In BIOACTIVE study, we evaluated vascular responses after the implant of biolimus A9-eluting stent (BES; BioMatrixTM) and the everolimus-eluting stent (EES; XIENCE VTM). In this study, we present the optical coherence tomography analysis (OCT) 6 months post-intervention.
MethodsPatients were randomized to treatment with BES (n = 22) or EES (n = 18). The primary outcome was the frequency of non-covered, poorly positioned struts by OCT.
ResultsOCT was performed in 26 patients (BES: n = 15; EES: n = 11) and 749 tomographic images and 7,725 stent struts were analyzed. BES and EES showed similar luminal and stent areas. Neointimal hyperplasia area, neointimal thickness and the percentage of in-stent obstruction (8.44 ± 5.10% vs. 9.21 ± 6.36%; p = 0.74) were similar. The rates of not covered struts (BES: 2.10 ± 3.60% vs. ESS: 2.46 ± 2.15%, p = 0.77) and poorly positioned struts (BES: 0.48 ± 1.48% vs. EES 0.44 ± 1.05%, p = 0.94) were similarly low. The frequency of frames with signs consistent with peri-strut inflammatory infiltrate was low and similar between BES (15.53 ± 20.77%) and EES (11.70 ± 27.51%; p = 0.68).
ConclusionsThe second-generation drug-eluting stents BES and EES were equally effective at suppressing the neointimal formation after 6 months, with favorable vascular responses. The frequency of frames with peri-strut infiltrate signals per patient was low, and lower than that observed historically with first-generation drug-eluting stents.
No estudo BIOACTIVE, avaliamos as respostas vasculares após implante do stent eluidor de biolimus A9 (SEB; BioMatrixTM) e o stent eluidor de everolimus (SEE; XIENCE VTM). Apresentamos a análise de tomografia de coerência óptica (OCT) 6 meses pós-intervenção.
MétodosOs pacientes foram randomizados para tratamento com SEB (n = 22) ou SEE (n = 18). O desfecho primário foi a frequência de hastes não cobertas e mal apostas pela OCT.
ResultadosA OCT foi realizada em 26 pacientes (SEB: n = 15; SEE: n = 11) e foram analisadas 749 imagens tomográficas e 7.725 hastes de stent. SEB e SEE apresentaram áreas luminais e dos stents semelhantes. A área de hiperplasia neointimal, a espessura neointimal e o porcentual de obstrução intra-stent (8,44 ± 5,10% vs. 9,21 ± 6,36%; p = 0,74) foram similares. As taxas de hastes não cobertas (SEB: 2,10 ± 3,60% vs. SEE: 2,46 ± 2,15%; p = 0,77) e mal apostas (SEB: 0,48 ± 1,48% vs. SEE 0,44 ± 1,05%; p = 0,94) foram baixas e semelhantes. A frequência de frames com sinais compatíveis com infiltrado inflamatório peri-haste foi baixa e similar entre SEB (15,53 ± 20,77%) e SEE (11,70 ± 27,51%; p = 0,68).
ConclusõesStents farmacológicos de segunda geração SEB e SEE se mostraram igualmente eficientes em suprimir a formação neointimal aos 6 meses, com respostas vasculares favoráveis. A frequência de frames com sinais de infiltrado peri-haste por paciente foi baixa, e menor do que a historicamente observada com os stents farmacológicos de primeira geração.
Drug-eluting stents (DES), designed under the concept of locally applying an antiproliferative agent on the vascular wall in a controlled manner, have met their primary endpoint of reducing excessive neointimal formation, commonly observed after coronary angioplasty with balloon and bare-metal stent (BMS) implantation.1,2 High antiproliferative efficacy has resulted in a significant reduction in restenosis rates and need for new coronary revascularization in a wide variety of clinical and anatomical scenarios,3–8 making DES implantation the standard treatment strategy during percutaneous coronary intervention (PCI) procedures in many regions of the world.
However, the widespread use of DES and longer follow-up of patients treated with this technology disclosed a significantly higher incidence of late- and very-late thrombosis than in those seen after BMS implantation.9–11
Although late/very late stent thrombosis is a multifactorial phenomenon, incomplete strut coverage due to delay in cellular matrix and functioning endothelium formation, chronic inflammatory reactions in the vessel wall, vascular remodeling, and acquired late malapposition were demonstrated after the implantation of first-generation DES.12–16 In general, these DES consisted of stainless steel platforms with relatively thick metal struts that eluted high doses of sirolimus or paclitaxel via durable polymeric carriers distributed around the entire metal surface. In particular, the durable polymers of the first-generation DES – in permanent contact with the vessel wall – were associated with local hypersensitivity reactions.12
These findings led to the development of new DES, aiming to reduce toxicity to the vascular wall and increase the biocompatibility of these devices, although without losing the antiproliferative efficacy displayed by the first-generation DES. In this sense, a number of modifications were implemented: platforms made of new metallic alloys; thinner struts; improved delivery systems; new antiproliferative drugs; use of lower doses of drugs; new polymeric matrices that are thinner, more biocompatible, and even bioresorbable; polymer applied directly to the abluminal side of the stent struts; and drug carrying and elution by non-polymeric platforms, etc.17
The BIOACTIVE study aimed to assess vascular responses after implantation of two second generation DES – BioMatrixTM (biolimus A9-eluting stent, utilizing a biodegradable polymer), and XIENCE VTM (everolimus-eluting stent, utilizing a durable, biocompatible fluoropolymer), which incorporated one or more of the aforementioned characteristics. For that purpose, optical coherence tomography (OCT) images were used, which, through high-resolution CT images, allow accurate assessment of the vascular repair process after coronary stenting.18,19 This study aimed to present the OCT analysis at 6 months post-intervention.
MethodsStudy design and populationBIOACTIVE is a prospective, randomized trial of the investigators’ initiative, in two centers (Instituto Dante Pazzanese de Cardiologia and Hospital Santa Marcelina), located in São Paulo, SP, Brazil, which aimed to assess the coronary vascular response six months after the implantation of the second-generation XIENCE VTM and BioMatrixTM DES. The primary endpoint of the study consisted of the combined assessment of two outcomes after six months: 1– evaluation of coronary endothelial function – quantified by quantitative coronary angiography (QCA) through changes in the peri-stent luminal diameter (5mm proximal and distal to the stent borders) between periods of rest, during sequential stimulation with cardiac pacemaker, and after administration of intracoronary nitroglycerin; and 2– quantification of the percentage coverage of the stent struts through OCT. Secondary endpoints consisted of the assessment of efficacy through QCA, intravascular ultrasound (IVUS), and OCT in the invasive 6 month follow-up.
Patients with de novo coronary lesions, with a maximum length of 20mm and located in native coronary arteries with a diameter of 3.0mm to 3.5mm, were included in the study. Diabetic patients were excluded, as well as patients treated within 72hours of an acute myocardial infarction (AMI) with ST-segment elevation. Patients with renal failure (serum creatinine > 2.0mg/dL or estimated creatinine clearance < 30mL/min) or severe left ventricular dysfunction (left ventricular ejection fraction < 30%) were also excluded. The main angiographic exclusion criteria were ostial lesions, bifurcation lesions, lesions in the left main coronary artery, or with the presence of thrombi or significant calcification.
The BIOACTIVE study was approved by the Research Ethics Committee of the participating institutions, and all patients signed an informed consent before randomization.
Characteristics of assessed stentsThe BioMatrixTM stent has a stainless steel platform, 120μm thick struts, coated with a 10μm thick durable primer (parylene C) and an 11μm thick polylactic acid (PLA) polymer, distributed only on the abluminal surface of the struts. This polymer carries the antiproliferative drug biolimus A9 and is designed to perform an initial fast release of approximately 40% of the drug. Subsequently, it is co-released with the remaining drug over a period of 6 to 9 months. Finally, the polymer is degraded into carbon dioxide and water.
The XIENCE VTM stent consists of a cobalt-chromium platform with thin struts (81μm), coated with a durable and highly biocompatible fluoropolymer, distributed around the entire surface of the struts. This polymer carries the antiproliferative drug everolimus at a dose of 100μm/mm2 and is designed to release 80% of the total drug dose in the first 30 days after implantation.
