This study aimed to histometrically evaluate the presence of gingival recession in the mesial surface of the teeth of rats experimentally subjected to primary occlusal trauma. This evaluation verified the distance from the cement-enamel junction (CEJ) to the free marginal gingiva (FMG) and to the height of the alveolar bone crest (CEJ-crest bone distance). There were 10 animals, randomly divided into 2 groups: occlusal trauma (OT) (n=5) – creation of an occlusal interference by fixing an orthodontic wire segment on the mandibular first molar occlusal face, which was randomly chosen, and a Control Group (CG) (n=5) – five animals with no exposure to the OT variable were euthanised after 14 days to obtain the initial parameters. The inter-group evaluation showed there was no significant difference between OT×CG when the CEJ-FGM distance (P=0.192) was evaluated after 14 days, but there was a significant difference between the two groups as regards the CEJ-alveolar crest bone distance (P=0.0142). Thus, it can be concluded that the OT induction model, after 14 days of experiment, promoted bone resorption. This was observed by the increase in the CEJ-alveolar crest bone distance. It also did not promote gingival recession, which was evaluated by the CEJ-FGM distance.
El objetivo de este estudio fue evaluar histométricamente en ratas la presencia de recesión gingival en la superficie mesial de los dientes sometidos experimentalmente a trauma oclusal primario a partir de la evaluación de la distancia desde la unión esmalte cemento (CEJ) a la encía marginal libre y la altura de la cresta ósea restante (distancia de la CEJ-cresta ósea) Con este fin, 10 animales fueron divididos al azar en 2 grupos: trauma oclusal (TO) (n=5) – creación de una interferencia oclusal mediante la fijación de un segmento de alambre de ortodoncia en la superficie oclusal del primer molar elegido al azar; y un grupo control (CO) (n=5) –5 animales sin la introducción de la variable TO fueron sometidos a eutanasia después de 14 días para obtener los parámetros iniciales. La evaluación intergrupo no mostró diferencias significativas entre los grupos TO×CO al evaluar después de 14 días la distancia de la CEJ-encía marginal libre (p=0,192) pero mostró una diferencia significativa entre los grupos TO×CO en cuanto a distancia de la CEJ-cresta ósea alveolar (p=0,0142). Por lo tanto, se concluye que el modelo de inducción del TO después de 14 días del experimento promueve reabsorción ósea siendo observado por el aumento en la distancia de la CEJ-cresta ósea alveolar y no promueve la recesión gingival evaluada a partir de la distancia de la CEJ-encía marginal.
Gingival recessions correspond to the migration of the free gingival margin apically to the cemento-enamel junction, resulting in the exposure of the root surface.1 Studies show that gingival recessions are frequent in patients with periodontal disease and their incidence, prevalence and extension are distinct between populations.2,3 However, there is an increase in their incidence and severity correlated with the aging of the individuals.4,5
Gingival recessions have a multifactorial nature and can be associated with predisposing factors related to periodontal biotype,6 presence of bone dehiscence and fenestration,7 patient's habits, such as: tobacco8–10 and cocaine use,11 abnormal mucogingival insertion and frenulum pull,2,12 malpositioned teeth.2,7 There are also trigger factors or primary etiological factors, which are those directly responsible for the beginning and evolution of the recessions: accumulation of plaque, calculus and presence of periodontal diseases,8,9 excessive orthodontic tooth movement, leading the tooth out of the alveolus13,14 and physical trauma, such as traumatic brushing,9,15 tongue and lip piercings9,16 and iatrogenicities.3,5
On the other hand, occlusal trauma (OT) is defined as a non-infectious lesion that affects the inserting periodontium (cementum, periodontal ligament and alveolar bone) due to occlusal forces that exceed its adaptive capacity.17 OT can occur in a tooth with normal support (primary occlusal trauma) or in a tooth with reduced support (secondary occlusal trauma).17 Some authors have suggested occlusal trauma would also be an etiological factor in the development and worsening of gingival recessions,18–20 however, data related to the OT influence on the gingiva seem controversial.21 Such controversy exists because of the impossibility of prospective monitoring patients with OT due to the experiment's ethical conduct. Therefore, there are doubts about the OT involvement in gingival recession's primary or secondary etiology: the clinical studies published are retrospective21 or case reports.18–20 Most are case reports and have low scientific evidence, as they do not isolate the variables studied, increasing the results biases. Thus, the doubts about the occlusal trauma influence on the development and worsening of gingival recessions persist in the periodontics and it is necessary to carry out studies to elucidate this topic.
