Mortality is high in patients with post-infarction cardiogenic shock. Reversal of tissue hypoperfusion is essential for organ preservation during the myocardial functional recovery period. The authors report the case of a female patient who, after consecutive episodes of cardiorespiratory arrest, developed cardiogenic shock secondary to spontaneous dissection of the left main coronary artery. After restoration of coronary flow through primary percutaneous intervention with stent implantation, the Impella™ 2.5 circulatory assist device was implanted, which allowed the patient's hemodynamic improvement, contributing to a favorable outcome.
Em pacientes com choque cardiogênico pós-infarto a mortalidade é alta. A reversão da hipoperfusão tecidual é essencial para a preservação orgânica durante o período de recuperação funcional do miocárdio. Relatamos o caso de uma paciente que, após seguidos episódios de parada cardiorrespiratória, evoluiu com choque cardiogênico secundário à dissecção espontânea do tronco de coronária esquerda. Após a restauração do fluxo coronariano, por meio da intervenção percutânea primária com uso de stent, optou-se pelo implante do dispositivo de assistência circulatória Impella® 2.5, que permitiu melhorar as condições hemodinâmicas da paciente, contribuindo para um desfecho favorável.
Individuals presenting with acute coronary syndrome and cardiogenic shock have high mortality rates, which may exceed 70%.1 The most common etiology of cardiogenic shock is acute myocardial infarction with ST-segment elevation.2 Coronary reperfusion strategies using the percutaneous approach, by restoring the infarction-related artery patency, can limit infarction size and improve ventricular function and prognosis of individuals in cardiogenic shock.2 Additionally, early reversal of tissue hypoperfusion is important in these cases. The use of mechanical support for circulatory assistance, such as the Impella™ device (Abiomed, Danvers, USA),3 is indicated when the cardiogenic shock does not respond to optimized pharmacological treatment and conventional measures, including volume infusion and use of vasopressors and inotropes, with or without the use of the intra-aortic balloon pump. This report aimed to present the case of a young female patient with refractory cardiogenic shock, in which the implementation of ventricular assistance through mechanical support device was crucial.