ProceduresPCI procedures were performed according to the routines of the institutions and in accordance with current recommendations.20,21 Pre-treatment with acetylsalicylic acid loading dose (300mg) and clopidogrel (300 or 600mg) was administered at least 24hours before PCI to patients who were not chronic users of these medications. At the start of the procedure, anticoagulation with unfractionated heparin (100 IU/kg) was carried out with administration of additional bolus, where necessary, to maintain an activated clotting time ≥ 250seconds.
After adequate positioning of the 0.014” guidewire in the distal portion of the target vessel, patients were randomized in a 1:1 proportion to receive the BioMatrixTM or XIENCE VTM stents. Predilation of target lesions was not mandatory and the direct stenting was allowed. The indication for stent post-dilation was made at the operating physician's discretion and, when performed, was done with balloons, preferably noncompliant, with a shorter length than the nominal length of the implanted stent, in order to prevent inflations out of the treated segment.
Follow-up and endpointsAll patients were assessed by clinical consultation at the medical office or by telephone calls at 1; 6 and 12 months after the index PCI. Angiographic restudy, with IVUS and OCT, was performed after 6 months.
The present analysis shows the comparison of vascular response assessment by OCT, for which the primary endpoint was the difference in the percentage of struts not covered by neointimal tissue in both treatment groups. The secondary outcomes of the OCT analysis were as follows: frequency of strut malapposition, neointimal tissue area, percentage of stent obstruction by neointimal tissue, and neointimal thickness. The occurrence of major adverse cardiac events (death, nonfatal myocardial revascularization, and target-vessel revascularization) at 12 months was also computed as a secondary outcome.
All adverse events were adjudicated and classified by an independent adjudication committee blinded to the type of stent received by patients.
Acquisition and analysis of optical coherence tomography imagesThe OCT images were acquired with the commercially available time-domain OCT system (M3 System; LightLab Imaging, Westford, USA) or frequency-domain optical coherence tomography system (C7 XR; St. Jude Medical, St. Paul, USA). The techniques used for image acquisition with both OCT systems have been previously described in detail.22,23 All images were stored in digital media and sent for analysis to an independent central laboratory (Cardiovascular Research Center, São Paulo, SP, Brazil). Analyses were performed using a dedicated and previously validated program24 (QIvusTM, version 3.0; Medis Medical Imaging, Leiden, Netherlands) and the operating physicians were blinded to the type of stent received by each patient.
After adjusting for the different pullback velocities of the imaging catheter provided by the two types of OCT equipment used, the analyses of tomographic vessel images were made at 0.6mm intervals in the longitudinal direction, along the entire treated segment.
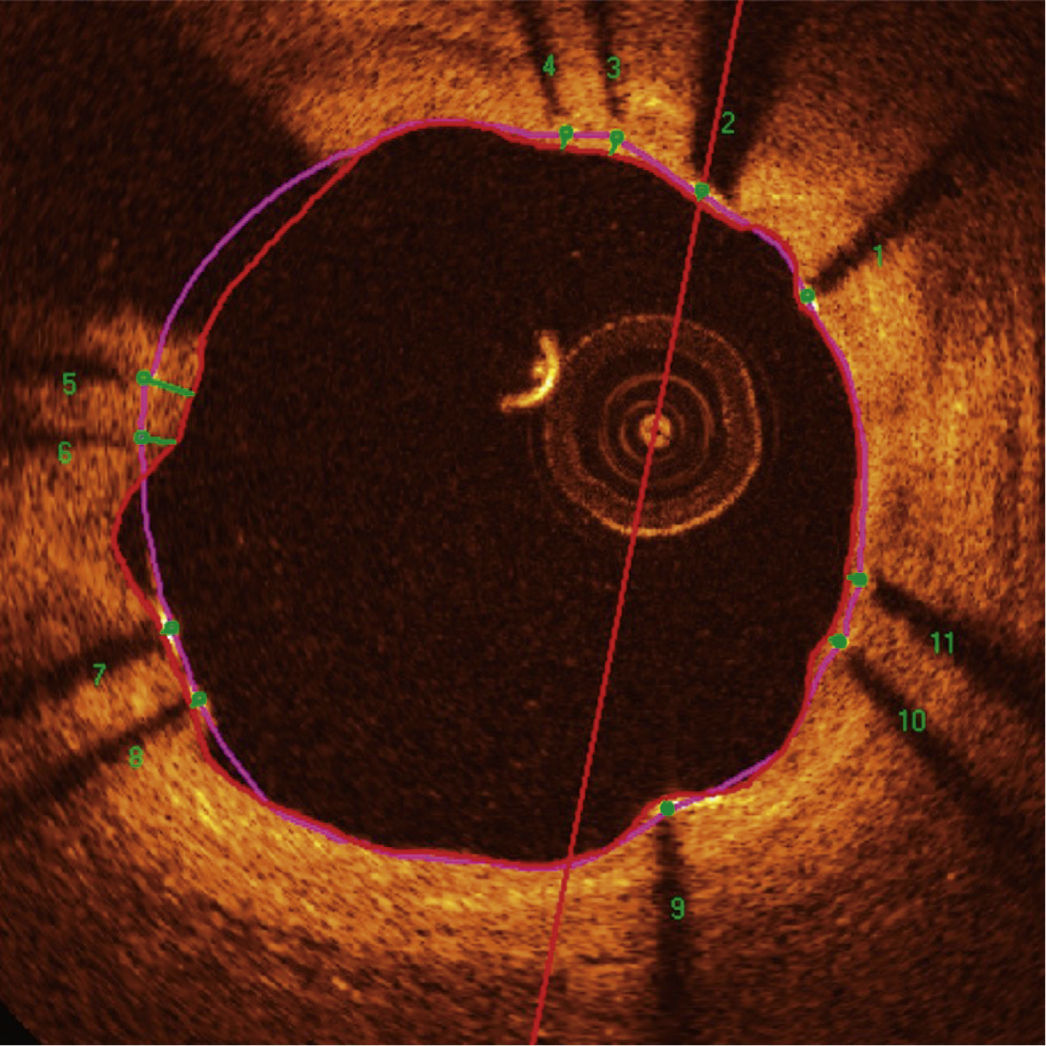
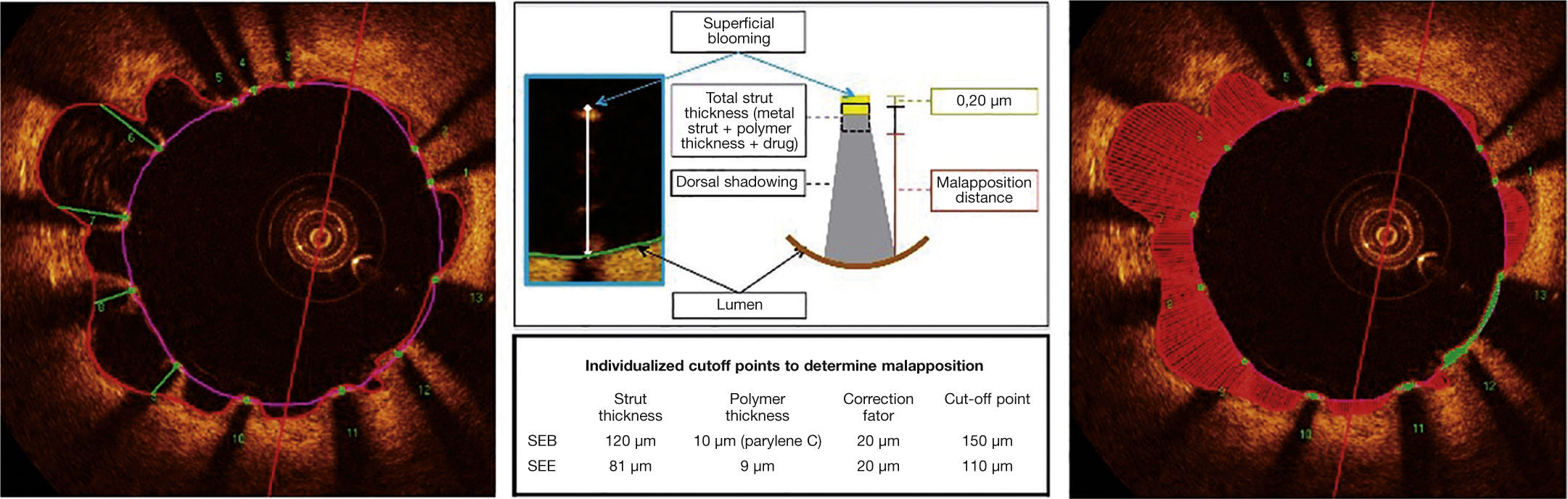
