We report the case of a pregnant woman at term with primary anti-phospholipid syndrome, portal vein thrombosis, massive splenomegaly, oesophageal varices and thrombocytopenia. The patient underwent an elective caesarean section under general anaesthesia to minimise the risk of spleen and variceal rupture, with a favourable outcome for both the mother and the newborn.
Chronic portal vein thrombosis is a rare condition, caused by various reasons, mainly thrombotic diathesis. It leads to increased portal pressure, with development of collateral circulation, splenomegaly and thrombocytopenia. Pregnancy in these conditions is considered high risk, but is not contraindicated if the underlying disorder is stabilised. The management of these patients should be multidisciplinary, under close monitoring; diagnosis and treatment of possible oesophageal varices is essential. The decision about mode of delivery and anaesthetic management must be individualised, depending on obstetric factors, the presence or absence of varices and thrombocytopenia, and associated comorbidities.
Presentamos el caso de una gestante a término con síndrome antifosfolípido primario, trombosis portal crónica, esplenomegalia masiva, varices esofágicas y trombocitopenia. La paciente fue sometida a una cesárea electiva bajo anestesia general para minimizar el riesgo de ruptura del bazo y de las varices, con un resultado favorable para la madre y el neonato.
La trombosis portal crónica es una patología poco frecuente, motivada por distintas causas, principalmente las diátesis trombóticas. Induce un aumento de la presión portal, con desarrollo de circulación colateral, esplenomegalia y trombocipenia. La gestación en estas condiciones se considera de alto riesgo, pero no está contraindicada si la patología está estabilizada. El manejo de estas pacientes debe ser multidisciplinar y su seguimiento estrecho; el diagnóstico y tratamiento de las posibles varices esofágicas es esencial. La decisión sobre el modo de finalizar la gestación y el manejo anestésico deben individualizarse en cada caso, en función de factores obstétricos, de la presencia o no de varices y trombocitopenia, y de las comorbilidades asociadas.
Having obtained approval from the Ethics Committee of our institution, we present the case of a 29 year-old, 74kg, pregnant woman at term, diagnosed three years before, because of miscarriage, with a primary anti-phospholipid syndrome, chronic portal vein thrombosis, massive splenomegaly and thrombocytopenia. This thrombosis led to portal hypertension with oesophageal varices, splenorenal collateral circulation and arterioportal fistulae. Anticoagulation with acenocoumarol was initiated following diagnosis. The patient was then switched to enoxaparin 60mg and acetyl salicylic acid 100mg daily when she expressed her wish of becoming pregnant again. She was also taken to oesophago-gastroscopy, ruling out the presence of varicose lesions requiring endoscopic treatment. The course of the pregnancy was uneventful and the patient was scheduled for elective caesarean section due to the risk of spleen rupture during labour.
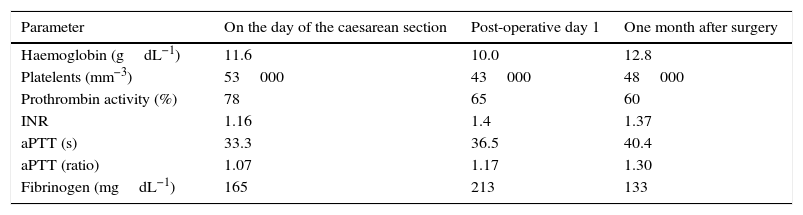
On exploration, there were no pathological cardiorespiratory findings or difficult airway predictors. On the day of surgery, the patient had 56000plateletsmm−3 (Table 1); SaO2 was 85%, and she had no dyspnoea, respiratory distress or cyanosis. The patient was pre-medicated with ranitidine 50mg, metoclopramide 10mg and cefazolin 2g IV 1h before the intervention. On arrival at the operating room, 2 large vein and radial artery lines were established, a bladder catheter was placed, and transfusion of a pool of platelets was initiated. After 3min of pre-oxygenation, rapid sequence induction was started using rocuronium 1mgkg−1, propofol 2mgkg−1, remifentanil 1mcgkg−1 and Sellick's manoeuvre; intubation was successful on the first attempt without substantial variations in blood pressure. Foetal delivery was assisted with a sucker, and no abdominal compression was used. Until that moment, anaesthesia was maintained with 100% O2 and 1% sevoflurane. FiO2 was then reduced to 50%, sevoflurane administration was interrupted and remifentanil perfusion was initiated (in order to avoid blood pressure or heart rate increases of more than 20%) together with propofol perfusion (for BIS between 40 and 60). Before the end of the intervention, the patient received IV paracetamol 1g, metamizol magnesium 2g, morphine hydrochloride 10g and ondansetron 4mg, as well as sugammadex 200mg before emergence and extubation, with full neuromuscular blockade recovery. Once the placenta was removed, oxytocin perfusion was initiated per protocol.
