending colon and caecal pole and 15−18cm of terminal ileum are isolated, which will act as a pouch. The ascending colon is detubularised to reduce peristalsis, giving it a spherical morphology. Subsequently, the terminal ileum is opened to the skin and the ureters are anastomosed to the proximal end of the pouch. Finally, the transit is reconstructed with ileocecal anastomosis (Fig. 2A–C).4,16–18
Continent urinary diversion techniques. Normal findings. (A–C) 51-year-old male patient with continent diversion with cutaneous stoma (Indiana pouch). A graphic representation (A), a coronal reconstruction in the excretory phase (B) and a three-dimensional volumetric reconstruction (C) are shown, where the pouch (arrowhead) and the ureterocolonic anastomoses (circle) are marked as points of special interest. (D–F) 45-year-old male patient with orthotopic neobladder continent diversion. A graphic representation (D), a coronal reconstruction in the excretory phase (E) and a three-dimensional volumetric reconstruction (F) are shown, where the neobladder (arrowhead) is marked as a point of special interest.
The excretory phase helps us differentiate the pouch from an abscess. In addition to fluid, the Indiana pouch may contain air bubbles secondary to catheterisation. Haustra can be confused with septa and mistakenly interpreted as an abscess.14,16
Continent diversions to the native urethra: orthotopic neobladder (Studer technique)It is performed in patients with low surgical risk and high life expectancy. It requires the resection of an ileal segment (≈50cm). A ≈40cm detubulated segment is used to create the pouch. An isoperistaltic intestinal segment (with the direction of peristalsis preserved congruent with the direction of intestinal transit) of approximately 10cm is placed proximal to the pouch to prevent urine reflux (generally to the right) and the ureters are anastomosed to it. Subsequently, the most tilted portion of the pouch is anastomosed to the proximal end of the native urethra. Finally, transit is restored with an ileoileal anastomosis (Fig. 2D–F).2,16–18
Identification of the afferent arm can help us not to confuse the neobladder with a native bladder. It must be remembered that a certain degree of pelviectasia is to be expected even in the late postoperative period.2,4,14 This may increase in late images (10−20min), given that the peristaltic capacity of the afferent arm is exceeded.2,4
ComplicationsBelow, the main complications observed after UDS are classified according to the anatomical region and the time of presentation (Table 2).
Postoperative complications of urinary diversion surgeries.
| Early (<30 days) | Late (>30 days) | |
|---|---|---|
| Urinary system | HydronephrosisUrinary leakComplications related to double J catheter | UrolithiasisUrethral stricture |
| Intestinal loops | Intestinal leakIntestinal rhythm alterations | Stomal strictureParastomal herniationEnterocutaneous and enterourinary fistulas |
| Infectious and other collections | Pyelonephritis, pyelitis, abscesses | |
| Haematoma | Lymphocele | |
| Tumoral | Tumour remnant | Locoregional recurrenceDistant recurrence |
For the post-surgical evaluation of the urinary system, the test of choice varies depending on the clinical suspicion. To evaluate hydronephrosis, urolithiasis or the position of a double J catheter, the initial test to be performed is ultrasound, while if a urinary leak is suspected, a CT scan with excretory phase should be performed.2,4
HydronephrosisIt is not necessarily a complication. In the early postoperative period, grade I–II hydronephrosis can be observed due to reflux of the urethral anastomoses that tends to spontaneously resolve three to six months after surgery19 (Fig. 3). In cases of continent bypass surgeries where an intestinal segment is detubularised, as is the case with the Studer technique, grade I–II hydronephrosis can be seen even years after UDS. However, in the event of new-onset hydronephrosis or a progressive increase in the degree of dilation of the pyelocalyceal system, obstruction of the anastomosis should be suspected.2,4,14
Hydronephrosis in different situations. (A) Bilateral grade I hydronephrosis expected in a normal postoperative course. (B) Grade II–III hydronephrosis with diffuse bilateral urothelial enhancement, secondary to stricture of the superinfected ureteroileal anastomosis in a patient with Bricker urinary diversion. (C) Grade III hydronephrosis secondary to lithiasis at the pyeloureteral junction (arrow). (D) Grade IV hydronephrosis secondary to a tumour implant (arrow).
It is a relatively common complication20 in which it is important to make an early diagnosis due to the risk of fibrosis and urethral stricture. The most common leak point is the urethral anastomoses with the pouch/neobladder.2,4 In the case of suspected diagnosis, the excretory phase is essential to demonstrate active extravasation of contrast6 (Fig. 4).