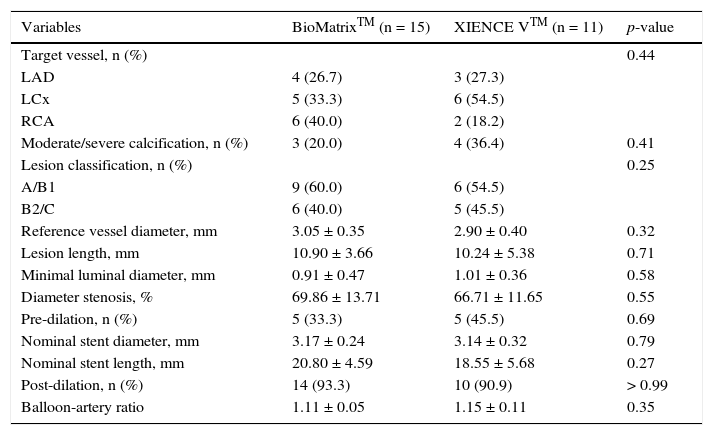
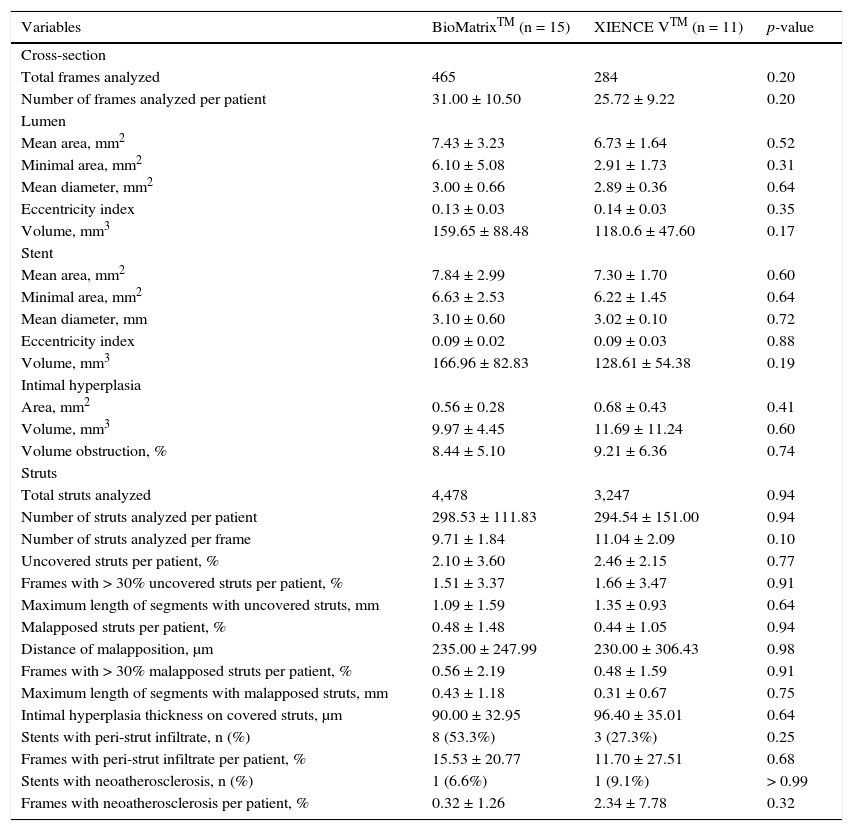
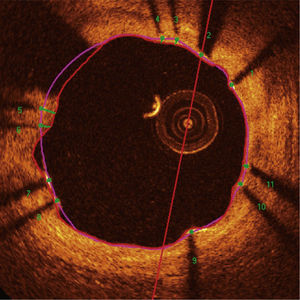
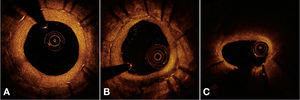
Automatic segmentation of the luminal contour (border between the lumen and the vessel intimal layer) was performed by the analysis program inside the predetermined segment, with manual adjustments made if necessary. Metal stent struts appear on the OCT as small points, or rounded or tapered structures that promote high reflection of the light signal emitted by the OCT catheter (blooming) and dorsal shadowing. Thus, a strut was considered for analysis only when the blooming + dorsal shadowing binomial was present. An automatic strut detection algorithm was used; false-positive or false-negative corrections were performed manually. The number of struts analyzed in each cross-sectional image along the segment of interest was then automatically computed. The contour traced along the inner surface of each strut was performed automatically and defined the stent area in each analyzed frame. Finally, the distance between the central points of the luminal face of each strut to the luminal contour was determined automatically by lines directed towards the center of gravity of the vessel (Fig. 1). When this distance was positive, the struts were classified as covered. Positive values of the distance between the stent strut and the lumen contour determined neointimal thickness in each individual strut. In case of negative distances, the struts were classified as uncovered (Fig. 2). If the negative distance was greater than the sum of the thickness of the strut + the thickness of the polymer (when present) + a correction factor for the minimum axial resolution of the OCT, the strut was classified as malapposed. Therefore, the cut-off points used to define strut malapposition varied according to the characteristics of each stent (BioMatrixTM = 150μm and XIENCE VTM = 110 μm) (Fig. 3).
Optical coherence tomography analysis. The luminal contour is segmented automatically, following the border between the lumen and the intimal layer (red line). Then the stent struts – highly light-reflective structures, with dorsal shade – are identified by an automated algorithm. The points located on the luminal surface are connected to determine the stent area (pink line). The distances between the luminal surface of each strut to the luminal contour are automatically determined by a trajectory directed towards the center of the vessel (green lines).
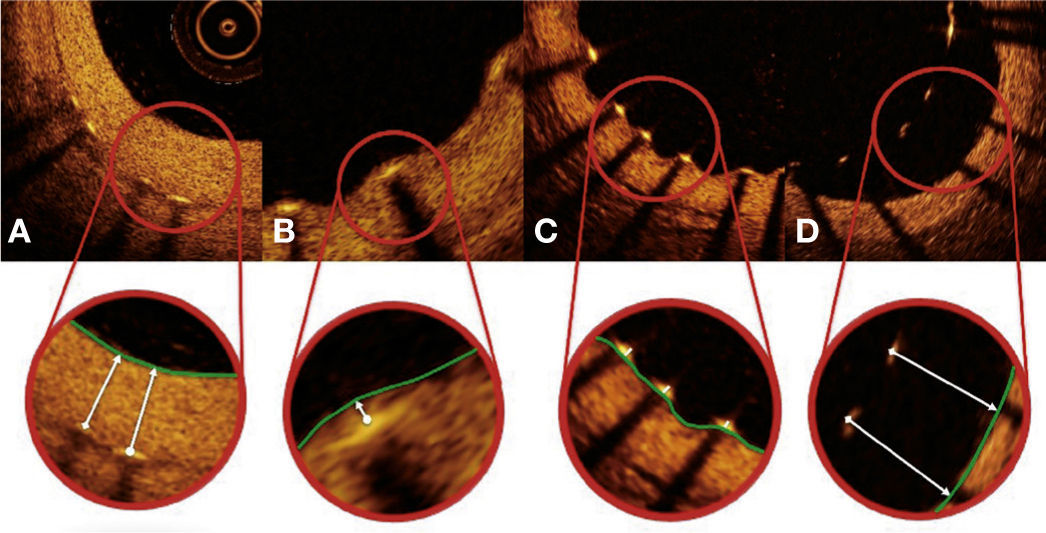
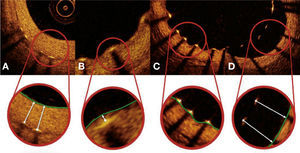
Strut coverage pattern. Each stent strut – present in each frame analyzed at 0.6mm intervals from the treated segment – was evaluated. When the distance between the central point of the strut on its luminal surface in relation to the contour of the vascular lumen was positive, the strut was classified as covered, and the tissue thickness was determined by the distance in micrometers (panels A and B). When the distance was negative, the struts were classified as non-covered (panels C and D). If this negative distance was greater than the sum of the strut thickness + polymer thickness + drug, this strut was further classified as malapposed (panel D).