Peri-operative test results.
| Parameter | On the day of the caesarean section | Post-operative day 1 | One month after surgery |
|---|---|---|---|
| Haemoglobin (gdL−1) | 11.6 | 10.0 | 12.8 |
| Platelents (mm−3) | 53000 | 43000 | 48000 |
| Prothrombin activity (%) | 78 | 65 | 60 |
| INR | 1.16 | 1.4 | 1.37 |
| aPTT (s) | 33.3 | 36.5 | 40.4 |
| aPTT (ratio) | 1.07 | 1.17 | 1.30 |
| Fibrinogen (mgdL−1) | 165 | 213 | 133 |
Source: Authors.
The procedure was uneventful, resulting in the birth of a female neonate who did not need resuscitation (1min and 5–10min Apgar 9 and 10, respectively). The immediate course was satisfactory, with normal bleeding and no need for additional platelet transfusion or the use of other blood products. Post-operative testing showed 43000plateletsmm−3 and 65% prothrombin activity, with the rest of the coagulation parameters being normal (Table 1). Enoxaparin treatment was resumed 14h after the intervention with 40mg/24h and 60mg/24h after the second day. The patient was discharged on day 5, with no remarkable events. Oral anticoagulation was restarted after one month, and enoxaparin was discontinued when the INR was higher than 2. There were no remarkable events during the postpartum period. At six months, the patient was readmitted twice due to abdominal pain associated with symptomatic cholelithiasis in the first instance, and with the degree of splenomegaly in the second instance. In view of the adequate response to the medical treatment and the high anaesthetic and surgical risk, the decision was to perform regular follow-up. At that time, the patient was Child–Pugh B7, and treatment with propranolol was initiated for primary prophylaxis of upper gastrointestinal bleeding.
DiscussionPortal vein thrombosis, in the absence of cirrhosis or hepatobiliary tumours, is an infrequent condition, usually associated with portal hypertension and hypersplenism with thrombocytopenia.1,2 Causes of this disorder include thrombotic diatheses,2 neonatal omphalitis,1 and infections.3 Thrombosis progression to the mesenteric vessels is associated with a high risk of intestinal ischaemia, with a mortality as high as 50%.2 Collateral circulation develops in the absence of revascularisation (cavernomatous transformation),1,2 creating the risk for upper gastrointestinal bleeding.
Contrary to the publications in older studies, it has been determined that portal flow increases significantly during normal pregnancy. It is not known whether that increase is also present in patients with hepatic vascular disease. Should that be the case, portal hypertension would be made worse.4,5 Pregnancy in those circumstances is very high risk,5,6 but there is no absolute contraindication in cases of stable portal thrombosis.2,6 Given that this condition is very infrequent, not many publications are available on this topic and there is no consensus regarding pregnancy management, although three essential points may be highlighted: optimisation of the patient's condition before term, a decision on how to deliver the patient (vaginal delivery or elective caesarean section) and related anaesthetic aspects (analgesic and anaesthetic techniques).
More than 43% of obstetric patients with non-cirrhotic portal hypertension and oesophageal varices will present with upper gastrointestinal bleeding, with a perinatal mortality of 35%.1 It has been suggested that upper gastrointestinal endoscopy for prophylactic treatment of varices before conception,1,2,5,7 or during the second trimester (when portal pressure increases the most), could reduce the risk of bleeding down to 8.6%.5 Sclerotherapy, as well as ligation, are considered safe procedures during pregnancy, although the latter is the preferred technique.5,7 The use of beta-blockers (non-selective, particularly propranolol) is also recommended for bleeding prevention and considered beneficial despite potential adverse foetal events, including intra-uterine growth retardation, bradycardia, and neonatal hypoglycemia.5,7 Portosystemic shunts are generally used only in bleeding that is refractory to medical and endoscopic treatment.5,7
There are no guidelines for decision-making regarding the delivery modality. This decision must be individualised in each case and is incumbent upon a multidisciplinary team.8 Some authors consider that vaginal delivery is the best option and that caesarean section must be reserved for obstetric indications.1,2,5,7,8 The expulsive stage must preferably be short and instrumented in order to avoid an excessive increase in abdominal and variceal pressures.1,5–8 Other authors advocate performing a caesarean section in patients with varices8 or massive splenomegaly,9 in order to avoid rupture due to increase in intra-abdominal pressure during labour and pushing. It is important to bear in mind that bleeding associated with caesarean section in these cases may be more abundant than usual as a result of thrombocytopenia and the presence of varices in the abdominal wall1; the risk of thromboembolism2 and decompensation of the patient's clinical status (ascites, encephalopathy)7 is also increased. The use of cups for foetal extraction in order to minimise abdominal wall compression has been documented.8
In the case of vaginal delivery, early initiation of analgesia is needed.6 Venous engorgement may also affect epidural and paraspinal vessels, with development of collateral circulation and increased risk of bleeding and systemic re-uptake of local anaesthetics when neuroaxial techniques are used1; if any of these techniques are planned, magnetic resonance imaging is desirable.1 Regional techniques are frequently dismissed due to the associated thrombocytopenia, although they may be preferred over general anaesthesia in certain circumstances10; risk and benefits must be weighed in each individual case.11,12 There have been no studies to date with a sufficient number of patients that may allow to determine the safety of these techniques in obstetric patients with platelet counts under 100000mm−3, although different authors consider figures between 75000 and 80000 as acceptable.13,14 Based on several studies,11,12,15–17 out of a total of 661 obstetric patients who received a neuroaxial technique with less than 100000 plateletsmm−3, there were no reported cases of spinal haematoma; also, there are no confirmed cases of haematomas in this type of patient in the absence of clinical signs of coagulopathy.18 Beilin et al.11 recommend requesting a platelet count as close as possible to the use of the technique in order to rule out a progressive drop in platelets, while other authors advocate performing a platelet function test such as thromboelastography.17,18 A relatively common practice consists of platelet transfusion before the puncture, although there is no strong evidence to support it.19 Interestingly, in the study by Hoekstra et al.,2 adverse pregnancy outcomes were associated with the highest platelet counts at the time of diagnosis.
