Journal Information
Vol. 22. Issue 6.
Pages 335-341 (November 2007)
Vol. 22. Issue 6.
Pages 335-341 (November 2007)
Full text access
Notificación de eventos adversos en un hospital nacional en Lima
Notification of adverse events in a national hospital in Lima
Visits
5679
Nora Espíritua, Gliceria Lavadob, Lilian Pantojac, Carmen Lamd, Mónica Barrientosd, Rigoberto Centenoe
a Área de Investigación. Hospital Nacional Dos de Mayo. Lima. Perú.
b Oficina de Gestión de la Calidad. Hospital Nacional Dos de Mayo. Lima. Perú.
c Oficina de Apoyo a la Docencia e Investigación. Hospital Nacional Dos de Mayo. Lima. Perú.
d Oficina de Epidemiología. Vigilancia epidemiológica. Hospital Nacional Dos de Mayo. Lima. Perú.
e Consultoría de servicios de salud. Organización Panamericana de Salud (OPS/OMS). Lima. Perú.
This item has received
Article information
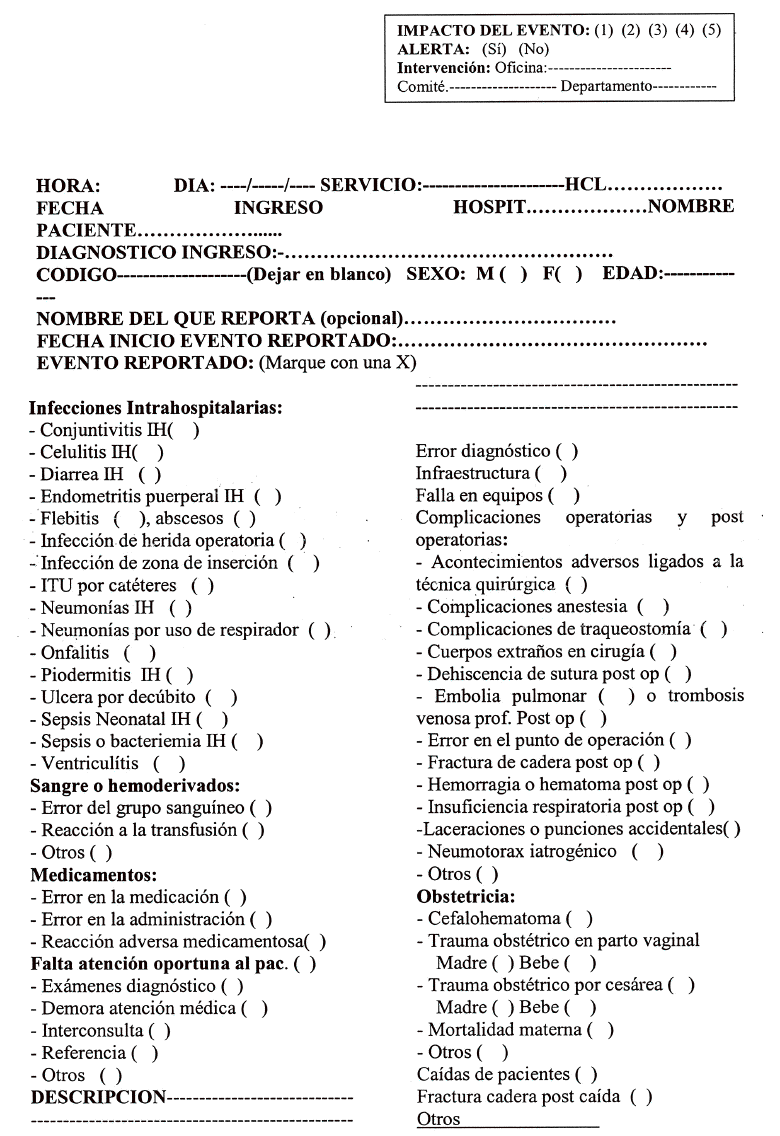
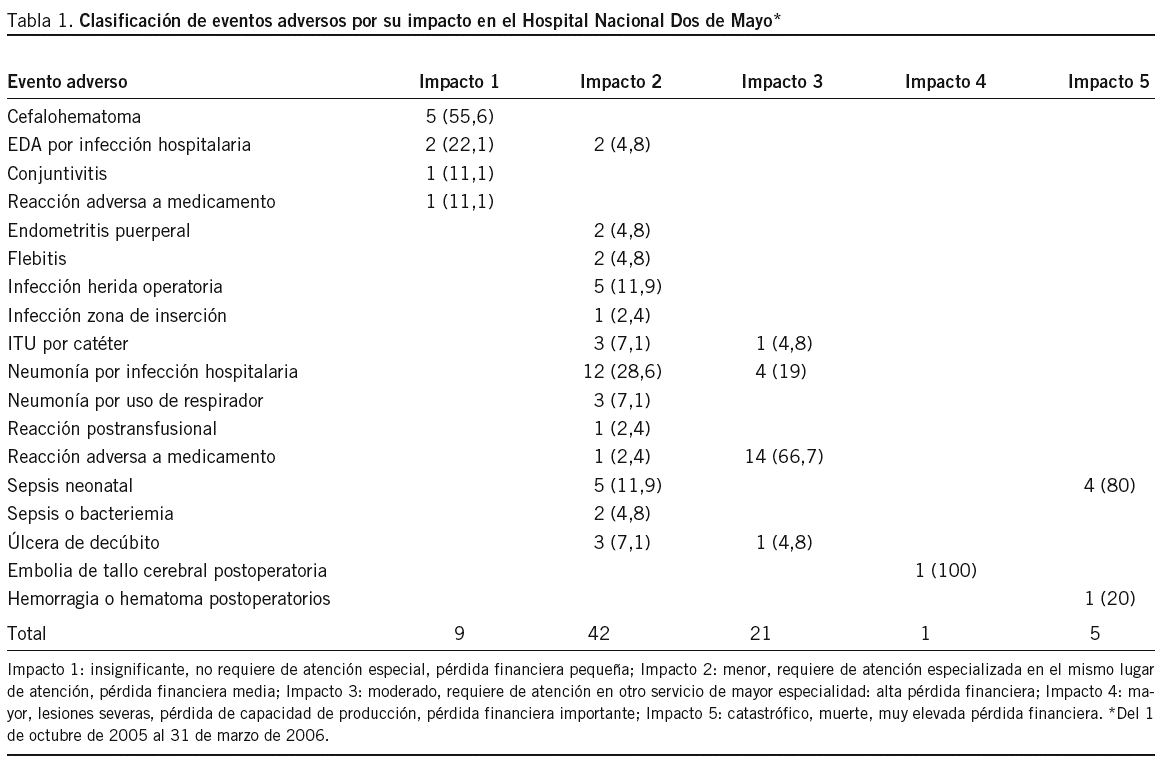
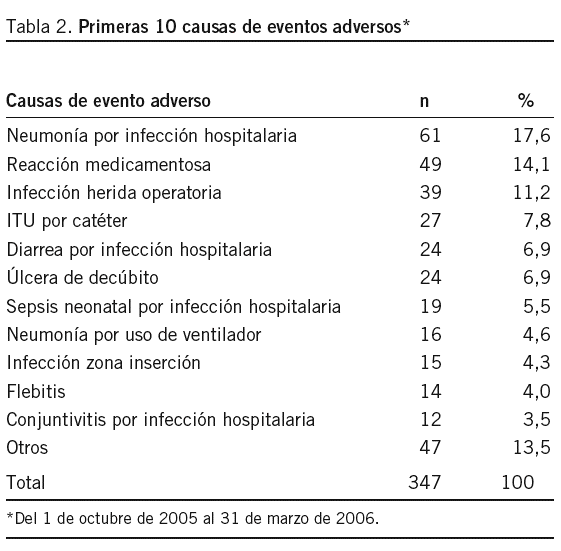
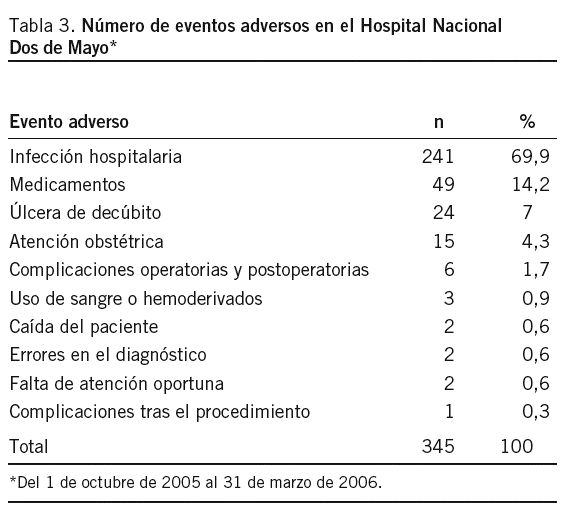
Objetivo: Establecer un sistema de notificación de eventos adversos (EA) en el Hospital Nacional Dos de Mayo (HNDM). Método: Investigación operativa, centrada en la puesta en funcionamiento de un sistema de notificación voluntario, confidencial y con vigilancia activa entre el 1 de octubre de 2005 y el 31 de marzo de 2006. El modelo para el abordaje de los EA fue de carácter sistémico. Resultados: El total de egresos fue 8.964 y la cantidad de EA reportados, 347. La tasa de EA fue del 3,9% y los EA potenciales, 12 (0,13%). Los EA más detectados fueron las infecciones hospitalarias, con 241 (70%), y