Malapposition. The metallic struts reflect all the light falling on their surface and produce a single hyperintense signal, which appears as intense glow (blooming), followed by intense dorsal shadowing. To determine the malapposition state, the authors used the distance from the luminal surface of the strut to the vascular lumen contour. When this distance was greater than the total strut thickness (metal strut + polymer thickness + drug), plus a 20μm correction factor to correct for the actual location of the strut surface, it was classified as malapposed (middle panel), and the malapposition distance was computed for each individual strut (left panel). Malapposition was also quantified as the area in each frame in which it is identified along the treated segment (right panel; hatched region in red). BES: biolimus A9-eluting stent (BioMatrixTM); EES: everolimus-eluting stent (XIENCE VTM).
The neointimal area was determined based on the measurement of the neointimal thickness, located between the stent and luminal contour. Regions around the vessel circumference, with a negative distance between the stent and the lumen contours (no tissue coverage), were included for neointimal quantification (Fig. 3). After the neointimal area was determined, stent obstruction percentage was computed for each analyzed frame by dividing the neointimal area by the stent area. The homogeneity of the circumferential and longitudinal distribution of neointimal hyperplasia along the treated segment was evaluated by visual assessment of scatter plots created for each case.
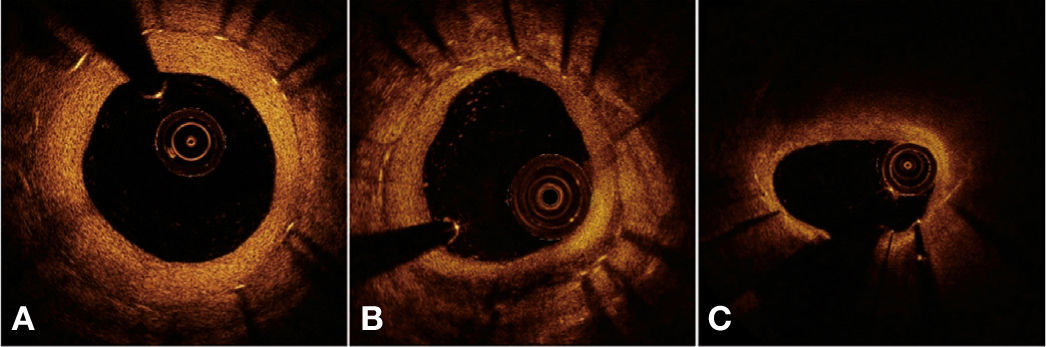
Finally, a qualitative assessment of neointimal tissue formed over the stent struts was performed. Peri-strut infiltrate regions were defined as homogeneous areas reflecting the optical signal with less intensity than the adjacent tissue, but without causing light attenuation.25 Neoatherosclerosis was defined as the presence of lipid infiltrate or neointimal calcification.26Figure 4 illustrates examples of qualitative assessment of neointimal tissue formed over the stent struts.
Qualitative assessment of neointimal tissue. In A, the cross-sectional image illustrates a pattern of normal vascular healing, characterized by high-intensity and homogeneous neointimal optical appearance. In B, an example of peri-strut infiltrate between 4 and 7 o’clock, characterized as a well-defined and homogeneous region that promotes low reflection of the optical signal, with less intensity than that of the surrounding tissue, but without causing lumen attenuation (allows for viewing of posterior structures). In C, an example of neoatherosclerosis, represented by lipid infiltration of neointimal tissue between 10 and 2 o’clock. Note significant attenuation of the optical signal, which prevents the visualization of the posterior structures in the region, including the stent struts.
Statistical analyses were performed using SPSS, version 20.0 (IBM Corp., Armonk, USA), and R software, version 3.1.1 (The R Foundation for Statistical Computing, Vienna, Austria).
Continuous variables are shown as mean and standard deviation and categorical variables as frequency and percentage. Categorical variables were analyzed by chi-squared test or Fisher's exact test, and continuous variables by Student's t-test and Mann-Whitney U-test, depending on variable distribution.
A generalized estimating equations model with first-order autoregressive covariance structure was used to adjust the analyses according to the pooled nature of the data from each patient (for instance, a patient with a stent placed in one vessel has dozens of frames and hundreds of struts analyzed). Thus, for a patient who received a long stent with more frames and struts analyzed, a different weight was computed as compared to another patient who received a short stent with fewer frames and struts analyzed.
All analyses were two-tailed and p < 0.05 was considered statistically significant.
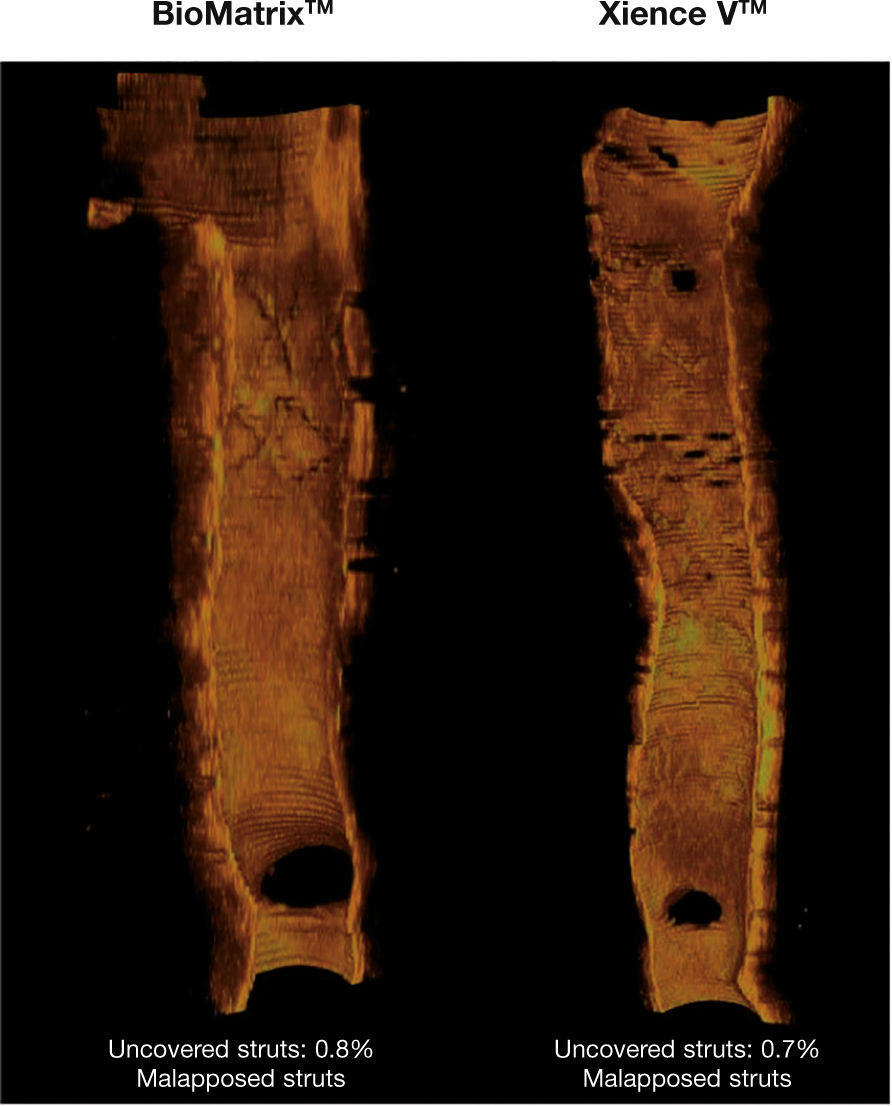
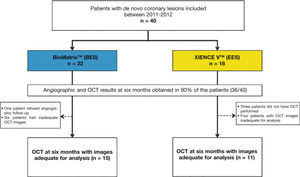
ResultsCharacteristics of the population and proceduresBetween July 2011 and April 2012, a total of 40 patients were included in the BIOACTIVE trial and randomized to receive the BioMatrixTM (n = 22) or the XIENCE VTM stent (n = 18). Of these, angiographic restudy at 6 months with IVUS and OCT was performed in 36 (90%) patients. After qualitative assessment of OCT images through core lab, a total of 26 patients were included for assessment of OCT outcomes (15 with BioMatrixTM and 11 with XIENCE VTM). A flowchart detailing the inclusion of the patients in the study is shown in Figure 5.
