The transition from ticagrelor to clopidogrel is not based on pharmacodynamic or clinical studies, but it is a common practice. The aim of the present study was to test, in an exploratory way, the transition to two different doses of clopidogrel at the time of hospital discharge in patients diagnosed with acute coronary syndrome submitted to percutaneous coronary intervention who were initially treated with ticagrelor.
MethodsPatients previously treated with ticagrelor were randomized to receive a loading dose of 300mg clopidogrel at hospital discharge, or 75mg without the loading dose. The primary endpoint was the incidence of cardiovascular adverse events or bleeding at 30 days.
ResultsOf 348 selected patients, 132 were enrolled and completed the study. The incidence of ischemic and hemorrhagic events at 30 days was similar between the groups, resulting in a rate of cardiac and cerebrovascular events of 6.1% vs. 9.1% (RR: 0.787; 95% CI: 0.361-1.715; p = 0.74).
ConclusionsThe transition to clopidogrel with a dose of 75mg at discharge, without a loading dose, appears to be a possible strategy. Studies with greater statistical power are needed to confirm these findings.
A transição do ticagrelor para o clopidogrel não está fundamentada em estudos farmacodinâmicos ou clínicos, mas é uma prática comum. O objetivo do presente estudo foi testar, de forma exploratória, em pacientes com diagnóstico de síndrome coronariana aguda submetidos à intervenção coronariana percutânea, inicialmente tratados com ticagrelor, a transição para duas diferentes doses de clopidogrel no momento da alta hospitalar.
MétodosPacientes previamente tratados com ticagrelor foram randomizados para receber uma dose de ataque de 300mg de clopidogrel no momento da alta hospitalar, ou 75mg, omitindo-se a dose de ataque. O objetivo primário foi a incidência de eventos adversos cardiovasculares ou sangramento aos 30 dias.
ResultadosDentre 348 pacientes selecionados, 132 foram incluídos e completaram o estudo. A incidência de eventos isquêmicos e hemorrágicos aos 30 dias foi similar entre os grupos, traduzindo-se em uma taxa de eventos cardíacos e cerebrovasculares de 6,1% vs. 9,1% (RR: 0,787; IC 95%: 0,361-1,715; p = 0,74).
ConclusõesA transição para clopidogrel com a dose de 75mg no momento da alta, omitindo-se uma dose de ataque, aparenta ser uma estratégia possível. Estudos com maior poder estatístico são necessários para confirmar estes achados.
In the management of patients with acute coronary syndrome (ACS), the use of a new P2Y12 receptor blocking agent such as ticagrelor, instead of clopidogrel, is the therapy of choice given the reduction observed in the incidence of severe adverse cardiac events, including cardiovascular mortality, without a significant increase in the risk of severe bleeding.1 Despite the proven cost-effectiveness,2,3 it is often the case that public healthcare policies or supplemental healthcare plans reimbursement policies do not provide for the supply of ticagrelor after discharge, even for patients to whom this use is indicated.4
Although part of the benefit of using ticagrelor in reducing mortality may depend on its possible pleiotropic effects, such as increased adenosine-mediated coronary flow velocity and fewer sudden deaths over 12 months,5 its rapid onset of action and potent platelet inhibition effect are associated with lower rates of acute complications, such as definitive stent thrombosis.6 Thus, even in a scenario in which the patient cannot afford long-term use of ticagrelor, this drug is used as the first treatment line for ACS during the in-hospital phase, in order not to deprive the patient of any advantages provided by the drug use during this period.
The transition from ticagrelor to clopidogrel is not based on pharmacodynamic or clinical studies, but it is common in clinical practice.7,8 Since ticagrelor shows a rapid termination of antiplatelet action (around 48 to 72hours),9 and considering that the daily administration of 75mg clopidogrel, not preceded by a loading dose, may take up to 7 days to reach its full effect,10 the question about the best transition strategy between the drugs persists, due to a theoretical time gap during which the patient would not be adequately receiving optimal platelet antiaggregation therapy.
