This report describes a patient with pulmonary hypertension secondary to schistosomiasis, who sought emergency care due to chest pain at rest. The clinical presentation and other information related to the case raised the suspicion of acute coronary failure, and a severe obstruction was identified in the left main coronary artery. The case report aimed to highlight the need for a differential diagnosis of chest pain complaints in these patients, and emphasizes the choice of percutaneous coronary intervention as an effective and safe treatment in this scenario.
Relatamos o caso de um paciente portador de hipertensão pulmonar de origem esquistossomótica, que procurou pronto atendimento por apresentar quadro de dor torácica em repouso. A apresentação clínica e os demais dados referentes ao caso levantaram a suspeita de insuficiência coronariana aguda, e foi diagnosticada uma obstrução grave do tronco da coronária esquerda. O relato do caso teve por objetivo destacar a necessidade do diagnóstico diferencial da queixa de dor torácica nestes pacientes e ressaltar a opção da intervenção coronariana percutânea como tratamento eficaz e seguro neste cenário.
Significant obstructions in the left main coronary artery are found in 5 to 7% of patients undergoing coronary angiography.1 Atherosclerotic involvement is the most frequent cause, but other causes can occur, such as extrinsic obstruction due to pulmonary artery dilation secondary to pulmonary hypertension.
Schistosomiasis is the third leading endemic parasitic disease in the world, and approximately 5.0% of infected patients also have pulmonary artery hypertension.2 Therefore, one of the main causes of pulmonary hypertension is secondary to schistosomiasis. Chest pain is one of the most common complaints in these patients, usually considered secondary to pulmonary hypertension.
The following case report draws attention to another possible cause of chest pain in these patients and emphasizes the important role of percutaneous coronary intervention in their treatment.
Case reportEAB, a 66-year-old male patient, was diagnosed with schistosomiasis at 10 years of age. At 38, he was diagnosed with pulmonary hypertension after transitory episodes of dyspnea. He remained asymptomatic until 53 years of age, when he started to have new episodes of intense dyspnea associated with atrial fibrillation. There was spontaneous reversion to sinus rhythm and the patient continued to receive warfarin, and he was followed-up by a pneumologist. At the time, pulmonary scintigraphy was negative for thromboembolism, and spirometry was normal. He was submitted to right heart catheterization during the same period, which disclosed pulmonary artery systolic pressure of 107mmHg (systolic pressure of 175mmHg) with no response at the vasodilator test. Routine echocardiograms showed right ventricular dilation and systolic dysfunction, dilation of the pulmonary artery and main branches, and pulmonary hypertension. Annual assessments of functional capacity with the 6-minute walk test showed values between 450 and 500 m for walked distance (reference values for age: 550 to 600 m).
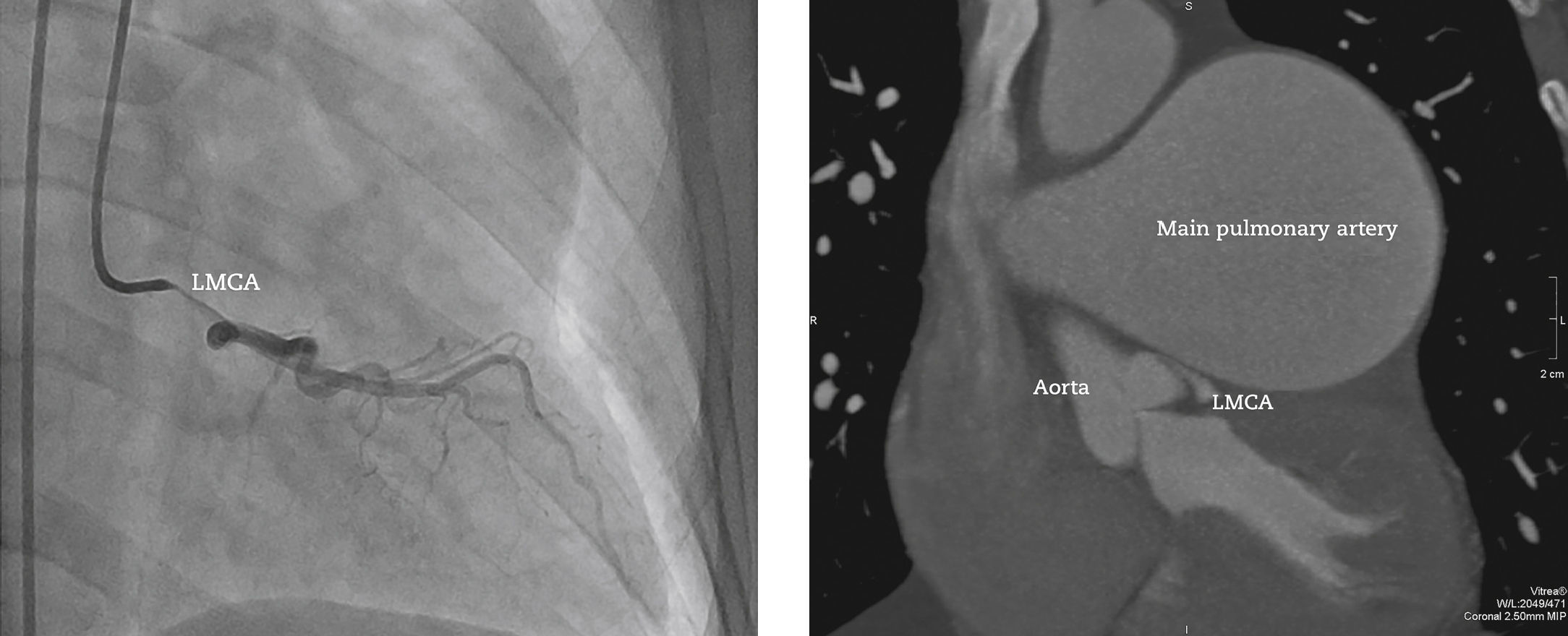
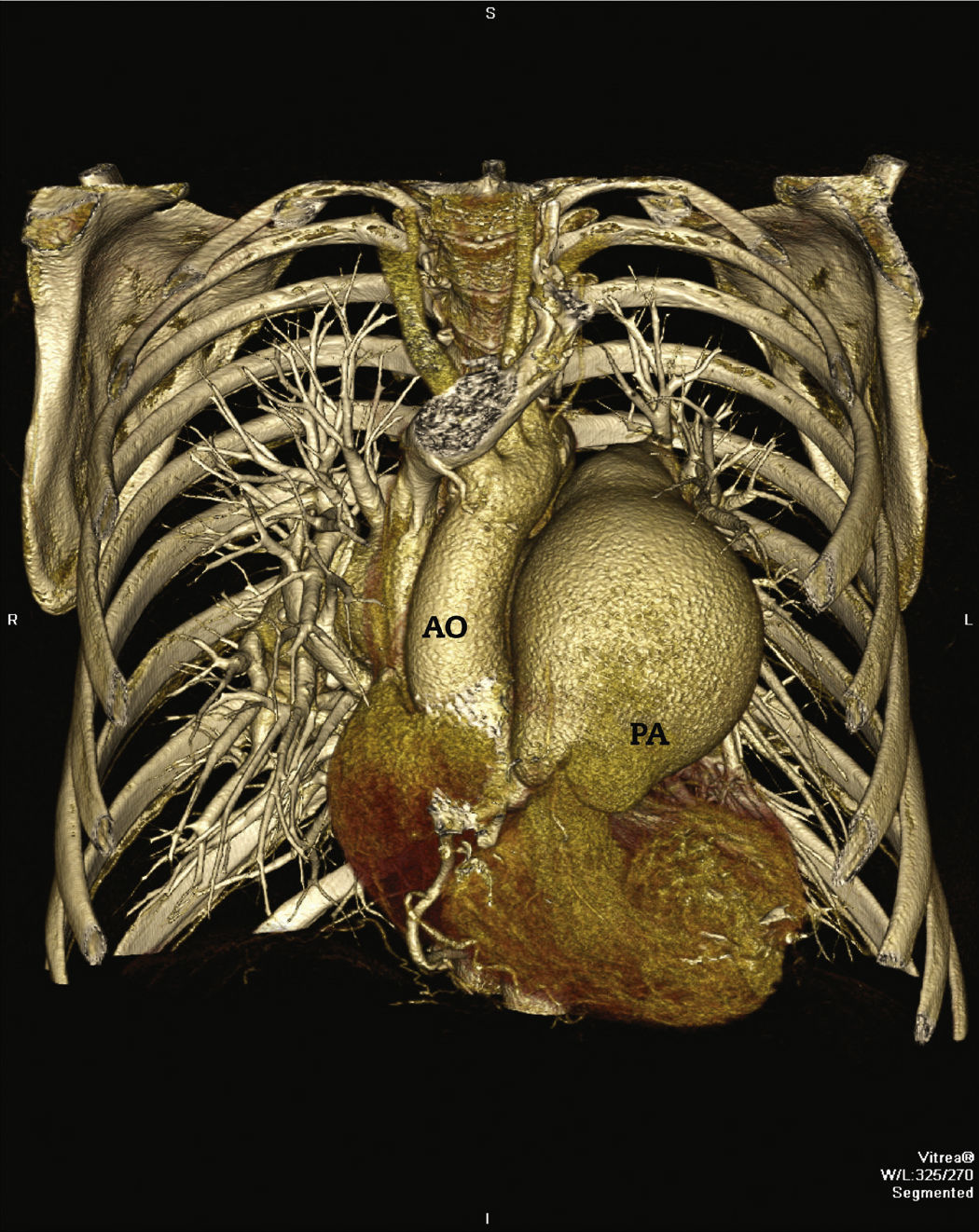