Study flowchart. Inclusion of patients in the study, identification of group to which they were allocated, and number of patients undergoing evaluation with optical coherence tomography (OCT). BES: A9-biolimus-eluting stent (BioMatrixTM); EES: everolimus-eluting stent (XIENCE VTM).
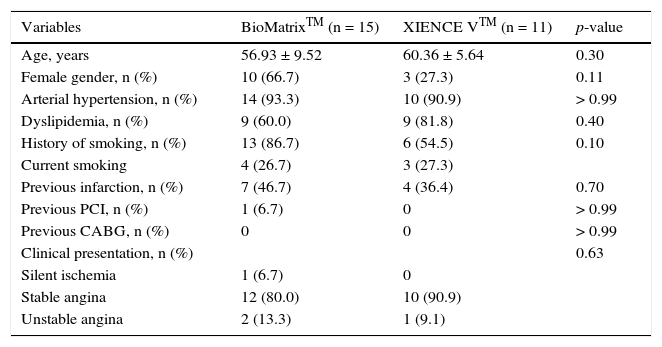
Table 1 shows the baseline demographic characteristics, while the angiographic and procedure variables are shown in Table 2. The mean age in both groups was 58.38 ± 8.15 years, and 50% of the patients included in the study were females. Stable angina was the predominant form of clinical presentation in both groups. There was a balanced distribution among the target vessels, and B2/C lesions accounted for 42.3% of the treated lesions. The target-vessel reference diameter was 2.99 ± 0.38mm and lesion length was 10.63 ± 4.39mm. Predilation of the lesions was performed in only five patients from each group, while the post-dilation was performed in 90% of cases in both groups, achieving a balloon-artery ratio of 1.13 ± 0.09.
Baseline demographic characteristics.
| Variables | BioMatrixTM (n = 15) | XIENCE VTM (n = 11) | p-value |
|---|---|---|---|
| Age, years | 56.93 ± 9.52 | 60.36 ± 5.64 | 0.30 |
| Female gender, n (%) | 10 (66.7) | 3 (27.3) | 0.11 |
| Arterial hypertension, n (%) | 14 (93.3) | 10 (90.9) | > 0.99 |
| Dyslipidemia, n (%) | 9 (60.0) | 9 (81.8) | 0.40 |
| History of smoking, n (%) | 13 (86.7) | 6 (54.5) | 0.10 |
| Current smoking | 4 (26.7) | 3 (27.3) | |
| Previous infarction, n (%) | 7 (46.7) | 4 (36.4) | 0.70 |
| Previous PCI, n (%) | 1 (6.7) | 0 | > 0.99 |
| Previous CABG, n (%) | 0 | 0 | > 0.99 |
| Clinical presentation, n (%) | 0.63 | ||
| Silent ischemia | 1 (6.7) | 0 | |
| Stable angina | 12 (80.0) | 10 (90.9) | |
| Unstable angina | 2 (13.3) | 1 (9.1) |
PCI: percutaneous coronary intervention; CABG: coronary artery bypass graft surgery.
Angiographic and procedural characteristics.
| Variables | BioMatrixTM (n = 15) | XIENCE VTM (n = 11) | p-value |
|---|---|---|---|
| Target vessel, n (%) | 0.44 | ||
| LAD | 4 (26.7) | 3 (27.3) | |
| LCx | 5 (33.3) | 6 (54.5) | |
| RCA | 6 (40.0) | 2 (18.2) | |
| Moderate/severe calcification, n (%) | 3 (20.0) | 4 (36.4) | 0.41 |
| Lesion classification, n (%) | 0.25 | ||
| A/B1 | 9 (60.0) | 6 (54.5) | |
| B2/C | 6 (40.0) | 5 (45.5) | |
| Reference vessel diameter, mm | 3.05 ± 0.35 | 2.90 ± 0.40 | 0.32 |
| Lesion length, mm | 10.90 ± 3.66 | 10.24 ± 5.38 | 0.71 |
| Minimal luminal diameter, mm | 0.91 ± 0.47 | 1.01 ± 0.36 | 0.58 |
| Diameter stenosis, % | 69.86 ± 13.71 | 66.71 ± 11.65 | 0.55 |
| Pre-dilation, n (%) | 5 (33.3) | 5 (45.5) | 0.69 |
| Nominal stent diameter, mm | 3.17 ± 0.24 | 3.14 ± 0.32 | 0.79 |
| Nominal stent length, mm | 20.80 ± 4.59 | 18.55 ± 5.68 | 0.27 |
| Post-dilation, n (%) | 14 (93.3) | 10 (90.9) | > 0.99 |
| Balloon-artery ratio | 1.11 ± 0.05 | 1.15 ± 0.11 | 0.35 |
LAD: left anterior descending artery; LCx: left circumflex artery; RCA: right coronary artery.
From a total of 823 frames available for analysis (respecting the interval between frames of 0.6mm), 75 frames were excluded from analysis for the following reasons: image with more than 45° of the circumference outside the field-of-view (n = 29 frames); presence of residual blood in significant amounts, preventing the visualization of the vessel intimal layer (n = 28 frames); and electronic artifacts (n = 18 frames). All 75 frames excluded were acquired using the first-generation equipment TD-OCT. In the 748 frames analyzed, 7,725 stent struts were evaluated individually. Table 3 shows the OCT analysis results.
Optical coherence tomography results.