The aim of the present study was to test, in an exploratory way, the transition to two different doses of clopidogrel at the time of hospital discharge and its impact on the rate of severe adverse cardiac events at 30 days in patients with a diagnosis of ACS submitted to percutaneous coronary intervention (PCI) and initially treated with ticagrelor.
MethodsStudy design and populationThis was a single-center exploratory study, which included patients with a diagnosis of non-ST elevation ACS (unstable angina or non-ST elevation myocardial infarction) submitted to invasive stratification and PCI with stent implantation, or ST-elevation acute myocardial infarction submitted to primary PCI; previously treated with ticagrelor with a loading dose of 180mg followed by a maintenance dose of 90mg every 12hours and acetylsalicylic acid with a loading dose of 300mg and maintenance dose of 100mg daily; and who could not afford ticagrelor after hospital discharge, requiring the transition to clopidogrel. The main exclusion criterion was the possibility of maintaining ticagrelor therapy for 12 months, constituting a representative sample of clinical practice.
Patients were randomized to receive a loading dose of 300mg of clopidogrel at hospital discharge, followed by 75mg for 12 months, or 75mg at discharge and for the next 12 months, without a loading dose. For randomization, a random sequence obtained from computational algorithms was used, kept in individual, sealed opaque envelopes, allowing concealment of the allocation process.
Objectives and definitionsThe primary efficacy endpoint of the study was the incidence of severe adverse cardiac events, defined as a combined outcome of cardiovascular mortality, acute myocardial infarction, stent thrombosis, or new target-vessel revascularization at 30 days. The primary safety objective was the incidence of severe bleeding at 30 days. Severe bleeding was defined as bleeding type 3 – (3a) bleeding with hemoglobin decrease ≥ 3 and < 5g/dL, or packed red blood cell transfusion; (3b) bleeding with hemoglobin decrease ≥ 5g/dL, or cardiac tamponade, or bleeding requiring surgical intervention, or bleeding requiring intravenous vasoactive drugs; (3c) intracranial hemorrhage, or subcategories confirmed by autopsy, imaging test or lumbar puncture, or intraocular bleeding with impaired vision; or type 5 – (5a) probable fatal bleeding, (5b) definitive fatal bleeding, as defined by the Bleeding Academic Research Consortium.11
ProceduresIn cases of non-ST elevation ACS, fondaparinux was the anticoagulant agent of choice in the pre-intervention management, except in patients with creatinine clearance < 20mL/min. During the PCI, supplementation with intravenous unfractionated heparin was implemented at the dose of 85 U/kg or 60 U/kg, when the use of glycoprotein IIb/IIIa inhibitors was planned. Anticoagulation of patients with ST-elevation acute myocardial infarction was obtained with intravenous unfractionated heparin at a dose of 100 U/kg in the interventional laboratory.
The PCI procedures followed the recommendations and practices established by the current guidelines.12 The radial approach was the first option for vascular access. Manual thrombus aspiration and use of glycoprotein IIb/IIIa inhibitors were performed at the interventionist's discretion. The 12-lead electrocardiogram was performed on admission, and at 30 to 60minutes after the end of the procedure. Creatinine kinase MB (CK-MB) isoenzyme and troponin measurements were performed every 6hours, until a decrease in levels of markers was observed; hemoglobin and hematocrit levels were measured between 12 and 24hours after the end of the procedure. Except when contraindicated, patient prescription included a statin, beta-blocker, and angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, in addition to antithrombotic agents. The study was approved by the local Research Ethics Committee and, prior to any procedure, the Free and Informed Consent form was signed.
Statistical analysisQualitative variables were shown as absolute frequencies and percentages. Quantitative data were described as means ± standard deviations or medians (25th–75th percentiles), according to each variable distribution. For the comparison of groups, the Chi-squared test or Fisher's exact test were used for the qualitative variables, and Student's t-test or the Mann-Whitney test were used for quantitative variables. The results with p-value < 0.05 were considered statistically significant. Estimates of event-free probability at 30 days were determined according to the Kaplan-Meier method.
