To establish the variability of vecuronium (lag-time, latency duration, clinical duration and complete recovery), original molecule, in a group of patients who received the agent prior to surgery under general anesthesia with endotracheal intubation.
Materials and methodsObservational, prospective study including 20 adult patients – ASAI or II classification – selected on the basis of suitability, who received general anesthesia and vecuronium as neuromuscular relaxant. Remifentanyl and propofol were the induction agents. The neuromuscular activity was quantified using a TOF-WATCH SX® stimulator, establishing lag-time, duration of latency, clinical duration and complete recovery. The data were analyzed using STATA 10.
ResultsThe data on lag-time to complete recovery are given as medians: lag-time: 32.5seconds; latency time: 240seconds; clinical length 25: 43.5min; clinical length 50: 57.5min; clinical length 75: 58.5min; clinical length 100: 55min; complete recovery: 87min; need for reversion: 55%; adverse effects: none.
ConclusionsThe results obtained suggest a broad variability between the time of administration of vecuronium, its clinical effect and duration of action, all of which were above the levels recorded in the literature. This suggests that the agent is long-acting and highly unpredictable. We recommend neuromuscular function monitoring as an indispensable routine and preferably quantitative.
Determinar la variabilidad del vecuronio (lag time, duración de latencia, duración clínica y recuperación completa), molécula original, en un grupo de pacientes que reciben este fármaco para ser intervenidos quirúrgicamente bajo anestesia general, con intubación endotraqueal.
Materiales y métodosEstudio observacional prospectivo hecho con 20 pacientes adultos, clasificados ASAI o II, seleccionados por conveniencia, quienes recibieron anestesia general y vecuronio usado como relajante neuromuscular. La inducción fue hecha usando remifentanilo y propofol. La actividad neuromuscular fue cuantificada mediante un estimulador TOF-WATCH SX® determinando lag-time, duración de latencia, duración clínica y recuperación completa. El análisis de datos se efectuó en STATA 10.
ResultadosLos datos referenciados desde lag time hasta recuperación completa están dados en medianas; lag time: 32,5s; tiempo de latencia: 240s; duración clínica 25: 43,5min; duración clínica 50: 57,5min; duración clínica 75: 58,5min; duración clínica 100: 55min. Recuperación completa: 87min. Necesidad de reversión: 55%. Efectos adversos: ninguno.
ConclusionesLos resultados obtenidos sugieren una gran variabilidad del tiempo entre la administración del vecuronio, su efecto clínico y la duración de acción, observándose superiores a los registrados en la literatura; esto lo hace ver más como un fármaco de larga acción y destacada imprevisibilidad, por lo que sugerimos el uso de monitorización de la función neuromuscular como elemento indispensable, preferiblemente de tipo cuantitativo.
Since the introduction of muscular paralysis as an adjunct to anesthesiology in the mid 1950s, the procedure was conceived as a way to reduce the depth of the anesthesia that patients required and theoretically, to reduce the risk of developing adverse cardiovascular consequences.1 Beecher and Todd published a study documenting a relative mortality risk of 6.6 in patients receiving muscular relaxants versus those who did not receive relaxing agents.2
These reports on adverse events continued during the next 2 decades and in 1972 Viby-Mogensen showed that 30% of the patients whose paralysis was considered adequately reversed, continued partially relaxed. As a result of this effect, neuromuscular relaxants are still today an independent factor for adverse respiratory events in the post-anesthesia care units.2–9 Thus, given the potential danger of relaxants, particularly the long-acting agents, the pharmaceutical industry has been focusing on the development of shorter acting molecules, with a more predictable pharmacodynamics, a larger safety margin and easily reversible one.10
This is why two intermediate action neuromuscular relaxants (atracurium and vecuronium) were introduced in the 1980s, with a view to reduce the complications related to residual relaxation11; these have been gaining their place in the contemporary clinical practice.
Vecuronium is a non-depolarizing amino-steroid neuromuscular blocker developed by Savant, Durant, Bowman and Marshall. It is an intermediate action muscle relaxant12–15 outstandingly noted for its absolute hemodynamic stability.16
This study shall establish the clinical and pharmacological characteristics of Vecuronium, 10mg (LP pharmaceutical form – lyophilized powder) through the observation of patients requiring the use of the drug for endotracheal intubation when general anesthesia is needed, for maintenance of anesthesia and during recovery.
The establishment of clinical measurements for vecuronium will also show the intubation conditions, the potential complications derived from its use, as well as the need for pharmacological reversal of the relaxant.
Materials and methodsAn observational clinical study was completed with the authorization of the Research Committee of the San José Hospital, administering vecuronium (original molecule). The patients included were 18–60years old, ASA I or II, undergoing low to intermediate risk procedures under the ACC/AHA classification, who required tracheal intubation for an estimate of 45min or more, has not taken any solids in the previous 8hours and who had signed the informed consent.