| Variables | BioMatrixTM (n = 15) | XIENCE VTM (n = 11) | p-value |
|---|---|---|---|
| Cross-section | |||
| Total frames analyzed | 465 | 284 | 0.20 |
| Number of frames analyzed per patient | 31.00 ± 10.50 | 25.72 ± 9.22 | 0.20 |
| Lumen | |||
| Mean area, mm2 | 7.43 ± 3.23 | 6.73 ± 1.64 | 0.52 |
| Minimal area, mm2 | 6.10 ± 5.08 | 2.91 ± 1.73 | 0.31 |
| Mean diameter, mm2 | 3.00 ± 0.66 | 2.89 ± 0.36 | 0.64 |
| Eccentricity index | 0.13 ± 0.03 | 0.14 ± 0.03 | 0.35 |
| Volume, mm3 | 159.65 ± 88.48 | 118.0.6 ± 47.60 | 0.17 |
| Stent | |||
| Mean area, mm2 | 7.84 ± 2.99 | 7.30 ± 1.70 | 0.60 |
| Minimal area, mm2 | 6.63 ± 2.53 | 6.22 ± 1.45 | 0.64 |
| Mean diameter, mm | 3.10 ± 0.60 | 3.02 ± 0.10 | 0.72 |
| Eccentricity index | 0.09 ± 0.02 | 0.09 ± 0.03 | 0.88 |
| Volume, mm3 | 166.96 ± 82.83 | 128.61 ± 54.38 | 0.19 |
| Intimal hyperplasia | |||
| Area, mm2 | 0.56 ± 0.28 | 0.68 ± 0.43 | 0.41 |
| Volume, mm3 | 9.97 ± 4.45 | 11.69 ± 11.24 | 0.60 |
| Volume obstruction, % | 8.44 ± 5.10 | 9.21 ± 6.36 | 0.74 |
| Struts | |||
| Total struts analyzed | 4,478 | 3,247 | 0.94 |
| Number of struts analyzed per patient | 298.53 ± 111.83 | 294.54 ± 151.00 | 0.94 |
| Number of struts analyzed per frame | 9.71 ± 1.84 | 11.04 ± 2.09 | 0.10 |
| Uncovered struts per patient, % | 2.10 ± 3.60 | 2.46 ± 2.15 | 0.77 |
| Frames with > 30% uncovered struts per patient, % | 1.51 ± 3.37 | 1.66 ± 3.47 | 0.91 |
| Maximum length of segments with uncovered struts, mm | 1.09 ± 1.59 | 1.35 ± 0.93 | 0.64 |
| Malapposed struts per patient, % | 0.48 ± 1.48 | 0.44 ± 1.05 | 0.94 |
| Distance of malapposition, μm | 235.00 ± 247.99 | 230.00 ± 306.43 | 0.98 |
| Frames with > 30% malapposed struts per patient, % | 0.56 ± 2.19 | 0.48 ± 1.59 | 0.91 |
| Maximum length of segments with malapposed struts, mm | 0.43 ± 1.18 | 0.31 ± 0.67 | 0.75 |
| Intimal hyperplasia thickness on covered struts, μm | 90.00 ± 32.95 | 96.40 ± 35.01 | 0.64 |
| Stents with peri-strut infiltrate, n (%) | 8 (53.3%) | 3 (27.3%) | 0.25 |
| Frames with peri-strut infiltrate per patient, % | 15.53 ± 20.77 | 11.70 ± 27.51 | 0.68 |
| Stents with neoatherosclerosis, n (%) | 1 (6.6%) | 1 (9.1%) | > 0.99 |
| Frames with neoatherosclerosis per patient, % | 0.32 ± 1.26 | 2.34 ± 7.78 | 0.32 |
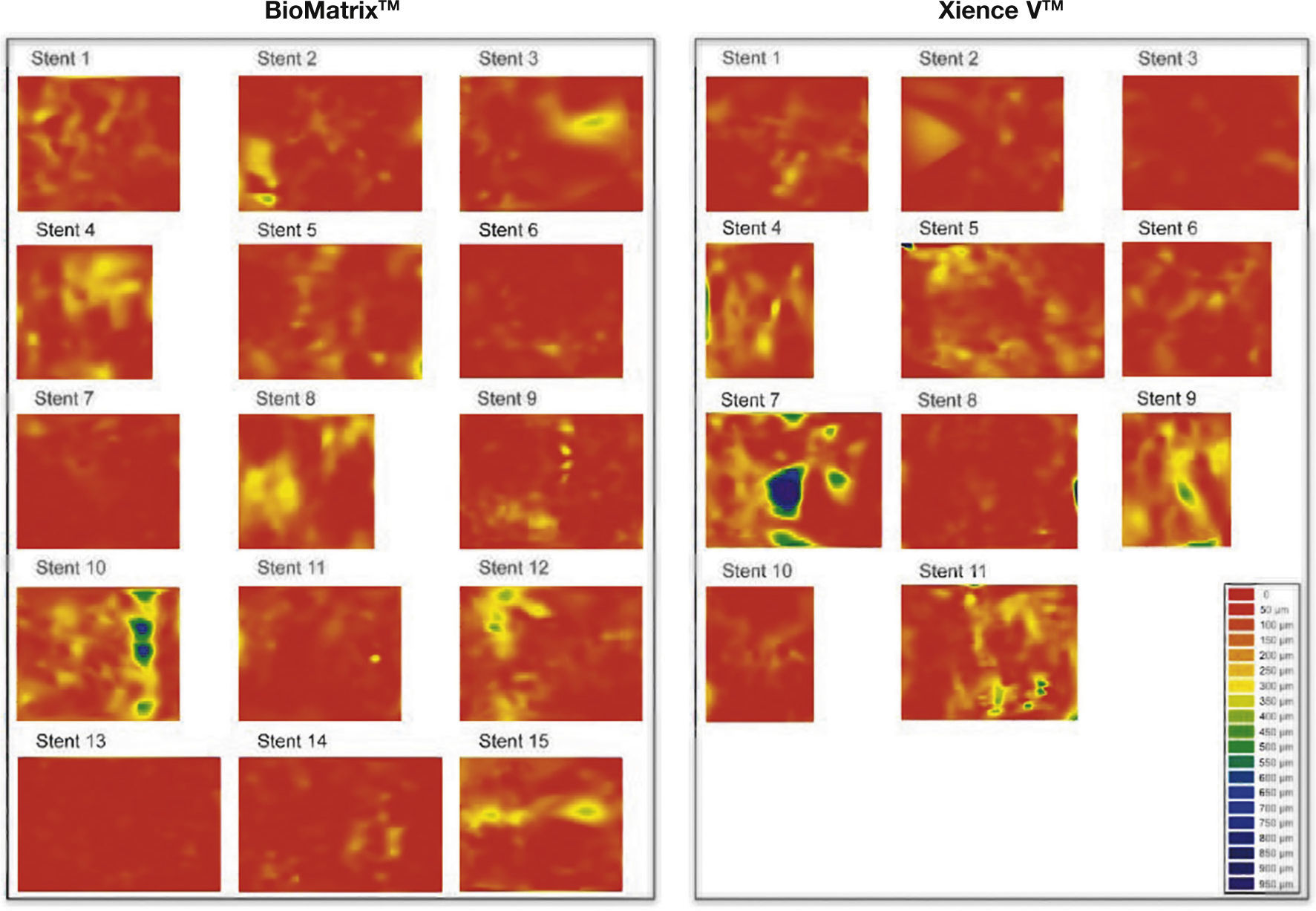
There were no significant differences between the groups regarding the planar quantitative morphometric analysis in cross-sectional level, as well as detailed analysis at strut level. Regarding the efficacy outcomes, both stent types showed marked antiproliferative potency, as shown by the small areas (0.56 ± 0.28mm2 vs. 0.68 ± 0.43mm2; p = 0.41) and thickness (90.00 ± 32.75μm vs. 96.40 ± 35.01μm; p = 0.64) of neointimal hyperplasia, which resulted in a small percentage of stent obstruction in both groups (8.44% ± 5.10% vs. 9.21% ± 6.36%; p = 0.74). It is noteworthy that, despite the small amount of neointimal tissue formed at the end of the 6 month period, its distribution was homogenous throughout the treated segment (Fig. 6). In fact, the percentage of uncovered struts per patient (primary surrogate safety endpoint) was low and similar between the BioMatrixTM and XIENCE VTM groups (2.10% ± 3.60% vs. 2.46% ± 2.15%; p = 0.77). Similarly, the incidence of malapposed struts was very low and similar in both groups (0.48% ± 1.48% vs. 0.44% ± 1.05%, p = 0.94) as well as the distance of malapposition of each individual strut to the vessel wall (235.00 ± 247.99μm vs. 230.00 ± 306.43μm; p = 0.98). Notably, the maximum longitudinal length of segments with uncovered (1.09 ± 1.59mm vs. 1.35 ± 0.93mm; p = 0.64) or malapposed struts (0.43 ± 1.18mm vs. 0.31 ± 0.67mm, p = 0.75) was small and similar between the groups. Figure 7 shows examples of good healing profile of the two types of stents.