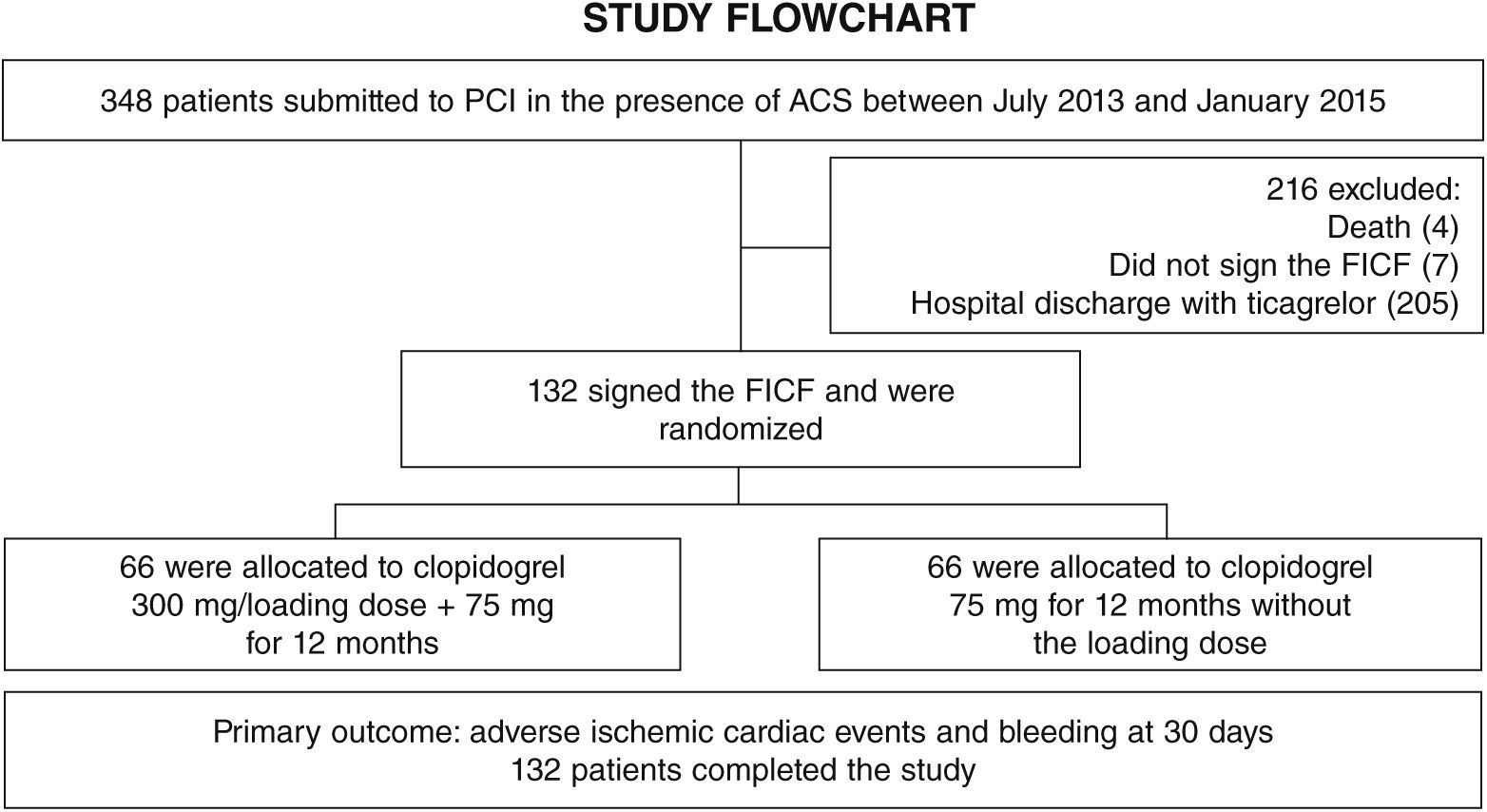
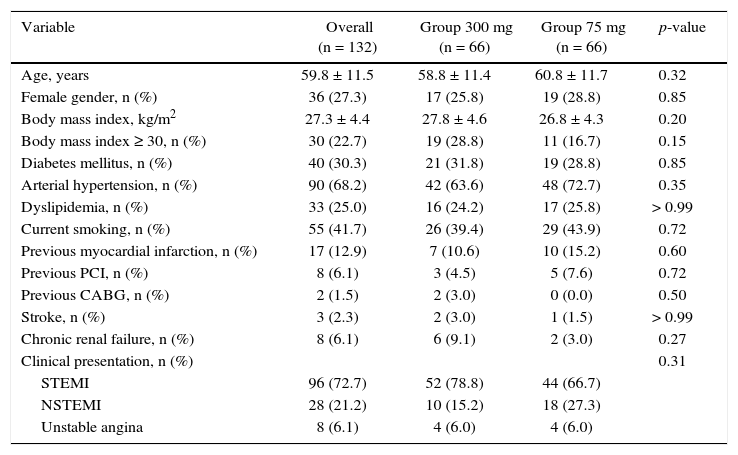
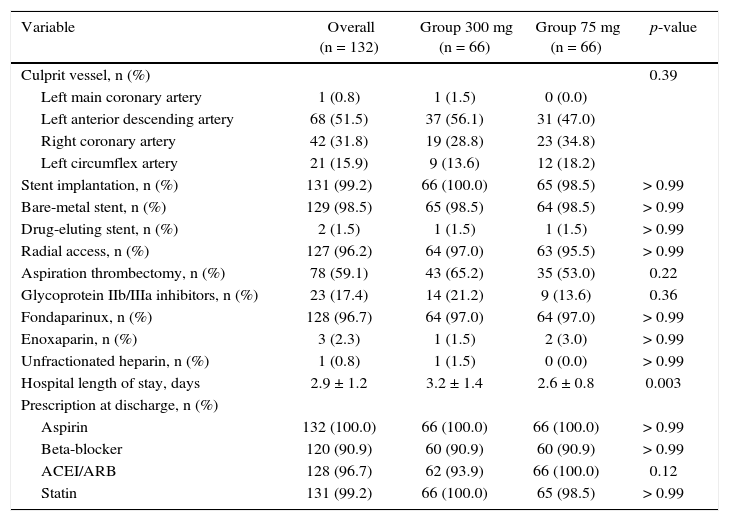
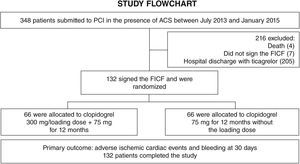
ResultsFrom July 2013 to January 2015, 348 patients diagnosed with ACS submitted to PCI received ticagrelor at hospital admission. Among those who were discharged, 132 could not afford outpatient drug maintenance, and were included in the present study (Fig. 1). The mean age of the patients was 60 years, with 27% females and 30% with diabetes mellitus, with no differences between groups (Table 1). Approximately three-quarters of the patients were submitted to primary PCI and, except for the longer hospital length of stay in the group that received 300mg clopidogrel, no difference was observed in the characteristics of procedures and concomitant medication at hospital discharge, with a high rate of pharmacotherapy prescription for secondary event prevention (Table 2).