Due to the doubts raised about the primary occlusal trauma and its influence on the gingiva, this study aimed to histometrically evaluate the primary occlusal trauma influence on the development of gingival recessions and alveolar bone resorption in rats.
Material and methodsAnimalsThe animals used were ten Wistar breed rats, 16-week-old male adults, weighing between 215g and 315g. The animals were kept in plastic cages with ad libitum access to food and water in the vivarium at the Universidade do Sagrado Coração. The animals used in this study are standard type and had systemic and oral health at baseline. This study was submitted to USC Ethics Committee in Animals’ Experiment and it was accepted under the protocol numbered 28/13.
Experimental designTo delineate the experiments, the 10 animals were randomly divided by sort into the following groups:
G1: OT primary test (N=5)In order to reproduce experimentally the primary occlusal trauma,22 five rats were randomly chosen to undergo occlusal interference. After 14 days, the animals were submitted to euthanasia by anesthetic depth through intraperitoneal injections of thiopental (150mg/kg of animal weight) with lidocaine (10mg/kg of animal weight).
G2: Control group (N=5)Five rats were randomly chosen to compose the negative control group. They were anesthetized in the baseline by intramuscular administration of ketamine (50–100mg/kg) (Dopalen®; Vetbrands LTDA, Jacareí, SP, Brazil) and Xylazine (10mg/kg) to simulate the same stress the test group was submitted to; however, they did not undergo occlusal interference. This group was created with the purpose of having initial parameters of the alveolar bone crest height and the free gingival margin. After 14 days, the animals were submitted to euthanasia by anesthetic depth through intraperitoneal injections of thiopental (150mg/kg of body weight) with lidocaine (10mg/kg of body weight).
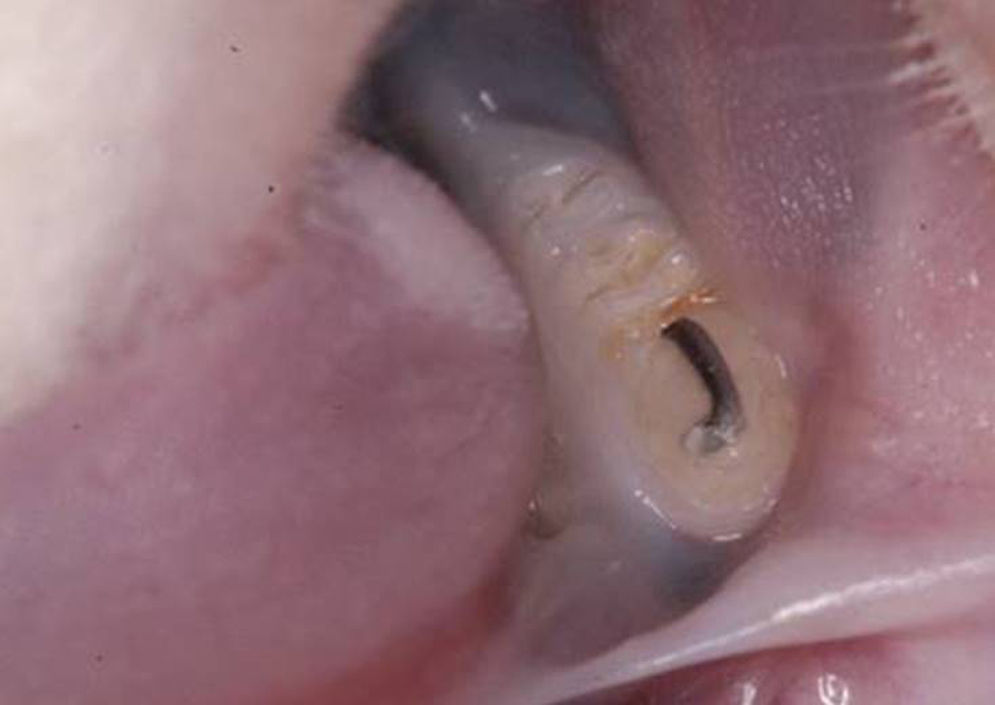
Oclusal trauma inductionIn the baseline, the animals were anesthetized by an intramuscular administration of ketamine (50–100mg/kg) (Dopalen®; Vetbrands LTDA, Jacareí, SP, Brazil) and Xylazine (10mg/kg). Their jaws were opened using the Doku apparatus (1966) to insert an orthodontic wire segment (0.5mm of diameter and approximately 1mm long) on the occlusal surface of the mandibular first molar, which was randomly chosen, to create an occlusal interference, using light-curing resin increments for the its fixation (Z100®; 3M, Sumaré, SP, Brazil).