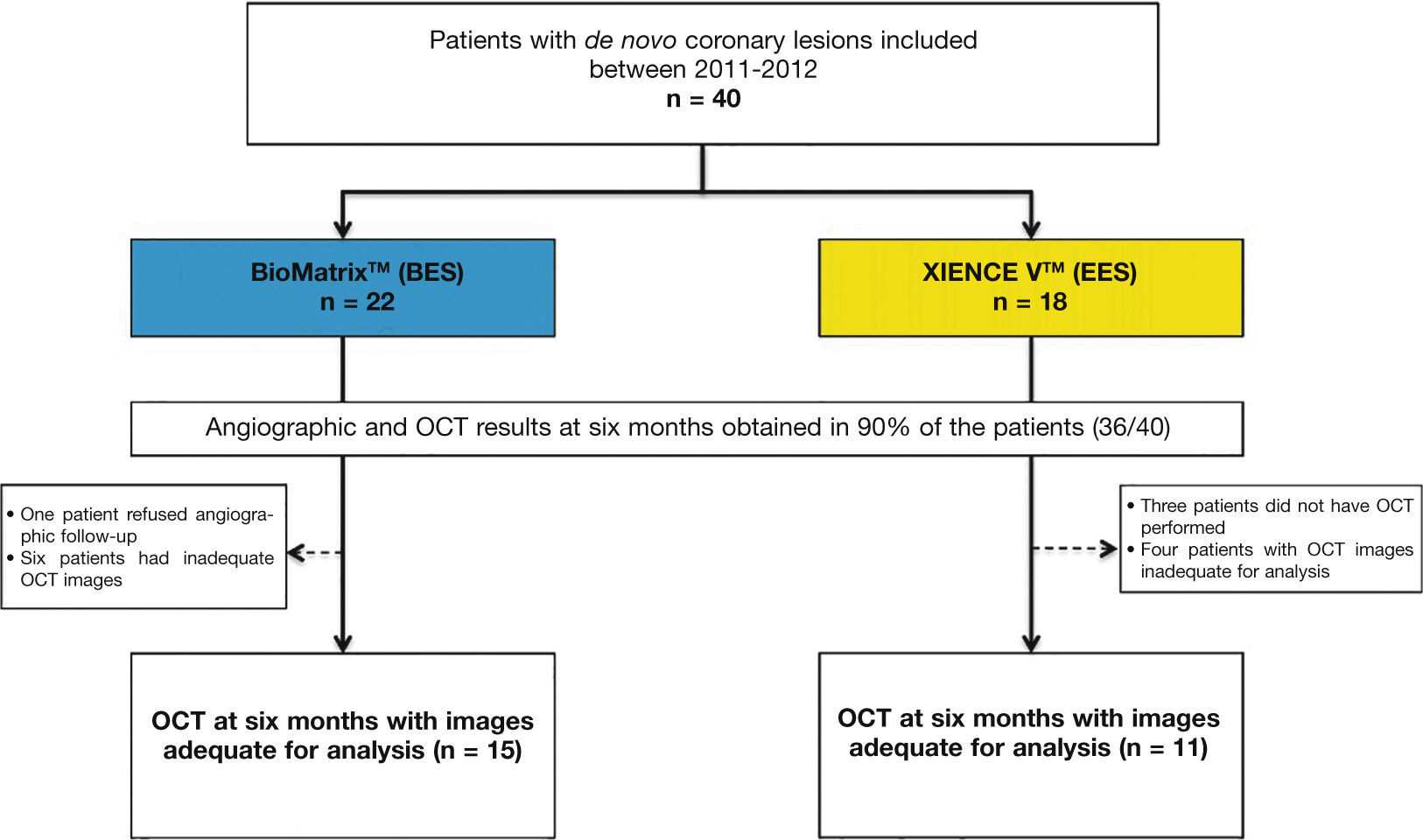
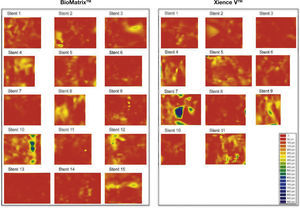
Dispersed neointimal topography. Neointimal tissue thickness was estimated around the 360° of circumference in each frame analyzed along the treated segment, and its distribution is shown in the circumferential and longitudinal directions of each evaluated stent. The graphs represent the stent cut longitudinally along the zero angle of the vessel circumference and arranged on a flat surface. The horizontal axis represents the stent length and the vertical axis, the 360° of its circumference. Neointimal tissue amount is represented by a color map, according to lesser (red) or greater (blue) thickness. It can be observed that both types of stents have low neointimal tissue thickness, with homogeneous distribution. Only two cases – one in each group – showed greater focal accumulation of neointimal tissue, identified by blue regions. These two patients had severe intra-segment restenosis and underwent new revascularization.
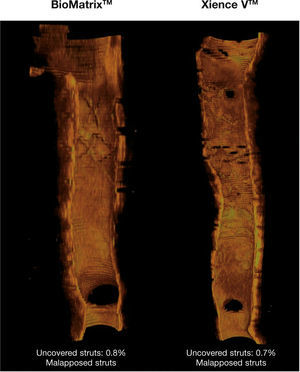
Healing profile of the stents. Three-dimensional reconstructions in the longitudinal direction of the BioMatrixTM and XIENCE VTM stents, illustrating the healing profile of these devices after 6 months. Note the presence of a thin neointimal tissue layer covering the entire treated segment, with a minimum percentage of uncovered struts.
In the analysis on the quality of neointimal tissue formed on the stents, the finding of peri-strut infiltrate was relatively rare, occurring in 15.53% of the frames analyzed in the BioMatrixTM group and in 11.70% of the frames analyzed in the XIENCE VTM group (p = 0.68). Neoatherosclerosis was observed in only one stent in each group.
Clinical outcomesThere were no cases of death or infarction during the 1-year follow-up. There were two target-lesion revascularizations – one in each group. One patient from the XIENCE VTM group had stable angina and at the time of restudy at 6 months had severe in-stent restenosis, with neoatherosclerosis findings on OCT, and thus was submitted to target-lesion revascularization. Another patient – from the BioMatrixTM group – also had stable angina at the 6-month restudy, which showed severe restenosis in the distal border of the previously implanted stent and was thus submitted to a new revascularization.
DiscussionDebates about DES safety are recurrent issues, but are still relevant. The present study used OCT to assess the antiproliferative efficacy of two types of second-generation DES, using surrogate endpoints to infer their safety in the medium term. The main findings were as follows: the incidence of uncovered or malapposed struts was low and similar in both groups after 6 months; the antipro-liferative efficacy was maintained in both groups, with minimal neointimal formation, evenly distributed along the treated segments, resulting in low percentage of obstruction; both DES, utilizing different technologies, promoted “healthy” optical healing aspect, with low incidence of peri-strut infiltrate.
Although the DES were successful in achieving their primary purpose of minimizing neointimal formation and reducing rates of restenosis and new revascularizations, the excess occurrence of late and very-late thrombotic events in these devices triggered a warning sign about their safety in the long-term. Although late stent thrombosis is a multifactorial phenomenon, it has been shown that the polymers used in DES to carry and control the release of antiproliferative agents locally in the vascular wall play a key role in the genesis and perpetuation of local inflammatory processes, potentially leading to the formation of late vascular remodeling and delayed healing.12,14,16
Aiming to minimize the deleterious effects of polymers found in first-generation DES, a series of modifications has been promoted in the design of new generations of DES. Among these modifications, the authors highlight the use of new metal alloys, with thinner struts; more biocompatible polymers, applied only to the abluminal surface of the struts (thus reducing their thickness); bioabsorbable polymers; non-polymeric drug carrier technology; and even totally bioabsorbable stents.17
Incorporating some of these technological developments, the BioMatrixTM stent has a bioabsorbable PLA polymer, applied to the abluminal surface of the stent platform's stainless steel struts, which carries and releases biolimus A9. The benefits of this new second-generation DES were demonstrated in the Limus Eluted from A Durable versus ERodable Stent coating (LEADERS) trial, which conducted a randomized comparison of the clinical outcomes of the BioMatrixTM stent with the first-generation DES CYPHERTM, a sirolimus-eluting stent released through a durable polymer. After 5 years, the group of patients treated with BioMatrixTM stent showed a trend to a lower incidence of major adverse cardiac events (22.3% vs. 26.1%; p = 0.071), in addition to a significant reduction in rates of late stent thrombosis after the first year (0.66% vs. 2.5%; p = 0.003).27 The OCT sub-analysis of this study showed a better healing profile with the BioMatrixTM stent, with a lower percentage of uncovered struts when compared with the CYPHERTM stent (0.6% vs. 2.1%, p = 0.04) at the end of 9 months.28
Similarly, the second-generation DES XIENCE VTM was also compared to the CYPHERTM stent in the randomized trial Scandinavian Organization for Randomized Trials with Clinical Outcome IV (SORT OUT IV). At the end of 9 months, the occurrence of the primary endpoint (cardiac death, myocardial infarction, definitive stent thrombosis, and target-vessel revascularization) was similar between the two types of DES (4.9% vs. 5.2%, p for non-inferiority = 0.01).29 These results were maintained until the end of the 2-year follow-up (8.3% vs. 8.7%; p = 0.66).30 Importantly, the XIENCE VTM stent, which similarly to the CYPHERTM stent has durable polymer, but is thinner and more biocompatible, showed a strong trend toward a lower incidence of definitive stent thrombosis at 9 months (0.1% vs. 0.7%, p = 0.05),29 which was significantly confirmed at the end of 2 years (0.2% vs. 0.9%; p = 0.02).30
After verifying the non-inferiority of the second-generation DES when compared to first-generation DES regarding their antiproliferative efficiency, but featuring a better safety profile,31 it was not long before the first comparisons between second-generation DES started to appear.