Clinical characteristics.
| Variable | Overall (n = 132) | Group 300 mg (n = 66) | Group 75 mg (n = 66) | p-value |
|---|---|---|---|---|
| Age, years | 59.8 ± 11.5 | 58.8 ± 11.4 | 60.8 ± 11.7 | 0.32 |
| Female gender, n (%) | 36 (27.3) | 17 (25.8) | 19 (28.8) | 0.85 |
| Body mass index, kg/m2 | 27.3 ± 4.4 | 27.8 ± 4.6 | 26.8 ± 4.3 | 0.20 |
| Body mass index ≥ 30, n (%) | 30 (22.7) | 19 (28.8) | 11 (16.7) | 0.15 |
| Diabetes mellitus, n (%) | 40 (30.3) | 21 (31.8) | 19 (28.8) | 0.85 |
| Arterial hypertension, n (%) | 90 (68.2) | 42 (63.6) | 48 (72.7) | 0.35 |
| Dyslipidemia, n (%) | 33 (25.0) | 16 (24.2) | 17 (25.8) | > 0.99 |
| Current smoking, n (%) | 55 (41.7) | 26 (39.4) | 29 (43.9) | 0.72 |
| Previous myocardial infarction, n (%) | 17 (12.9) | 7 (10.6) | 10 (15.2) | 0.60 |
| Previous PCI, n (%) | 8 (6.1) | 3 (4.5) | 5 (7.6) | 0.72 |
| Previous CABG, n (%) | 2 (1.5) | 2 (3.0) | 0 (0.0) | 0.50 |
| Stroke, n (%) | 3 (2.3) | 2 (3.0) | 1 (1.5) | > 0.99 |
| Chronic renal failure, n (%) | 8 (6.1) | 6 (9.1) | 2 (3.0) | 0.27 |
| Clinical presentation, n (%) | 0.31 | |||
| STEMI | 96 (72.7) | 52 (78.8) | 44 (66.7) | |
| NSTEMI | 28 (21.2) | 10 (15.2) | 18 (27.3) | |
| Unstable angina | 8 (6.1) | 4 (6.0) | 4 (6.0) |
PCI: percutaneous coronary intervention; CABG: coronary artery bypass grafting; STEMI: ST-segment elevation myocardial infarction; NSTEMI: non-ST-segment elevation myocardial infarction.
Angiographic and procedure characteristics.
| Variable | Overall (n = 132) | Group 300 mg (n = 66) | Group 75 mg (n = 66) | p-value |
|---|---|---|---|---|
| Culprit vessel, n (%) | 0.39 | |||
| Left main coronary artery | 1 (0.8) | 1 (1.5) | 0 (0.0) | |
| Left anterior descending artery | 68 (51.5) | 37 (56.1) | 31 (47.0) | |
| Right coronary artery | 42 (31.8) | 19 (28.8) | 23 (34.8) | |
| Left circumflex artery | 21 (15.9) | 9 (13.6) | 12 (18.2) | |
| Stent implantation, n (%) | 131 (99.2) | 66 (100.0) | 65 (98.5) | > 0.99 |
| Bare-metal stent, n (%) | 129 (98.5) | 65 (98.5) | 64 (98.5) | > 0.99 |
| Drug-eluting stent, n (%) | 2 (1.5) | 1 (1.5) | 1 (1.5) | > 0.99 |
| Radial access, n (%) | 127 (96.2) | 64 (97.0) | 63 (95.5) | > 0.99 |
| Aspiration thrombectomy, n (%) | 78 (59.1) | 43 (65.2) | 35 (53.0) | 0.22 |
| Glycoprotein IIb/IIIa inhibitors, n (%) | 23 (17.4) | 14 (21.2) | 9 (13.6) | 0.36 |
| Fondaparinux, n (%) | 128 (96.7) | 64 (97.0) | 64 (97.0) | > 0.99 |
| Enoxaparin, n (%) | 3 (2.3) | 1 (1.5) | 2 (3.0) | > 0.99 |
| Unfractionated heparin, n (%) | 1 (0.8) | 1 (1.5) | 0 (0.0) | > 0.99 |
| Hospital length of stay, days | 2.9 ± 1.2 | 3.2 ± 1.4 | 2.6 ± 0.8 | 0.003 |
| Prescription at discharge, n (%) | ||||
| Aspirin | 132 (100.0) | 66 (100.0) | 66 (100.0) | > 0.99 |
| Beta-blocker | 120 (90.9) | 60 (90.9) | 60 (90.9) | > 0.99 |
| ACEI/ARB | 128 (96.7) | 62 (93.9) | 66 (100.0) | 0.12 |
| Statin | 131 (99.2) | 66 (100.0) | 65 (98.5) | > 0.99 |
ACEI: angiotensin-converting enzyme inhibitor; ARB: angiotensin-receptor blocker.