The occlusal surface of the selected molar was previously cleaned with the aid of a microbrush, followed by the conditioning of the occlusal surface with phosphoric acid 37% (Villevie® Dentalville do Brazil, Joinville, SC, Brasil), rinsing and application of the adhesive (Single Bond®; 3M, Sumaré, SP, Brazil) according to the manufacturer specifications. The orthodontic wire's diameter standardized the height of the occlusal interference; the resin was inserted until the limit of the wire height, without exceeding it. The period of the primary occlusal trauma induction was 14 days (Fig. 1).
Euthanasia and sample gatheringThe animals were submitted to euthanasia by anesthetic depth through intraperitoneal injection (thiopental 150mg/kg of body weight with lidocaine 10mg/kg of body weight). They had their jaws removed and hemisectined in the symphysis. The hemimandibles were fixed in formilun 10% for 48h. After that, the hemimandibles were rinsed in PBS, and, then, decalcified with EDTA 10%. This solution was weekly changed during 30 days and it was kept in room temperature.
After being demineralized, the specimens were dehydrated in increasing concentrations of ethanol, diaphonized in xylene and embebeded in paraffin. Serial sections (6μm) were obtained (Leica RM2155; Leica Microsystems GmbH, Wetzlar, Germany) in a mesio-distal direction.
Histometric analysisAfter choosing the most convenient orientation, 6-μm-thick sections from the buccal bone plate were obtained. Equidistant sections were sorted according to the total of histologic cuts per tooth.
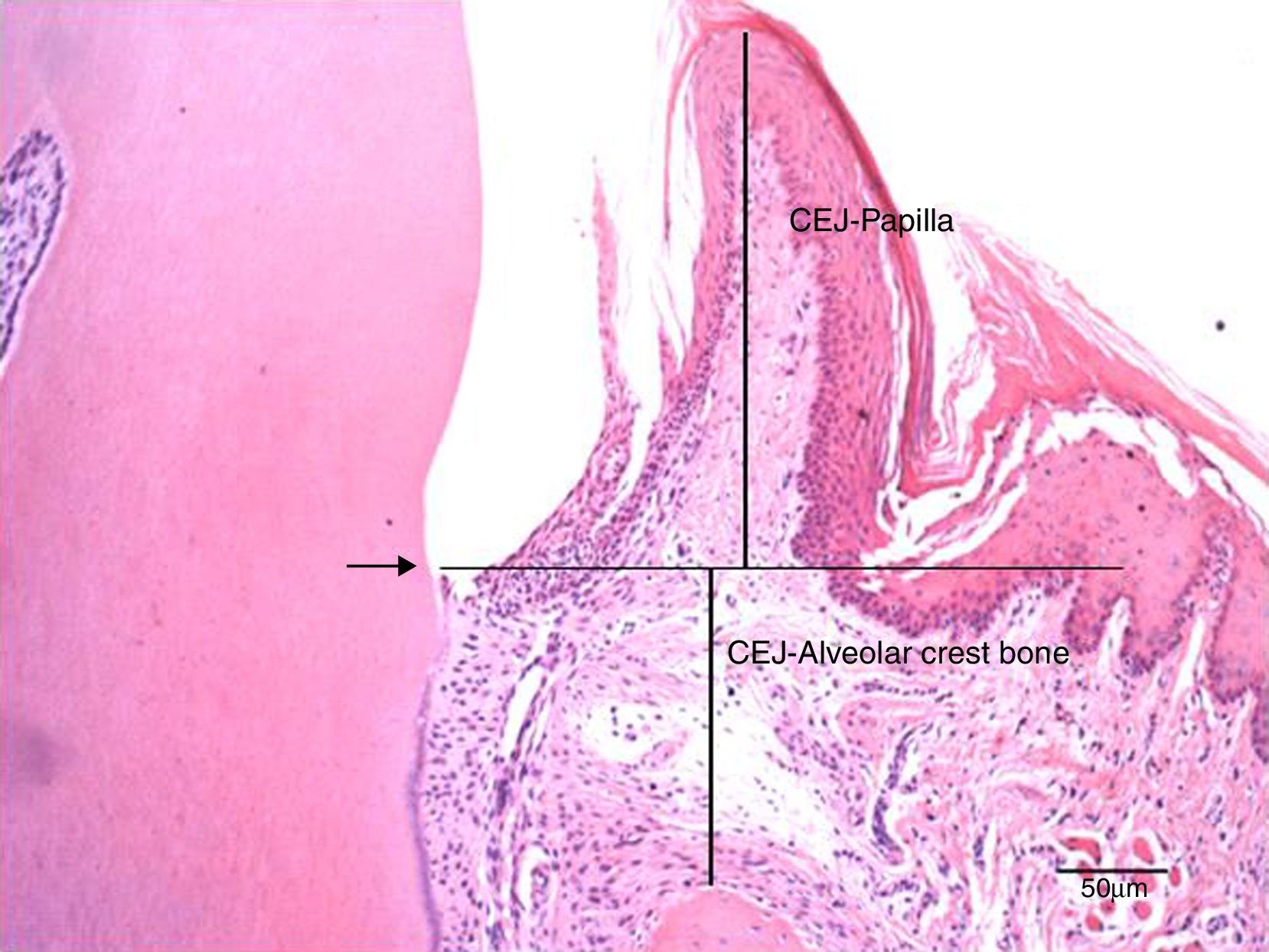
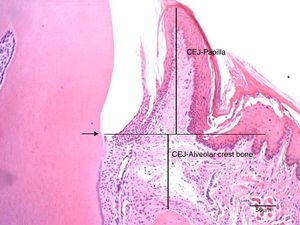