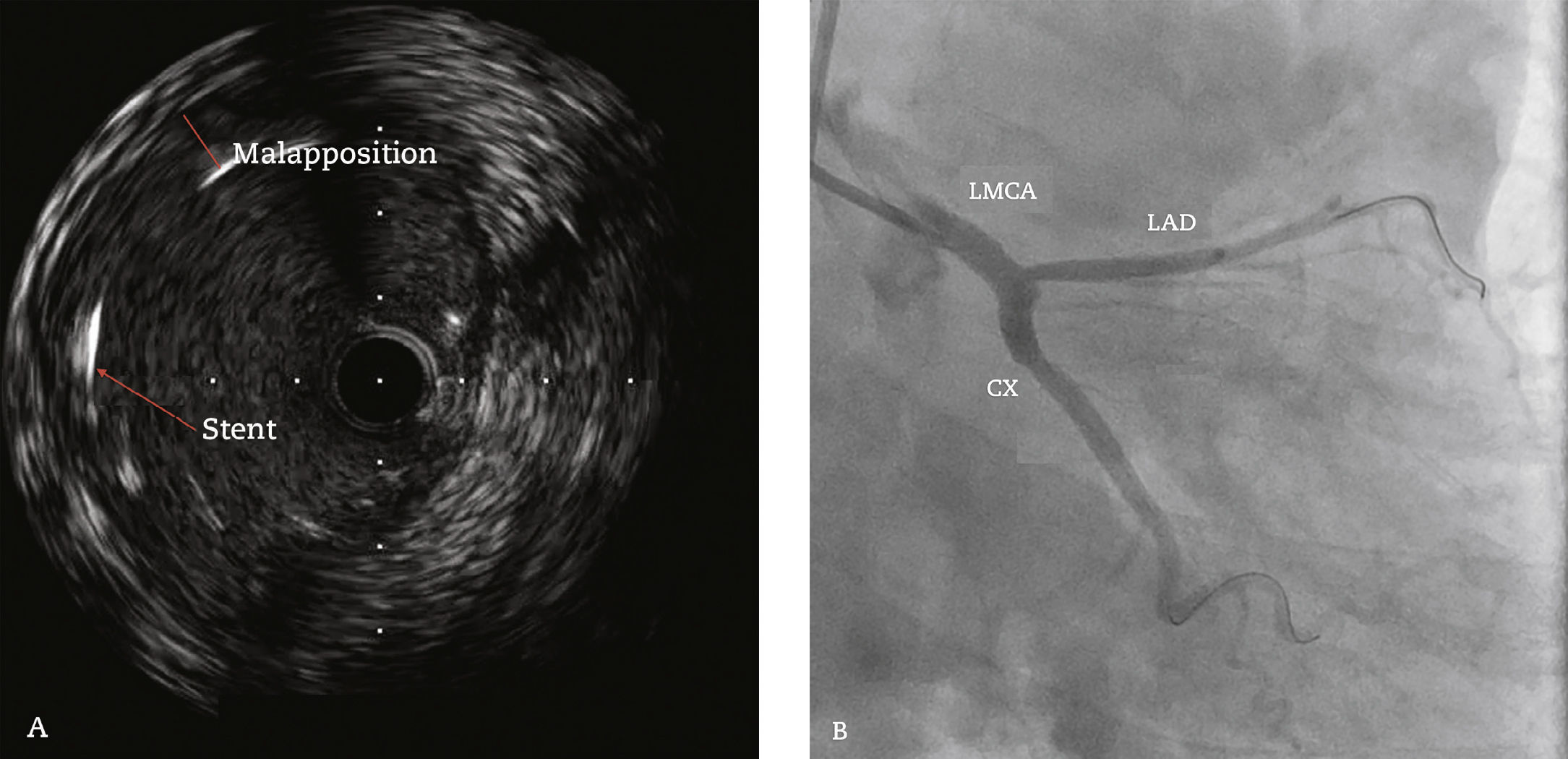
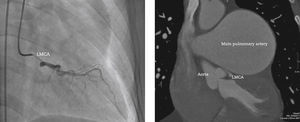
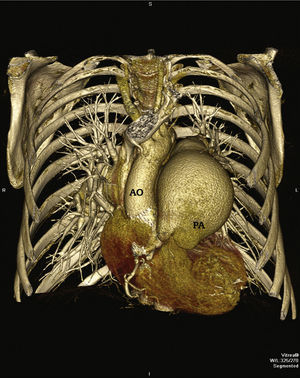
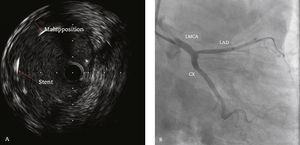
In 2011, at age 62, his dyspnea worsened, this time associated with atypical chest pain. Treatment was started with sildenafil, with significant improvement to functional class II of the New York Heart Association (NYHA). In July 2014, he developed an episode of acute chest pain at rest and sought emergency care. An electrocardiogram showed right bundle branch block, ST-segment depression, and negative T wave from V1 to V5 (alterations that had been previously present), with sequential troponin I values of 34.7 pg/mL, 44.8 pg/mL, and 46.2 pg/mL (RV<34.2 pg/mL). The patient was referred to this service with a diagnosis of acute coronary syndrome. Coronary angiography disclosed a severe, subocclusive lesion in the left main coronary artery, without other significant obstructions, and left ventriculography showed preserved systolic function (Fig. 1). Considering the previous history of significant pulmonary artery dilation and few angiographic signs of coronary atherosclerosis, it was decided to perform a coronary computed tomography angiography, which showed an 80.4-mm main pulmonary artery dilation (RV<26mm) causing extrinsic compression of the left main coronary artery (Fig. 2). The case was discussed with the pneumologist and the cardiovascular surgeon. Considering the high operative risk, it was decided to perform a percutaneous coronary intervention. A 4.0×28mm PROMUS Element™ drug-eluting stent (Boston Scientific – Natick, USA) was