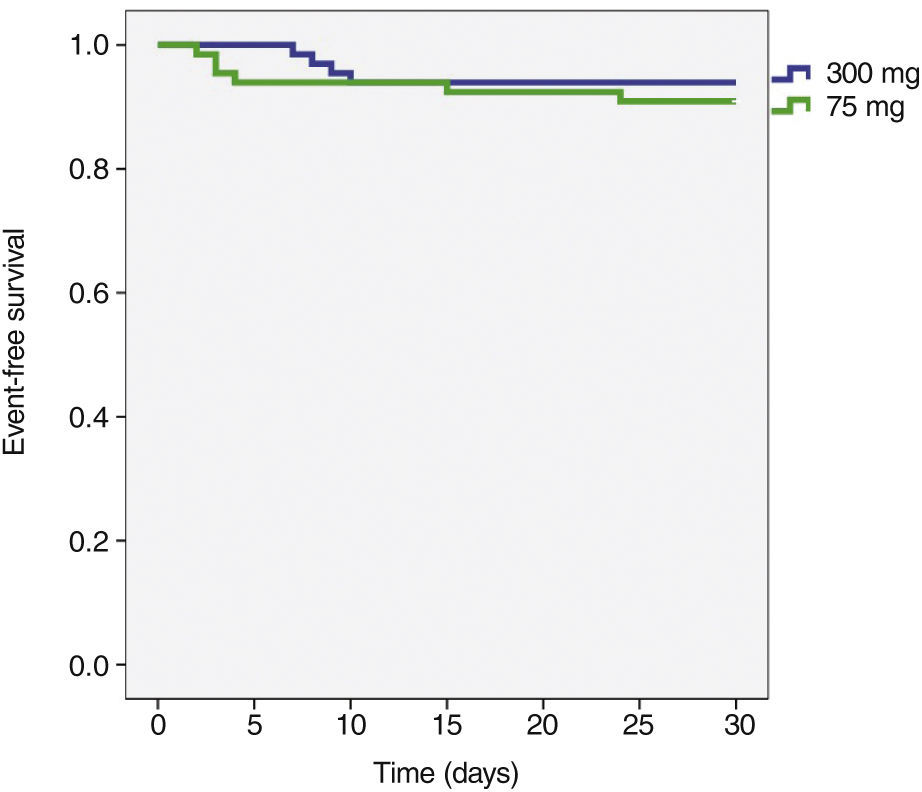
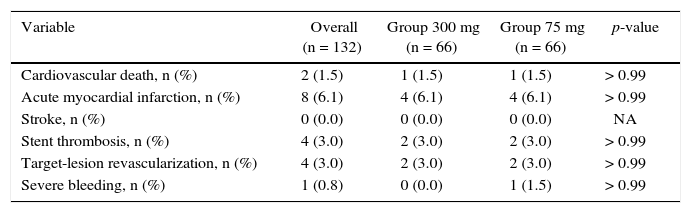
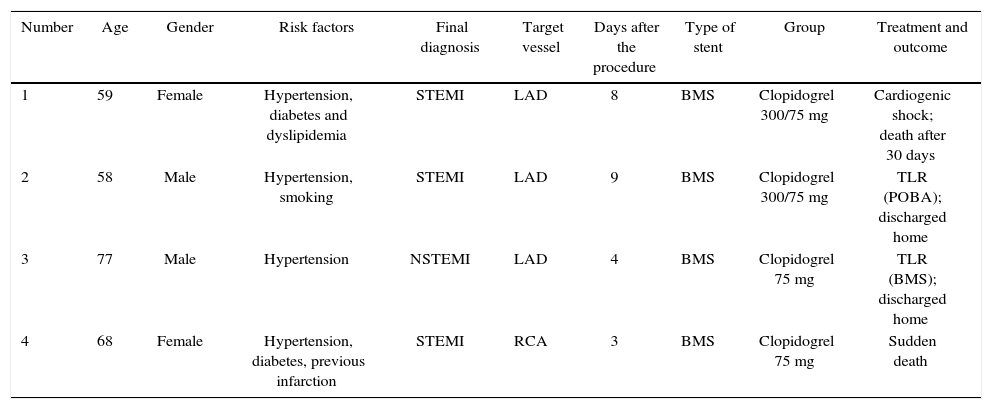
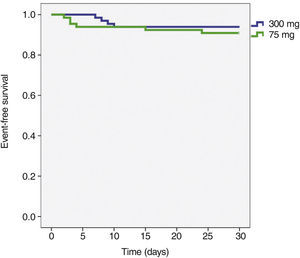
The incidence of ischemic and hemorrhagic events at 30 days was similar between groups, translating into a clinical and cerebrovascular event rate of 6.1% vs. 9.1% (relative risk – RR: 0.787, 95% confidence interval – 95% CI: 0.361-1.715, p = 0.74) (Table 3). The Kaplan-Meier analysis showed a similar probability of 30-day cerebrovascular adverse events (93.9% vs. 90.9%, log-rank test; p = 0.50) (Fig. 2). Four cases of subacute stent thrombosis were observed (two in each group), and are shown in Table 4.
Adverse cardiac and cerebrovascular events and bleeding at 30 days.
| Variable | Overall (n = 132) | Group 300 mg (n = 66) | Group 75 mg (n = 66) | p-value |
|---|---|---|---|---|
| Cardiovascular death, n (%) | 2 (1.5) | 1 (1.5) | 1 (1.5) | > 0.99 |
| Acute myocardial infarction, n (%) | 8 (6.1) | 4 (6.1) | 4 (6.1) | > 0.99 |
| Stroke, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
| Stent thrombosis, n (%) | 4 (3.0) | 2 (3.0) | 2 (3.0) | > 0.99 |