Case reportA 33-year-old female patient was admitted on December 9, 2013 at the emergency unit of a cardiac hospital in Aparecida de Goiânia (GO, Brazil), with a complaint of oppressive chest pain, without irradiation, which started after mild exertion, approximately 20minutes before arrival. The electrocardiogram showed abnormal ventricular repolarization in anteroseptal leads. Biochemical markers of myocardial necrosis were negative. A chest X-ray showed no significant alterations. After admission, she had two cardiorespiratory arrests in ventricular fibrillation, which were promptly reversed with defibrillation and resuscitation maneuvers. She was admitted to the intensive care unit under mechanical ventilation with severe hypotension; central venous access, invasive blood pressure catheter, and intravenous noradrenaline administration were performed. Echocardiogram at the bedside showed ejection fraction of 29% and akinesia of the apical region, in addition to akinesia of the middle region of the anteroseptal, inferoseptal, and anterior left ventricle walls.
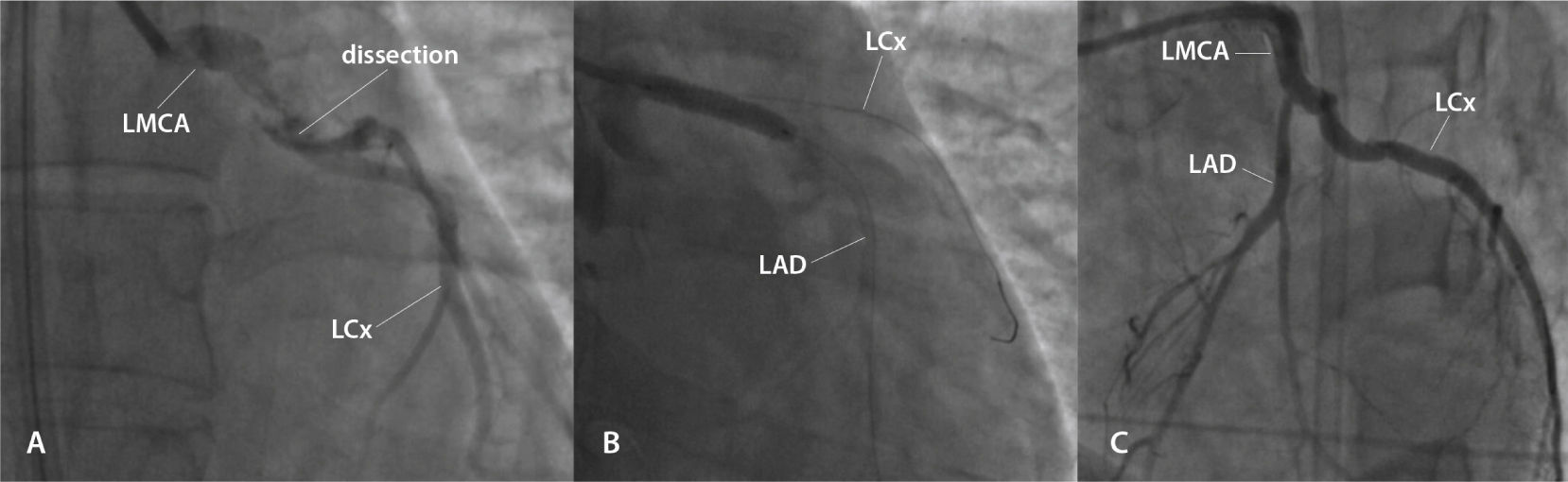
She was referred to an emergency coronary angiography, which revealed a subocclusive lesion, suggesting a spontaneous dissection in the left main coronary artery, with minimal flow in the left anterior descending and left circumflex arteries (Fig. 1A). She immediately underwent primary percutaneous coronary intervention with implantation of a bare-metal stent in the left main coronary artery (Fig. 1B and 1C). During the procedure, she had five episodes of pulseless electrical activity cardiac arrest, promptly reversed.
(A) Lesion showing the aspect of spontaneously dissection in the left main coronary artery (LMCA), with minimal flow in the left anterior descending artery (LAD) and left circumflex (LCx) artery. (B) Percutaneous coronary intervention with implantation of a bare-metal stent in the LMCA. (C) Outcome after percutaneous coronary intervention.
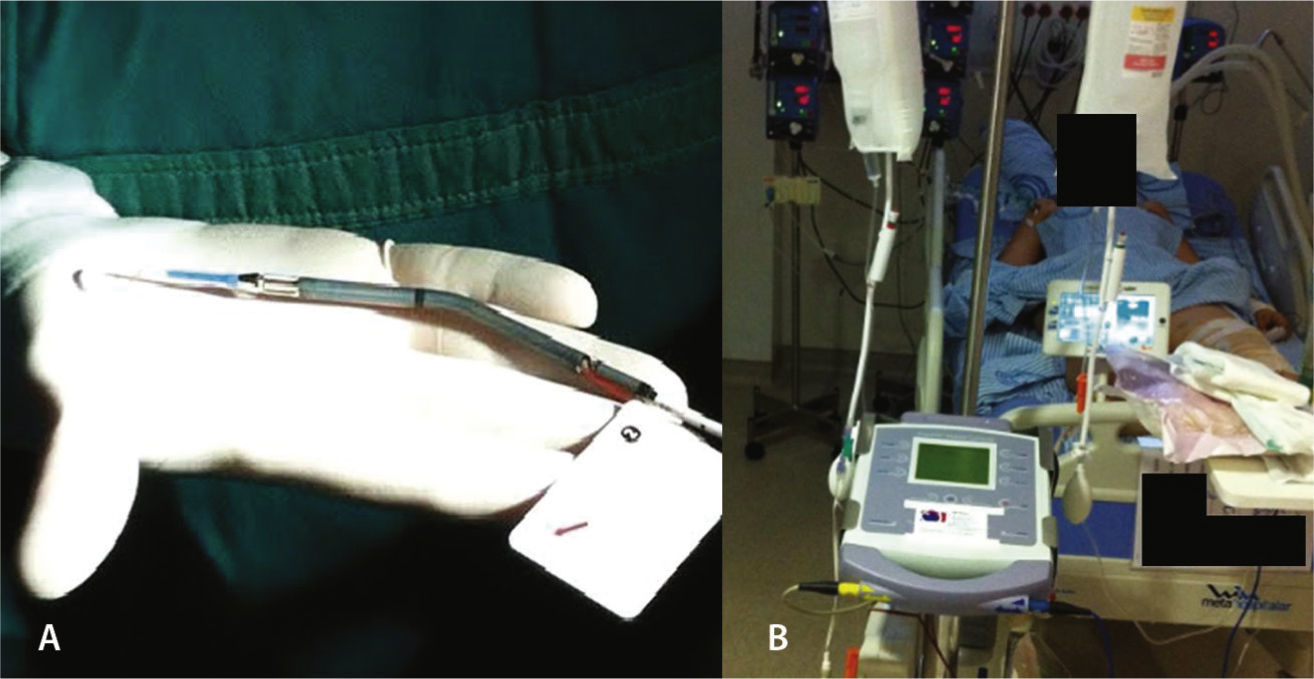
On the following morning, in the presence of refractory cardiogenic shock, with maximum doses of noradrenaline and dobutamine, the use of a circulatory assist device was considered, and the Impella™ device, 2.5 liters/minute, was installed approximately 18hours after admission, through the left femoral artery (Fig. 2). The hemodynamic instability was reversed, and the patient was successfully extubated on the fourth day of hospitalization without any neurological deficit. She subsequently developed hemolysis, hemoglobinuria, and worsening renal function without the need for hemodialysis. The Impella® device had its parameters gradually reduced, followed by withdrawal 5 days after its installation, maintaining hemodynamic stability with cardiogenic shock resolution.