el servicio de neonatología es el que tiene el mayor número de casos: 47 (19,5%); entre éstos, la sepsis hospitalaria neonatal (19 casos) fue la más frecuente. La segunda causa de EA fue las reacciones adversas a medicamentos (RAM), con 49 (14,2%) casos, de los cuales 39 (79,6%) ocurrieron durante el tratamiento antirretroviral. Casi todas las notificaciones fueron obtenidas por vigilancia activa y hubo sólo 5 reportes voluntarios. Encontramos información fragmentada de otros subsistemas, como el del comité de infecciones hospitalarias, hemovigilancia, farmacovigilancia, sistema de quejas y auditorías. Se buscaron EA en las reintervenciones quirúrgicas no programadas, y se clasificaron los EA por frecuencia e impacto. Conclusiones: La tasa general de EA estimada (3,9%) es menor que la de países desarrollados cuyas condiciones sanitarias son mejores que las nuestras, lo que indica un probable subregistro. La vigilancia activa de EA e integración de subsistemas es la forma más adecuada en el momento para obtener información de EA.
Palabras clave:
Eventos adversos
Sistema de notificación
Seguridad del paciente
Objective: To establish a system for notifying adverse effects (AE) at the Dos de Mayo National Hospital. Method: We performed an operative investigation of the implementation of a confidential, voluntary reporting system with active surveillance from 1 October 2005 to 31 March 2006. A systems-based model was used for the management of AE. Results: Among a total of 8,964 hospital admissions, 347 AE were reported. The rate of AE was 3.9% and there were 12 near misses (0.13%). The most frequent