implanted in the left main coronary artery. An intravascular ultrasound was performed after implantation, which showed stent malapposition; the issue was resolved after stent dilation with a 4.5×15mm balloon catheter (Fig. 3). Hospital discharge occurred 2 days after the procedure.
Coronary angiography to the left (right anterior oblique caudal projection) showing subocclusive obstruction in the left main coronary artery (LMCA). Coronary computed tomography angiography on the right. Note the significant main pulmonary artery dilation, causing extrinsic obstruction of the LMCA.
Intravascular ultrasound performed after stent implantation in the left main coronary artery (LMCA) showing image suggestive of stent strut malapposition (A). Coronary angiography (right anterior oblique caudal projection) after the 4.0 x 28mm drug-eluting stent implantation in the LMCA, followed by post-dilation with a 4.5 x 15mm non-compliant balloon catheter (B). LAD: left anterior descending artery; CX: circumflex artery.
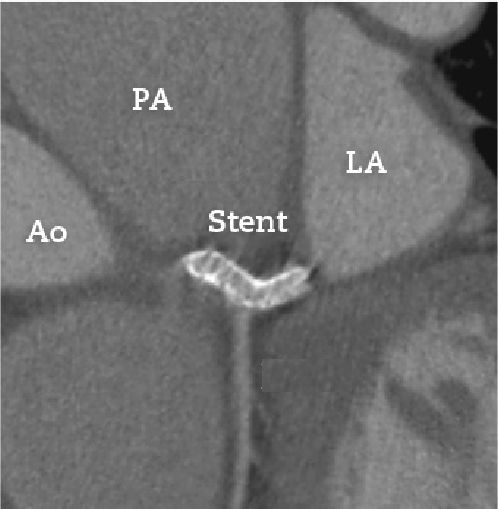
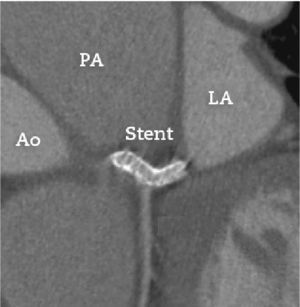
The patient had no recurrence of chest pain and the dyspnea improved to functional class I (NYHA). He is currently asymptomatic and receives metoprolol, furosemide, simvastatin, clopidogrel, warfarin, and sildenafil. The patient received acetylsalicylic acid for 30 days after the procedure. Imaging follow-up was carried out with coronary computed tomography angiography performed 8 months after the procedure (Fig. 4), which showed the well-positioned stent with preserved lumen, with no signs of deformation or restenosis.