Urinary leaks. (A and B) 75-year-old male patient with Indiana pouch reconstruction with diffuse abdominal pain. CT obtained in the excretory phase (B) shows contrast leakage into the peritoneal cavity at the point of anastomosis of the ureters to the pouch (arrow) in relation to urinary leakage. (C–E) 55-year-old male patient with reconstruction using the Bricker procedure with abdominal pain and increased acute-phase reactants. Coronal CT in portal phase (C) shows a thin-walled hypodense collection adjacent to the right ureter (arrowheads). Coronal CT obtained in the excretory phase (D) shows that the excreted contrast fills the collection (arrowheads) in relation to the post-surgical urinary leakage at the level of the middle third of the ureter (arrow, D). (E) Three-dimensional volumetric reconstruction representing the relationship of urinary leakage (arrowhead) with the excretory system.
UDS patients have an increased incidence of urolithiasis compared to the normal population, and it is more frequent in patients with an ileal conduit.20 Most stones are struvite (infection stones). It should be remembered that non-contrast CT improves their detection (Fig. 5), although it may be difficult to differentiate radiopaque suture material from stones. Comparison with early postoperative CT may be helpful.2,4,14,16
Urolithiasis. (AC) 60-year-old male patient with reconstruction with neobladder (star) who presents with positive left renal fist percussion and cramping pain. A 21mm non-obstructive stone is observed in the lower calyceal group of the left kidney (arrow) seen on ultrasound (A), MVRP (multiplanar volume rendered projection) reconstruction (B) and coronal CT without contrast (C).
This is a relatively frequent complication which occurs in the first few postoperative years. The most common location is the ureteroenteric anastomosis of the left ureter due to its angulation. It is mainly caused by urethral ischaemia and consequent fibrosis or tumour recurrence.2,4,14,16
On imaging, it appears as a new-onset hydronephrosis and delay in the excretory phase, with thickening of the urothelium (Fig. 6). The coexistence of associated soft tissue suggests a malignant stricture.2,4
Urethral stricture. (A–C) 55-year-old male patient with reconstruction with Indiana pouch reservoir who presents a progressive increase in serum creatinine levels. Signs of left obstructive uropathy (A, rectangle) secondary to bifocal tumour progression in the enterourethral anastomosis (B and C, arrows) are observed.
These complications are rare. They include iatrogenic urethral perforation with consequent urinary leakage/urinoma (Fig. 7), malposition or migration of the endoprosthesis and urinary infections.21
Complications from the placement of a double J catheter. (A–E) 70-year-old male patient with Bricker reconstruction (early postoperative) who presents with diffuse abdominal pain and worsening renal function. (A and B) Migration of the proximal end of the urethral stent (arrow). The excretory phase (D and E) shows contrast extravasation from a urethral perforation (arrowhead) as a complication of double J catheter placement. (C) Percutaneous nephrostomy showing active contrast leak (arrow).
When the leaking of intestinal contents, mechanical obstruction or fistula is suspected, CT is the technique of choice for diagnosis.
Leaking of intestinal contentsIt is a rare complication. The most common leak point is the ileoileal anastomosis. Risk factors are ischaemia, previous radiotherapy or the presence of intestinal obstruction distal to the anastomosis4,17,22 (Fig. 8).
Leakage of intestinal contents. (A–E) 58-year-old male patient with Bricker diversion for muscle-invasive bladder carcinoma. Chest X-ray (A), CT in portal (B) and excretory (C) phases. Postoperative pneumoperitoneum is observed (arrow, A) and contrast extravasation in the excretory phase from the junction of the left ureter to the neobladder towards the peritoneal cavity (B and C, rectangle). The patient undergoes re-operation to close the leak. The CT images in the portal phase (D and E) correspond to the same patient 24h after re-operation. The inflammatory changes secondary to postoperative faecal peritonitis are shown: marked enhancement of the peritoneal laminae (dashed arrow), free fluid (arrow) and hypoenhancement of the intestinal loops (arrowhead).
Normal intestinal rhythm is usually recovered five days after surgery. Its absence suggests:
- -
Paralytic ileus: This is the most common postoperative intestinal complication. A generalised dilation of all intestinal loops is observed, without identifying a transition zone. In conventional radiography and CT, multiple air-fluid levels are observed4,20 (Fig. 9A).
Figure 9.Alterations of intestinal transit. (A) 61-year-old female patient with recent post-surgical changes after Bricker urinary diversion (arrow, double J catheter directed to RIF) with absence of bowel movements 7 days after the intervention. Diffuse dilation of the loops of the small intestine (double arrow) and the gastric chamber (wavy arrow) is observed, with the presence of gas at a distal level, without a point of change in calibre, in relation to paralytic ileus. (B and C) 58-year-old female patient with neobladder diversion with diffuse abdominal pain, vomiting and absence of gas/bowel movements 8 days after the intervention. (B) X-ray of the abdomen in the supine position in which multiple air-fluid levels are observed as a sign of mechanical obstruction. (C) Study is completed with CT in the portal phase where multiple loops of small intestine dilated retrogradely at the point of sudden change in calibre (arrow) are observed in relation to mechanical intestinal obstruction secondary to flanges.