AE were nosocomial infections 241 (70%). The highest number of cases (47 [19.5%]) occurred in the neonatology department and the most frequent condition was neonatal sepsis (19 cases). The second most frequent AE were adverse drug reactions, amounting to 49 cases (14.2%). Of these, 39 (79.6%) were caused by antiretroviral therapy. Almost all notifications were obtained by active surveillance and there were only five voluntary reports. We found additional information in fragmented subsystems such as the nosocomial infections board, blood surveillance, pharmacology surveillance, the complaints system and audits. In addition, we searched for AE in non-elective surgical re-interventions. All AE were classified by frequency and impact. Conclusions: The estimated overall rate of AE (3.9%) was lower than that in developed countries with better health conditions, suggesting a possible under-reporting bias. We infer that active surveillance of AE coupled with integration of subsystems is currently the most appropriate way to obtain information on AE.
Keywords:
Adverse events
Notification systems
Patient safety
Full text is only aviable in PDF
Bibliografía
[1]
World Alliance for Patient Safety, Forward Programme 2005. Geneva: WHO; 2005.
[2]
Disponible en: www.who.int/patientsafety
[3]
Patient safety. Washington: Committee on Data Standards for Patient Safety, Institute of Medicine; 2004.
[4]
Medication errors and organizational culture in the pharmacy. En: ECRI. Improving patient safety. London: Department of Health; 2002.
[5]
Brennan TA, Leape LL, Laird NM, Hebert L, Localio AR, Lawthers AG, et al..