The cuts were stained with hematoxylin and eosin according to the routine protocol used at the Histologic Laboratory of the Universidade do Sagrado Coração. Three equidistant histologic sections were selected per tooth for the histometric analysis. The sections were digitalized at 100× magnification (10× objective lens and 10× ocular lens). Using the linear measuring system (Image-Pro®, Media Cybernetics, Silver Spring, MD, USA), we measured the distance from the free gingival margin (FGM) to the cemento-enamel junction (CEJ) to evaluate the gingival recession, and, also, the distance from CEJ to the reminiscent alveolar crest (Fig. 2). The measures were carried out on the mesial surface of the mandibular first molar and they were expressed in micrometers (μm).
Photomicrography at 100× magnification (10× objective and 10× ocular) of the mesial region of the mandibular first molar. It shows the measurements of the distance from the cemento-enamel junction to the papilla (CEJ-papilla) and the cemento-enamel junction to the bone crest. Arrow: cemento-enamel junction (CEJ); Bar: 50μm.
Prior to the final readings, the intraclass correlation was performed to evaluate the calibration of the examiner. Ten sections were randomly chosen; their measurements were made and repeated one week after the first measuring. The high value of correlation coefficient (r=0.989; confidence interval 95%: 0.972–0.996) shows the reading reproducibility was consistent, demonstrating a good calibration of the examiner.
Data processingThe distance from JCE-FGM and JCE-crest bone were initially assessed for homogeneity by Shappiro–Wilk test and to equal variance by Brown–Forsythe test. Due to the homogeneity detected in JCE-FGM (P=0.339) and JCE-crest bone distance (P=0.526) and due to have passed the variance test (P=0.746 and P=0.882, respectively), unicaudal T test was performed. The level of significance considered was 5%.