(0.33MB). - -
Mechanical obstruction: The loops are dilated to a point where a sudden change in calibre is observed (transition zone) and which is where the cause of the obstruction is located. It must be remembered that the most frequent point of obstruction is located in the enteroenteric anastomosis (due to oedema or stricture) or close to the anastomoses due to adhesions2,4 (Fig. 9B and C).
It is the most frequent complication observed in continent diversions.17,22 It involves a narrowing of the distal end of the duct, near the skin surface. This stricture can lead to subsequent complications: obstruction of the ileal conduit, hydronephrosis, renal failure and superinfection (Fig. 10A and B).2,14,22 Fluoroscopy may be useful, especially the sagittal plane.
Stomal complications. (A and B) 72-year-old male patient with Bricker urinary diversion with discomfort at the stoma level and slight worsening of serum creatinine. Grade III–IV hydronephrosis (B, arrows) secondary to thickened and reduced cutaneous stoma (A, box) with associated locoregional inflammatory changes. (C) 68-year-old female patient with Bricker-type urinary diversion with abdominal lump and diffuse discomfort. Right hydronephrosis (star) secondary to change in calibre at the level of the parastomal herniation in the right iliac fossa (arrow).
It is a common complication (5–25%), of which the risk factors are obesity and age. CT is useful to detect hernias, analyse their contents and rule out associated complications2,4 (Fig. 10C).
FistulasThey can be an early or late complication. There are different types of fistulas (enterourinary, enterogenital, enterocutaneous). It must be remembered that enterourinary fistula is the most common and occurs most frequently after the creation of a neobladder, with the leak located between this and the ileoileal anastomosis2,4,14,22 (Fig. 11).
Urinary fistulas. (A and B) 58-year-old male patient with Bricker urinary diversion complicated by an enterourinary fistula. Intravenous contrast leakage is observed through the ileostomy loop (yellow arrowhead) into an adjacent small bowel loop (white arrowhead), suggesting an enterourinary fistula. The leak point is marked with an arrow. The fistula was surgically repaired and, although the intervention was successful, the patient had an unfavourable evolution and eventually died after 22 days. (C–E) 66-year-old female patient with Bricker urinary diversion complicated with an enterocutaneous fistula. The ileostomy loop (arrowhead) is fixed, adjacent to the abdominal incision area. One week later (D), this loop is in contact with the anterior abdominal wall at the lower end of the midline laparotomy wound, forming a well-organised collection (rectangle). The patient was treated using the open vacuum-pack technique and progressed favourably.
When an infectious complication is suspected, the technique of choice is ultrasound, which can be completed with CT based on the visualised findings or according to the patient's clinical context.2,4
Urinary tract infections. Pyelonephritis and pyelitisUrinary tract infections can occur as early or late complications. The CT manifestations of pyelonephritis are nephromegaly, delayed nephrogram, focal wedge-shaped hypodensities with loss of corticomedullary differentiation and locoregional inflammatory changes (perinephric fat stranding, fluid, etc.)4,16,23 (Fig. 12A–C).
Infectious complications. (A) 65-year-old male patient with cystectomy and prostatectomy with Bricker diversion (arrow) who presents with pyuria and leukocyturia with positive left renal fist-percussion (A). An enlarged left kidney is observed with delayed nephrogram and contrast excretion (arrowheads) compatible with pyelonephritis. (B and C) Male and female patients, 42 and 45 years old, respectively, with delayed nephrogram in relation to unilateral (C) and bilateral (B) pyelonephritis (rectangle). (D) 71-year-old female patient with Bricker urinary diversion who presents with increased acute phase reactants and abdominal pain. Adjacent to the point of the ureteroileal anastomosis there is an air-fluid collection with capsular enhancement (star). Axial CT obtained in the delayed excretory phase shows that the excreted high-attenuation contrast material fills the collection (arrows), suggesting an abscessed urinoma.
It is a common complication. The unenhanced and excretory phases of CT urography help determine the content of the collection in patients who generally share the same symptoms (fever, peritonism and abdominal pain).2,4,14,23,24
- -
Urinoma: The excretory phase helps with its categorisation. In UDS with a stoma, air bubbles may be found within the collection (Fig. 12D).
- -
Haematoma: Complex and heterogeneous collection near the surgical bed that appears hyperdense on baseline CT. To rule out active bleeding, the examination is completed with arterial and venous phases (Fig. 13A and B).