The Comparison of the Everolimus Eluting with the Biolimus A9 Eluting Stent (COMPARE-II) trial compared, in a randomized manner (2:1), 2,707 patients for treatment with NoboriTM stents (n = 1,795; Terumo, Tokyo, Japan), which, similarly to BioMatrixTM stents, release biolimus A9 through a bioabsorbable polymer, or with everolimus-eluting stents through a durable fluoropolymer (XIENCE VTM or XIENCE PRIMETM; n = 912). The occurrence of the primary endpoint (cardiac death, nonfatal myocardial infarction, and target-vessel revascularization guided by ischemia) at 12 months was similar in both groups (NoboriTM: 5.2% vs. XIENCE VTM: 4.8%; p for non-inferiority < 0.0001).32 These results are echoed in the OCT evaluation of these two stent types performed by Tada et al., who found no significant differences regarding the percentage of strut coverage between the second-generation DES NoboriTM and XIENCE VTM (OR = 1.54; 95%CI 0.63-3.79; p = 0.34) after 6 to 8 months.
The present study confirmed the excellent healing profile of XIENCE VTM and BioMatrixTM stents, which demonstrated potent suppression in neointimal formation intensity without affecting the healing profile, as demonstrated by the low percentage of uncovered struts. However, a longer follow-up is required to assess the potential benefits of DES with durable polymers.
Evolution in the evaluation of new drug-eluting stents and the contribution of optical coherence tomographyThe evolution of invasive imaging methods has coincided with technological advances in interventional cardiology. Throughout history, intravascular imaging methods have contributed to the understanding of the mechanism of action in different devices, helping to understand possible failure mechanisms and providing for technological advancement.
Historically, the late performance of a coronary stent was evaluated for its efficacy in suppressing neointimal tissue formation – often evaluated by late luminal loss at angiography and quantification, by IVUS, of neointimal hyperplasia volume and the degree to which this tissue obstructs stent area (the percentage of obstruction). However, the demonstration, through histopathological studies, that the absence or delay in stent strut healing was among the most powerful pathological predictors for the occurrence of late thrombosis in these devices13,16 induced reflection about the in vivo evaluation of coronary stents. The focus was no longer only the assessment of the antiproliferative stent potency, but also the quantitation of morphometric markers indicative of safety. Thus, the percentage of coverage and apposition of coronary stent struts started to be used as a primary endpoint in a number of randomized studies designed to evaluate the performance of different DES over variable periods.33
While BMS usually develop a circumferential coverage of neointimal tissue with a mean thickness of 500μm (late luminal loss of approximately 1mm) – easily identified and quantified by angiography and IVUS – DES show a delayed and even null hyperplastic response that is so intense that their struts are often covered by a thin tissue layer, whose thickness is well below the detection limits of angiography and IVUS. Due to its high axial resolution (ten times greater than that provided by IVUS), OCT was demonstrated to be more sensitive and accurate than the IVUS to identify coverage and malapposition of stent struts,34–36 showing good correlation with histological assessment18,36–38 and high reproducibility.18,38 Therefore, OCT allows for the assessment of complex stent-vessel interaction in vivo with an unprecedented level of detail, and thus became the standard imaging modality for vascular response assessment after stent implantation in a number of randomized studies.33 In the present study, the mean thickness of the neointimal tissue formed at the end of 6 months was less than 100mm and was below the detection limits of the IVUS.
In addition to the abovementioned accurate quantitative assessment, OCT also allows for the identification of the quality of the neointimal tissue formed. At the beginning of DES evaluations with OCT, it was not rare to observe areas of low optical signal intensity in the neointimal tissue around the stent struts. These findings, known as peri-strut infiltrates (or PLIA, peri-strut low intensity areas) showed correspondence, at the histological findings, with hypocellular regions and the presence of fibrinoid material and proteoglycans, surrounded by a polymorphic healing response with macrophages and lymphocytes.25 A pathological study carried out by van Beusekom et al. showed the presence of acellular areas after first-generation DES implantation, which did not exist with BMS.39 These findings were confirmed in vivo in the assessment of 36 stents, in which peri-strut infiltrates were more frequently identified in DES (65%) than in BMS (19%; p < 0.001), suggesting a local adverse reaction to the drug and/or polymer.25 Neoatherosclerosis is a phenomenon that refers to the formation of new atherosclerotic plaque inside an already-formed neointima. The degeneration of a normal neointima, with the development of vulnerable plaques and their eventual rupture, has been recently identified as a cause of late stent failure – again, more frequently identified after DES implantation.40 In the present study, the incidence of peri-strut infiltrate was lower than that observed after first-generation DES implantation, suggesting, in theory, a more benign vascular response after second-generation DES implantation. The actual clinical impact of these findings remains to be investigated in larger studies with a longer follow-up period. In the present population, the incidence of neoatherosclerosis was low, represented by one case in each group. However, it was associated with more exacerbated neointimal proliferation in one case, which required revascularization.
LimitationsSome limitations of the present study are worth mentioning. First, although the included population provided enough data (749 frames and 7,725 struts analyzed) for evaluation of surrogate endpoints of efficacy and safety by OCT, the number of patients included is insufficient to assess the clinical impact of the findings on OCT. Second, non-performance of the OCT after the index procedure prevents a temporal classification of observed malappositions; it is not possible to determine whether a malapposition identified at 6 months is only the residual effect of an acute malapposition, or whether it was acquired later. However, the low incidence and magnitude of the malappositions, observed in the context of favorable stent healing, suggest that the few malappositions observed in this study do not represent unfavorable local vascular reactions. Third, the 6-month evaluation period appears to be a short time to detect potential differences between the two stents evaluated. It is unknown whether the favorable vascular responses observed in the present study will remain in the long-term follow-up. Fourth, the population included in this analysis consisted predominantly of stable patients without diabetes and with relatively short lesions in larger vessels. This selection was aimed to minimize the inclusion of patients with more advanced disease, which could impact the assessment of endothelial function – one of the primary BIOACTIVE study endpoints. Thus, extrapolation of the results shown here to more complex populations should be performed with caution.
ConclusionsThe second-generation drug-eluting stents BioMatrixTM and XIENCE VTM showed favorable vascular response after 6 months. These two types of drug-eluting stents demonstrated excellent efficacy profiles, with effective suppression of neointimal formation and low percentage of in-stent obstruction, without causing delay in strut coverage, as shown by the low and comparable rates of non-covered and malapposed struts. In addition, the healing quality was also favorable, with reduced rates of peri-strut infiltrate and neoatherosclerosis.
Funding sourcesNone declared.
Conflicts of interestThe authors declare no conflicts of interest.
Peer Review under the responsability of Sociedade Brasileira de Hemodinâmica e Cardiologia Intervencionista.