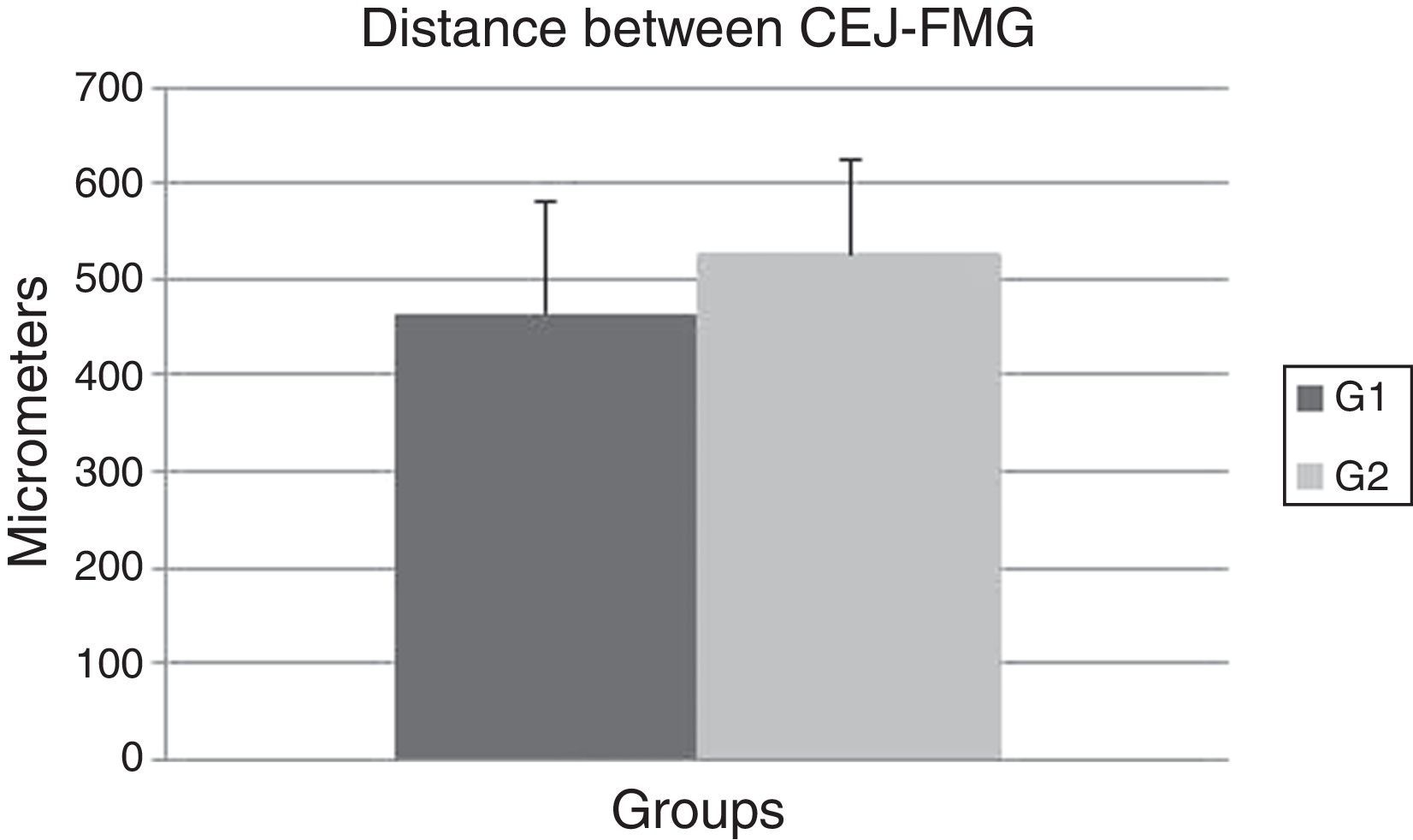
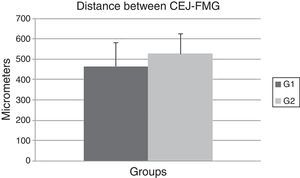
ResultsIn this experiment, no significant difference was observed between the OT (464.7±116.04) and the CG (527.2±97.19) (P=0.192) groups when the CEJ–FGM distance was evaluated after 14 days (Fig. 3). These data show that the OT model failed to promote gingival recession in the OT group after 14 days.