| Target-lesion revascularization, n (%) | 4 (3.0) | 2 (3.0) | 2 (3.0) | > 0.99 |
| Severe bleeding, n (%) | 1 (0.8) | 0 (0.0) | 1 (1.5) | > 0.99 |
NA: not applicable.
Findings in the four patients with stent thrombosis.
| Number | Age | Gender | Risk factors | Final diagnosis | Target vessel | Days after the procedure | Type of stent | Group | Treatment and outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 59 | Female | Hypertension, diabetes and dyslipidemia | STEMI | LAD | 8 | BMS | Clopidogrel 300/75 mg | Cardiogenic shock; death after 30 days |
| 2 | 58 | Male | Hypertension, smoking | STEMI | LAD | 9 | BMS | Clopidogrel 300/75 mg | TLR (POBA); discharged home |
| 3 | 77 | Male | Hypertension | NSTEMI | LAD | 4 | BMS | Clopidogrel 75 mg | TLR (BMS); discharged home |
| 4 | 68 | Female | Hypertension, diabetes, previous infarction | STEMI | RCA | 3 | BMS | Clopidogrel 75 mg | Sudden death |
STEMI: ST-elevation myocardial infarction; LAD: left anterior descending artery; BMS: bare-metal stent; TLR: target lesion revascularization; POBA: plain old balloon angioplasty; NSTEMI: non-ST-elevation myocardial infarction; RCA: right coronary artery.