A new echocardiogram performed after Impella™ device withdrawal demonstrated an improvement in left ventricular ejection fraction (46%), akinesia of the apical region, akinesia of the middle region of the left ventricular anteroseptal wall, and hypokinesia of the middle region of the anterior and inferoseptal walls of the left ventricle.
The patient was asymptomatic on discharge from the hospital 15 days after admission.
DiscussionTemporary ventricular assist devices, such as Impella™ 2.5, are often used as circulatory rescue therapy in the face of refractory hemodynamic conditions that may induce systemic-organ failure resulting from tissue hypoperfusion.4 Cardiac output must be quickly reestablished in these patients, in order to maintain systemic perfusion. For this purpose, vasoactive and inotropic drugs are regularly used and, in the presence of persistent hemodynamic instability, the use of devices such as Impella™ 2.5 is effective, reducing ventricular load and providing the necessary circulatory support to allow myocardial recovery.5,6
Impella™ 2.5 is a centrifugal-flow pump, embedded in a tube, which aspirates blood from the left ventricle through an inflow area, near the tip, and expels the blood from the catheter to the ascending aorta, decompressing the left ventricle, improving coronary perfusion and decreasing the need for inotropic drugs. The patient must remain anticoagulated with an activated coagulation time (ACT) of around 180seconds through continuous heparin infusion.
The device can be inserted in a standard catheterization procedure through the femoral artery, ascending aorta, aortic valve, and positioned in the left ventricle. It can be implanted quickly, is easy to maintain in intensive care units, and promotes immediate hemodynamic condition improvement.7
The main indication that should guide the implementation of mechanical circulatory support after myocardial infarction (with or without ST-segment elevation) is the presence of persistent cardiogenic shock, even after early revascularization (through coronary artery bypass surgery or percutaneous coronary intervention).8
It is noteworthy that the use of such device is not free from adverse effects. Events like hemolysis, acute renal dysfunction, thrombocytopenia, bleeding, aortic valve injury, stroke, vascular access complications, and others have been described.9
Clinical trials have been carried out to compare different approaches to cardiogenic shock secondary to acute myocardial infarction.8,10 The IABP-SHOCK II (Intra-aortic Balloon Pump in Cardiogenic Shock II) study included 598 patients randomized between intra-aortic balloon group and a control group. Thirty-day mortality was similar (39.7% vs. 41.3%, p = 0.69). No differences were observed between secondary outcomes, such as time spent in the critical-care unit, serum lactate, doses and time of use of vasoactive drugs, time for hemodynamic stabilization, and others.8
Another study carried out with 26 patients with cardiogenic shock sought to compare the use of Impella™ 2.5 and the intra-aortic balloon pump (IABP), investigating hemodynamic outcomes (pre- and 30minutes after installation), lactic acidosis, hemolysis, and mortality. Better hemodynamic support was observed in the group of patients treated with Impella™ 2.5 compared to IABP, with higher values of cardiac index, cardiac output, and mean arterial pressure 30minutes after its installation. No significant differences were observed regarding the other assessed parameters, and mortality was similar between the groups (46%).6
It is worth mentioning the role of percutaneous coronary intervention with stent implantation in cardiogenic shock, with a fundamental objective of reestablishing Thrombolysis in Myocardial Infarction (TIMI) III flow and myocardial perfusion, followed by hemodynamic support with circulatory assist devices when necessary, in selected cases (early use in refractory cases, in young patients, and in those without organ dysfunction, among others).
Therefore, the authors conclude that the use of the Impella™ device allowed rapid improvement in the patient's hemodynamic condition, preserving vital functions until the myocardium could fully resume its contractile function. Mechanical ventricular assist devices should be considered as an alternative in similar cases.
FundingNone declared.
Conflicts of interestThe authors declare no conflicts of interest.
Peer review under the responsibility of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.