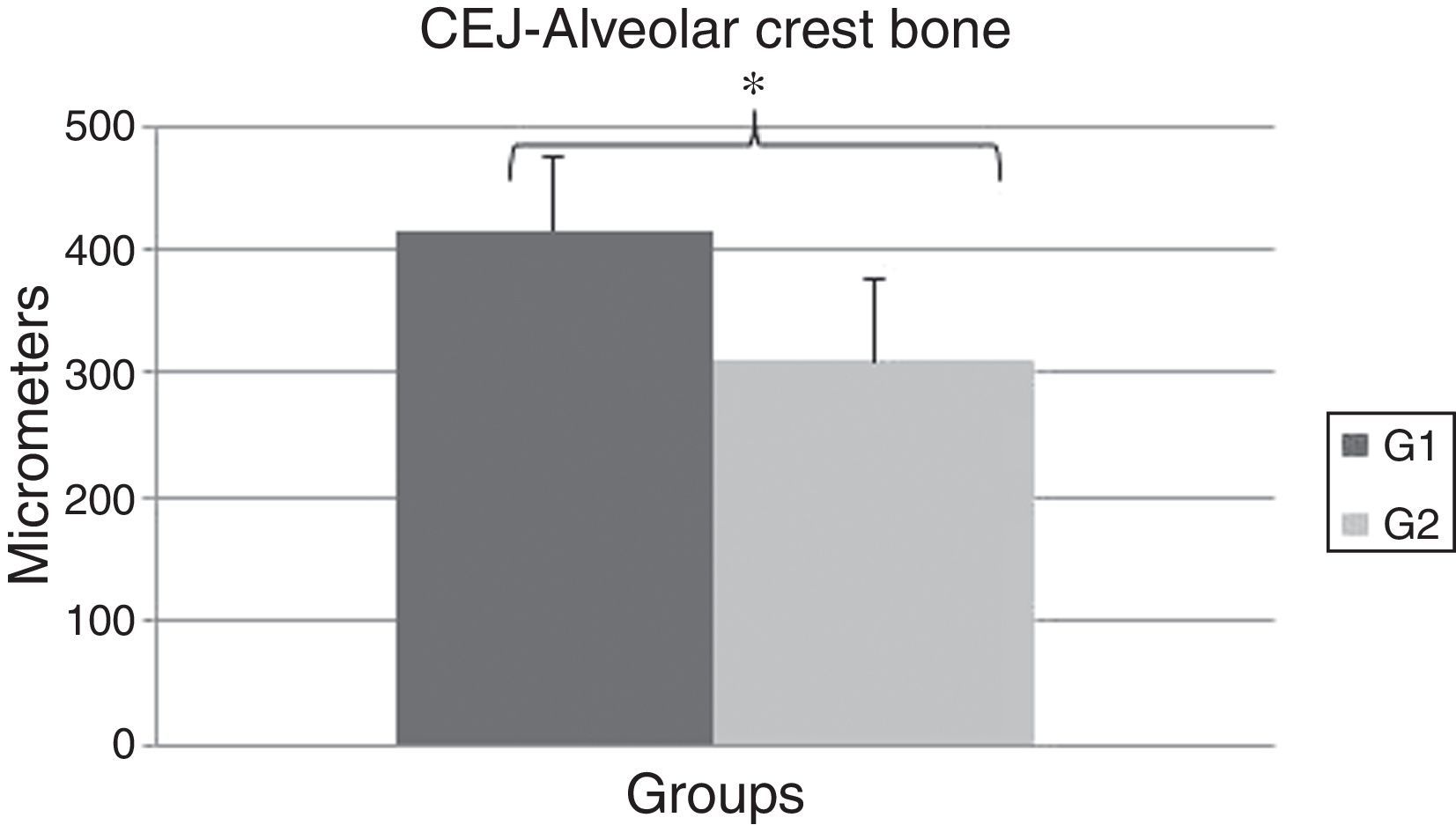
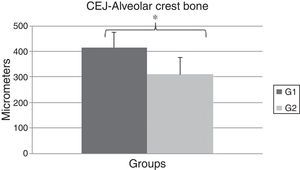
A significant difference was observed between the OT (416.0±59.69) and the CG (310.0±65.78) groups regarding the distance between CEJ and the alveolar crest bone (P=0.0142) (Fig. 4). These data show the OT model was able to promote bone resorption of the alveolar crest bone after 14 days of experiment.
DiscussionOcclusal forces are absorbed and distributed to the cementum, the periodontal ligament and the alveolar bone. When the forces applied to a tooth or group of teeth exceed the adaptive capacity of the inserting periodontium, a non-infectious lesion called occlusal trauma17 settles in these tissues.