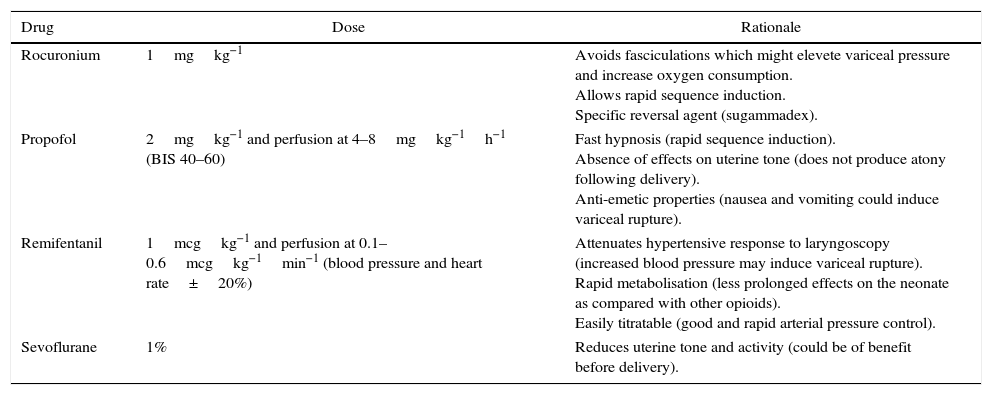
As for general anaesthesia, adequate anaesthesia depth is critical in order to avoid the development of hypertension and tachycardia, which could favour variceal bleeding; this is especially important when a significant stimulus is applied, as is the case at the time of laryngoscopy and intubation, skin and uterine incisions, and foetal extraction.6 Because of its pharmacokinetic properties, remifentanil can be very useful,6 while thiopental and succinylcholine have also been used safely6 (Table 2). It is important to bear in mind that, in these patients, anaesthetic agents may trigger or worsen an encephalopathy.5
Anaesthetic drugs used.
| Drug | Dose | Rationale |
|---|---|---|
| Rocuronium | 1mgkg−1 | Avoids fasciculations which might elevete variceal pressure and increase oxygen consumption. Allows rapid sequence induction. Specific reversal agent (sugammadex). |
| Propofol | 2mgkg−1 and perfusion at 4–8mgkg−1h−1 (BIS 40–60) | Fast hypnosis (rapid sequence induction). Absence of effects on uterine tone (does not produce atony following delivery). Anti-emetic properties (nausea and vomiting could induce variceal rupture). |
| Remifentanil | 1mcgkg−1 and perfusion at 0.1–0.6mcgkg−1min−1 (blood pressure and heart rate±20%) | Attenuates hypertensive response to laryngoscopy (increased blood pressure may induce variceal rupture). Rapid metabolisation (less prolonged effects on the neonate as compared with other opioids). Easily titratable (good and rapid arterial pressure control). |
| Sevoflurane | 1% | Reduces uterine tone and activity (could be of benefit before delivery). |
Source: Authors.
Regardless of the form of delivery, close surveillance of postpartum bleeding is critical because of its higher incidence.5 Antibiotics must be administered, especially in cases of ascites, in order to prevent spontaneous bacterial peritonitis.5 If indicated, anticoagulation must be resumed as soon as possible in the absence of bleeding data, ideally within the first 24h.7
From the above, it may be concluded that, although in cases of portal thrombosis pregnancy must be considered of high risk, it is not contraindicated if the underlying disorder is stabilised. These patients require a multidisciplinary approach and close follow-up. An upper gastrointestinal endoscopy is mandatory in order to diagnose and treat potential oesophageal varices. The decision regarding the mode of delivery and anaesthetic techniques must be individualised in each case on the basis of obstetric factors, the presence or absence of varices and/or thrombocytopenia, and any associated comorbidities.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare having no disclosures.
FundingThe authors declare having no source of funding.
Please cite this article as: López Correa T, Sastre Rincón JA. Caesarean section in a patient with chronic portal vein thrombosis and thrombocytopenia: Case report. Rev Colomb Anestesiol. 2017;45:251–255.