DiscussionPulmonary hypertension is triggered by the remodeling of the intimal and medial layers of the pulmonary vascular bed, with consequent increase in vascular resistance, increased pulmonary artery diameter, right ventricular enlargement, and dysfunction, which can cause heart failure (cor pulmonale) and death.2 The most common causes of pulmonary hypertension are primary pulmonary hypertension, congenital heart disease, chronic thromboembolic disease, and advanced pulmonary parenchymal disease. Causes of extrinsic compression of the main pulmonary artery are thoracic outlet syndrome, syphilis, metastatic carcinoma and bronchogenic cyst.3 However, in countries where schistosomiasis is endemic, due to its high prevalence, this parasitic disease is one of the main causes of pulmonary hypertension.
Pulmonary artery dilation can cause extrinsic compression of the left main coronary artery, resulting in significant lumen reduction. The accurate incidence of this condition is not known, and it can occur in 5 to 44% of patients with pulmonary hypertension.3 Its development depends on a chronic increase in the pulmonary artery diameter, usually associated with severe pulmonary hypertension. Compression of the artery becomes probable when the pulmonary artery diameter is >55mm, or when the ratio between the latter and the aortic root diameter is at least 1.98.3
The most common clinical presentation is the occurrence of anginal chest pain, with or without dyspnea. However, syncope, severe arrhythmias, myocardial infarction, or ventricular dysfunction may also be present.3 In the event of any of these signs or symptoms in patients with severe pulmonary hypertension, physicians should suspect the possibility of left main coronary artery obstruction.
The definitive diagnosis of compression is attained by angiography, whether or not associated with the intravascular ultrasound.3 The most appropriate projection is the left anterior oblique cranial view. Coronary computed tomography angiography has some advantages: in addition to depicting the obstruction, it can assess the pulmonary artery, its association with the left main coronary artery, and left ventricular function. It has the disadvantage of exposing the patient to radiation and iodinated contrast. Cardiac magnetic resonance imaging can evaluate the characteristics of the myocardial muscle, adding little to the diagnosis of extrinsic compression. Due to its non-invasive characteristic, coronary computed tomography angiography should be initially requested and used during follow-up after treatment. Imaging tests that assess myocardial ischemia have not been efficient in the evaluation of myocardial perfusion in these patients.3 A likely hypothesis is that extensive ischemia caused by the obstruction would lead to a balanced in myocardial perfusion in several left ventricular segments, which would cause difficulties when analyzing these images.
The best treatment for the extrinsic obstruction of the left main coronary artery by pulmonary artery dilation is yet to be established. The performance of randomized controlled trials is difficult due to the small number of cases.
Myocardial revascularization can be performed through conventional surgery or percutaneous stent implantation. Several authors consider the latter the best option, for different reasons.3–6 First, the risk that is inherent to the conventional surgery in these cases is increased due to the presence of severe pulmonary hypertension. Second, the obstruction physiopathology is not atherosclerotic, which may result in lower rates of stent restenosis and an easier-to-perform percutaneous procedure. According to the reported cases, this type of treatment has shown to be effective and safe during the in-hospital and follow-up periods.3,4,6
The choice between bare-metal stent and drug-eluting stents is also yet to be defined. Some authors prefer bare-metal stents due to the non-atherosclerotic characteristic of the lesion and the large left main coronary artery diameters, which would make restenosis unlikely.4,6 However, the high efficacy and safety of drug-eluting stents in the treatment of atherosclerotic lesions in the left main coronary artery make these the most often used devices in the reported cases.3 It has not been established whether routine angiographic control during follow-up of cases is necessary. As described above, coronary computed tomography angiography would probably be sufficient to rule out restenosis or late complications, considering the recent advances in the method. Nevertheless, in view of the morbidity and large area of myocardium at risk, the use of dual antiplatelet therapy for at least 1 year is recommended.
The treatment of pulmonary hypertension should be performed according to the etiology. Clinical control with vasodilators associated with percutaneous treatment of the lesion has also been described.3 In schistosomiasis cases, treatment should involve specific measures for the parasitic infection. The pharmacological measures include antiparasitic treatment (praziquantel and artemether), even in newly diagnosed chronic forms. The treatment eliminates the adult parasites, which produces eggs, minimizing harm to the lung parenchyma. However, vascular remodeling establishes an irreversibility point, in which the antiparasitic treatment alone is ineffective in preventing progressive worsening. The definitive treatment is heart-lung transplantation.7
Funding sourceNone declared.
Conflicts of interestThe authors declare no conflicts of interest.
Peer Review under the responsability of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.