Due to the lack of prospective clinical studies in the current literature because of the ethical issues involved, little is known about the influence of occlusal trauma in the gingiva and, as a consequence, its influence in the development of gingival recession. It has been proposed that occlusal trauma could be associated to periodontal deterioration in patients with a good plaque control, but due to lack of evidence the same authors concluded their study that soft tissue trauma could be explained only because local trauma as aggressive oral hygiene combined with a possible genetically determined enhanced susceptibility leading to soft tissue breakdown.23 Therefore, experimental researches able to limit the problem, isolate the variables and limit the biases of the results are the first step to elucidate the effects of occlusal trauma on the gingiva.
The period of 14 days after OT induction was chosen to perform the histometric evaluation because in a previous study it was observed that 14 days was sufficient to detect histometric OT lesion detected by measuring alveolar bone loss in furcation region in a rat model.22 It is relevant to know that 1 day in adult rats life is comparable to 1 month in human's life and the period chosen in this study reflects 14 months in human analogy.24,25
The results of this study showed the proposed model was able to promote resorption of the alveolar crest bone mesial in the first molars’ mesial surfaces after 14 days of OT induction, verifying an increase in distance from the alveolar crest bone to the CEJ for the OT group compared to the CG (P=0.0142).
Other studies have also identified, in other experimental models, the influence of OT in the alveolar bone resorption in Macaca irus,26 in the destruction of inserting periodontium of Callithrix jacchus.27 Other authors also reported its influence over the increase of RANKL levels and the decrease of osteopontin levels after 1 and 7 days, showing there is an influence on bone resorption of the OT model caused by the production of an occlusal interference.28 These authors did not find any influence of these markers after the 14th day of the experiment, revealing a functional adaptation of the periodontium due to traumatogenic demand. In the occlusal interference model implemented in this study, we were able to verify the influence on bone resorption after 14 days, similar to the study by Campos et al.,23 showing the lesion had not undergone functional adaptation and had returned to the initial height dimensions of the mesial alveolar crest.
The OT model implemented in this study failed to promote gingival recession on the first molars’ mesial surfaces after 14 days, as there were no significant differences between the OT and the CG regarding the distance between CEJ and FGM (P=0.192). Data from the present study are corroborated by the results of Ericsson and Lindhe29 which observed in an experimental study in dogs that occlusal trauma developed by the application of jiggling forces developed by a insertion of occlusal interference was unable to produce additional clinical attachment loss in teeth with reduced but healthy periodontium and also verified by radiographs that the proposed OT model was able to produce lesion detected by bone resorption and periodontal ligament enlargement, showing that OT does not act as a primary etiologic factor in clinical attachment loss and this can be translated that OT does not produce gingival recession, as the apical displacement of the free gingival margin is counted in the results of clinical attachment loss. In a posterior study, the same authors verified that in the presence of periodontitis, OT produced additional attachment loss acting as a co-factor of destruction.30
Our findings contradicts the results of clinical case reports published in the literature18–20 that associate occlusal trauma with the development of gingival recession. It should be taken into consideration the lack of scientific evidence of case reports, because there is no control of bias; therefore, these case reports findings should be evaluated with caution. As the results of this study, a retrospective clinical study21 showed no significant relationship between the presence of occlusal discrepancies and changes in the gingival levels, corroborating the findings of this study, which did not identify influence of the primary OT on the gingiva.
Despite the small number of animals used in this study, we could detect significant differences between groups, showing the number of rats used in the sample was enough to assess the proposed lesion. This study data should also be evaluated with caution because the dynamics of mastication in rats are different from humans; therefore, a direct derivation of the results cannot be used. However, animal studies are essential for the scientific literature, since they allow the evaluation of physiological and pathological mechanisms that could not be evaluated clinically, allowing the improvement of prevention methods, diagnosis and treatment of infectious and non- infectious lesions in humans.
Further studies using different experimental times should be performed to evaluate the influence of OT on the buccal surface of the teeth and their proximal surfaces, as well as evaluate gingival and periodontal inflammatory markers to better understand whether there is an interaction between OT and the presence of gingival recessions.
ConclusionsWithin the limits of this study, it was possible to conclude the OT model, after 14 days:
- (a)
Promoted bone resorption observed by the increase of the distance between the CEJ and the alveolar crest bone;
- (b)
Did not promote gingival recession, assessed through the distance between CEJ and FGM.
The authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestsThe authors declare no conflict of interest.