Clinical practice registries show that the transition between P2Y12-receptor inhibiting drugs can occur in up to one-third of hospitalized patients. Although the most frequent change is from clopidogrel to ticagrelor or prasugrel, concerns about the risk or occurrence of bleeding, older age, previous cerebrovascular events, need for triple antithrombotic therapy, and coverage by public or supplementary health policies mean that approximately 5 to 10% of patients initially taking the new antiplatelet agents are discharged on clopidogrel.7,8 Different transition scenarios between P2Y12 receptor inhibitors have been reported in clinical and pharmacodynamic studies,13–15 but there have been few reports on the transition from ticagrelor to clopidogrel.
In the present series, the predominant reason for the transition from ticagrelor to clopidogrel was the lack of economic resources to acquire the drug after hospital discharge, since the local public healthcare system does not reimburse for ticagrelor. Nevertheless, the authors opted for hospital administration of the new antiplatelet so as not to deprive the patients of the possible benefits provided by its rapid action onset and the potent platelet inhibition achieved with ticagrelor. This decision was based on the results observed in the PLATO (PLATelet inhibition and patient Outcomes) randomized study, in which ticagrelor, when compared to clopidogrel, resulted in a 40% reduction in the risk of stent thrombosis between the 4th hour and 30 days (RR: 0.60; 95% CI: 0.39-0.93).16 Even when the agent used for comparison was ticagrelor itself, its earlier administration in the pre-hospital phase was associated with a reduction in cases of definitive stent thrombosis when compared with the in-hospital administration, both in the first 24hours (0.0% vs. 0.8%; p = 0.008) and at 30 days (0.2% vs. 1.2%; p = 0.02), as demonstrated in the ATLANTIC study.6
A pharmacodynamic study could demonstrate the behavior of platelet aggregation inhibition during the transition from ticagrelor to clopidogrel. It has recently been shown that, despite the formal recommendation of the PLATO study regarding the administration of an 180-mg dose of ticagrelor, even in patients pre-treated with clopidogrel, no difference was observed in platelet inhibition when the loading dose is omitted, starting the treatment with 90mg.14 Faced with the impossibility of pharmacodynamic evaluation, the authors chose to perform the present exploratory study, which suggests the safety and efficacy of the transition from ticagrelor to clopidogrel, without the need for a loading dose of the latter. A similar rate of ischemic complications was observed, which would reflect inadequate antiaggregation, such as stent thrombosis. Although a thrombosis rate of 3% may seem high, the study had a representative real-world sample, with 73% of patients with a final diagnosis of ST-elevation acute myocardial infarction, disclosing several predictors of this complication. The low rate of hemorrhagic complications may be justified by the use of strategies to reduce bleeding, such as radial access, fondaparinux, and low use of glycoprotein IIb/IIIa inhibitors.17
Study limitationsThe main limitation of this study was its exploratory characteristic and its small sample size. However, as the transition from ticagrelor to clopidogrel has been practiced in this center for approximately 2 years, and in the absence of robust ongoing studies, the authors aimed to obtain a pragmatic answer to a routine situation. The randomization did not follow sampling calculation criteria, as well as of expected outcomes. Failure to perform a pharmacodynamic study is also an important limitation, although the clinical outcomes suggest a more applicable response to the daily practice. The short follow-up period of 30 days may be a limitation, but it reflects the effects of the transition from ticagrelor to clopidogrel, since clopidogrel action reaches its plateau after 30 days and the effects of its long-term prescription are scientifically known. The group of patients who continued using ticagrelor was not followed, which would allow the comparison of the observed outcomes, representing another limitation of the present study.
ConclusionsIn patients with acute coronary syndrome submitted to percutaneous coronary intervention and treated with ticagrelor during the in-hospital phase, the transition to clopidogrel with a 75-mg dose at hospital discharge without a loading dose appears to be a safe and effective strategy. The result should be interpreted as a hypothesis generator, and studies with greater statistical power are necessary to confirm these findings.
Funding sourcesNone declared.
Conflicts of interestThe authors declare no conflicts of interest.
Peer review under the responsibility of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.