Figure 13.Differential diagnosis of intra-abdominal collections. (A and B) 55-year-old female patient with right nephrectomy and cystectomy with Bricker reconstruction who presents with progressive anaemisation. A hyperdense retroperitoneal collection (≈50 HU) is observed in the surgical bed (arrow), compatible with a post-surgical haematoma (A and B). (C) 60-year-old female patient with cystectomy with Bricker reconstruction. A well-defined, low-density collection is observed adjacent to the right external iliac vessels (arrow) consistent with a lymphocele (C). (D) Intralesional air bubbles are not specific for abscesses. They can be seen both in abscesses and in drained collections, in urinomas (when there is a stoma) or in stoma bags without pathology, as in the case of the image.
(0.61MB). - -
Lymphocele: Low-density homogeneous collection near surgical clips, more common after lymphadenectomy14 (Fig. 13C).
- -
Abscess: Collection with irregular ring enhancement and air bubbles inside. Fluid collections can become superinfected, resulting in an abscess23 (Fig. 12D and E). It should be noted that intralesional air bubbles are not specific for an abscess; they are also detected in drained collections, in pouches and in urinomas with a stoma.2 Furthermore, there are abscesses/collections without intralesional gas (Fig. 13D).
Transitional cell carcinoma is the most common histological type of bladder cancer (up to 90% of cases). At the time of diagnosis, 2% of patients present a synchronous tumour of the UUT and up to 6% will develop a metachronous lesion.5,25 Furthermore, the overall recurrence rates are high, up to 60–70%. The test of choice for assessing tumour recurrence is CT urography.5,25
- -
Local recurrence (pelvis): It can take on different radiological manifestations, such as a soft tissue mass at the level of the pelvis, an obstructive urethral stricture (commonly associated with a soft tissue mass), pelvic lymphadenopathy or stricture of the ureterointestinal anastomosis.2,26 In the case of a reconstruction with a neobladder, it is essential to carefully review the urethral anastomosis, since it is the most frequent location of recurrence.14,26–28 (Fig. 14)
Figure 14.Locoregional tumour recurrence. (A and B) 68-year-old male patient with radical cystoprostatectomy and neobladder reconstruction. Lobulated soft tissue lesion with heterogeneous enhancement in the surgical bed (arrows) suspicious of tumour recurrence (A). At 6 months of follow-up (B), central ulceration (arrowhead) and increase in size of the mass are observed. (C and D) 71-year-old male patient with radical cystoprostatectomy and neobladder reconstruction. Pseudonodular thickening of the walls of the neobladder (arrow) in relation to local tumour recurrence.
(0.54MB). - -
Recurrence in the UUT (urethral): It can be seen as an intraluminal mass/nodule, thickening of the urothelial wall with luminal narrowing or as an infiltrating mass.2,14,28
- -
Metastatic recurrence: Up to half of cystectomy patients will relapse with distant disease. It includes recurrence at the lymph node level (inguinal lymphadenopathy, interaortocaval lymphadenopathy, etc.), bone, lung, liver or peritoneal level2,14,28 (Fig. 15).
Figure 15.Locoregional and distant tumour recurrence. (AD) 62-year-old male patient in postoperative follow-up after radical cystectomy and prostatectomy for bladder carcinoma with Bricker reconstruction. (EH) Follow-up study performed 3 months later, confirming locoregional and distant tumour progression. Coronal and axial sections acquired in the portal venous phase are shown. (A and E) Increased degree of bilateral hydronephrosis (arrow). (B and F) Peritoneal implants (box) in the anastomosis between the ureters and the neobladder that cause retrograde hydronephrosis. (C and G) Cluster of subcutaneous nodules in relation to tumour implants (box). (D and H) Newly appearing hypodense hepatic lesions with a bilobar distribution in relation to metastatic deposits (arrowheads).
(0.57MB).
The evaluation of UDS often represents a radiological challenge. Knowledge of the main surgical techniques and the expected postoperative findings in the different imaging techniques is essential for correct assessment. Early identification of both early and late postoperative complications is essential to reduce patient morbidity and mortality.
Author contributionsResponsible for the integrity of the study: ASP.
Study conception: ASP, EAM, AGH.
Study design: ASP, EAM, AGH.
Data collection: ASP, EAM, AGH.
Data analysis and interpretation: N/A.
Statistical processing: N/A.
Literature search: ASP, ECL, RGL, MCGG.
Drafting: ASP, RGL, ECL, MCGG.
Critical review of the manuscript with intellectually relevant contributions: ASP, RGL, ECL, MCGG.
Approval of the final version: ASP, RGL, ECL, MCGG.
Conflicts of interestThe authors declare that they have no conflicts of interest.