Incidence of adverse events and negligence in hospitalized patients: Results of the Harvard Medical Practice Study I-II..
N Engl J Med, 324 (1991), pp. 370-84
[6]
To err is human: building a safer health system. Washington: Committee on Quality of Health Care in America, Institute of Medicine; 1999.
[7]
Ottwara: Canadian Institute for Health Information; 2004.
[8]
The Stationery Office. London: Department of Health; 2000.
[9]
Washington: Veterans Health Administration; National Center for Patient Safety; 2001.
[10]
Discussion paper on adverse event and error reporting in healthcare. Washington: Institute for Safe Medication Practices; 2000.
[11]
Statement before the subcommittee on oversight and investigations, Committee on Veterans' Affairs. Washington: US House of Representatives; 2000.
[12]
Thomas EJ, Studdert DM, Burstin HR, Orav EJ, Zeena T, Williams EJ, et al..
Incidence and types of adverse events and negligent care in Utah and Colorado..
Med Care, 38 (2000), pp. 261-71
[13]
Wilson RM, Harrison BT, Gibberd RW, Hamilton JD..
An Analysis of the causes of adverse events from the Quality in Australian Health Care Study..
Med J Aus, 170 (1999), pp. 411-5
[14]
Foster AJ, Asmis TR, Clark HD, Al Saied G, Code CC, Caughey SC, et al..
Ottawa Hospital Patient Safety Study: incidence and timing of adverse events in patients admitted to a Canadian teaching hospital..
CMAJ, 170 (2004), pp. 1235-40
[15]
Washington: Institute of Medicine; 2001.
[16]
Choi SS, Jazayeri DG, Mitnick CD, Chalco K, Bayona J, Fraser HS..
Implementation and initial evaluation of a Web-based nurse order entry system for multidrug-resistant tuberculosis patients in Peru..
Medinfo, 11 (2004), pp. 202-6
[17]
Costa LA, Loureiro S, De Oliveira MG..
Errores de medicación de dos hospitales de Brasil..
Farm Hosp, 30 (2006), pp. 235-9
[18]
Carvalho PR, Carvalho CG, Alievi PT, Martinbiancho J, Trotta EA..
Prescription of drugs not appropriate for children in a Pediatric Intensive Care Unit..
J Pediatr, 79 (2003), pp. 397-402
[19]
Carvalho FM, Widmer MR, Cruz M, Palomo V, Cruz C..
Clinical diagnosis versus autopsy..
Bull Pan Am Health Organ, 25 (1991), pp. 41-6
[20]
Valdez-Martínez E, Arroyo-Lunagómez E, Landero-López L..
Concordancia entre el diagnóstico clínico y el patológico por necropsias..
Salud Publica Mex, 40 (1998), pp. 32-7
[21]
Coradazzi AL, Morganti AL, Montenegro MR..
Discrepancies between clinical diagnoses and autopsy findings..
Braz J Med Biol Res, 36 (2003), pp. 385-91
[22]
Rugeles S, Castro JF, Borrero AJ..
Errores en la atención en salud: estudio piloto para el diseño de procesos más seguros en el Hospital Universitario San Ignacio..
Rev Colomb, 19 (2004), pp. 125-32
[23]
Pena-Viveros R, Rodriguez-Moctezuma JR, Lopez-Carmona JM..
Factors associated with complaints against physicians working at Mexican Institute of Social Security hospitals..
Salud Publica Mex, 46 (2004), pp. 210-5
[24]
Disponible en: http://es.wikipedia.org/wiki/Daniel_Alcides_Carri%C3%B3n
[26]
Geneva: World Alliance for Patient Safety; 2005.
[27]
PriceWaterhouseCoopers' Health Research Institute. Disponible en: www.pwc.com/health
[28]
What are the best strategies for ensuring quality in hospitals? Geneva: WHO Regional Office for Europe's Health Evidence Network; 2003.
[29]
Estudio Nacional sobre los Efectos Adversos ligados a la Hospitalización. ENEAS 2005. General Técnica. Madrid: Ministerio de Sanidad y Consumo; 2006.
[30]
Washington: OPS; 2007. Disponible en: http://www.paho.org/ spanish/gov/csp/csp27-16-s.pdf