Patients with suspect or confirmed pregnancy, 20% or more over the ideal body weight for height, neuromuscular or neurological disorders, liver, renal cardiovascular, respiratory of psychiatric disorders, a clinical history of chronic alcoholism of drug abuse, gastro-esophageal reflux, hypothermia (temperature in the thenar region below 32°C) during the intraoperative period, a history of exposure to antibiotics (except for penicillin, tetracycline or cephalosporins), quinidine, lidocaine, antihistamine agents (H1 or H2), trimethaphan during the 48hours prior to surgery, or patients in whom the use of these drugs was planned for during the study period were all excluded.
Patients with a history of exposure to antidepressants, carbamazepine or phenytoin in the week prior to the trial, predictors of difficult airway or a history of difficult airway were also excluded.
Furthermore, those patients who in the opinion of the anesthesiologist in charge, required an airway manipulation procedure different from the trial standard (intubation with the patient awake, intubation using other instrumentation in addition to the laryngoscope, for instance the fiber optic bronchoscope, illuminating stylet, laryngeal mask), grade III or IV laryngoscopies because of inability to see the vocal chords under direct vision, were additional exclusion criteria.
The surgical procedures were monitored to determine the electrical cardiac activity, pulse oximetry, non-invasive blood pressure, heart rate, capnography and finally monitoring the neuromuscular function and the thenar temperature using a TOF WATCH SX® monitor according to the following protocol:
- 1.
Immobilization of the arm leaving the thumb free and immobilization of the other four fingers.
- 2.
Skin cleansing using cotton and 40% alcohol.
- 3.
Application of electrodes: A. Two electrodes are placed at the ulnar level, the distal at 1cm from the wrist. B. Proximal at 3cm from the first electrode.
- 4.
Electrode connection: A. Negative: Distal (Black). B. Positive: Proximal (White).
- 5.
Connect the accelerometer to the distal aspect of the thumb pad with micropore.
The induction of anesthesia was done with a bolus dose of 1–2μg/kg of remifentanil, propofol 2mg/kg, and the TOF WATCH monitor was calibrated and 15seconds later a 0.1mg/kg dose of vecuronium was administered. Then followed a supramaximum sequence of 1Hz stimulus and from thereon measurement of lag time (onset of response decline) and latency time (95% of response lost). The intubation of the trachea was under laryngoscope and the quality of the intubation was measured according to Cooper's scale.17,18 Monitoring changed to train of four (TOF) and the duration of the clinical action was measured through the gradual recovery of the contraction strength in percentages, i.e. 25, 50, 75 and 100%, respectively.
The anesthesia was maintained with isoflurane 0.5% MAC and remifentanil 0.25 micrograms per kilogram per minute, adjusting to the patient's requirements and the type of surgery. All patients were under controlled with mechanical ventilation maintaining the expired CO2 within 30–38mmHg. Depending on the relationship between the duration of the relaxant and the surgical time, the patient was allowed to evolve spontaneously. If an additional dose of relaxant was needed, the patient was excluded from the study.
At the end of the case, the patients were evaluated in accordance with the acceleromiography monitoring19–21 and those who did not show a T1/T4 ration of over 75% were pharmacologically reverted with 0.04mg/kg using neostigmine plus atropine 1mg. Reversal complications were described if any.
Finally, the researchers examined the patients in the post anesthesia recovery room to rule out any residual paralysis, making sure that they had fully recovered awareness and establishing the ability to raise the head from the stretcher for 5seconds.
Analysis of the dataThe descriptive statistics for quantitative variables were calculated as means and ranges, and qualitative variables as proportions. The analysis was done with the STATA 10 software.
ResultsTable 1 describes the most salient demographic characteristics considered for this study. 20 patients were included (85% females). The age distribution was similar and 50% of the patients weighted 64kg or less (45–83). The mean thenar skin temperature was 34°C (33–35).
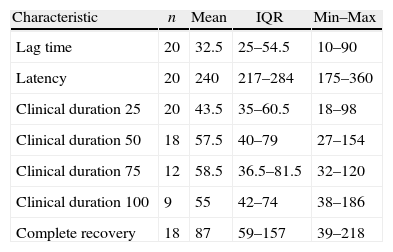
The surgical procedures were diverse, with a predominance of abdominal surgery – hysterectomy in 7 patients representing 35% of the cases. Table 2 shows the data for starting times, duration and neuromuscular recovery following the administration of vecuronium. The characteristics with largest spread were latency (Min: 175–Max: 360) and complete recovery (Min: 39–Max: 128). The characteristics with the narrowest range were lag time and clinical duration 25. Of the total number of patients in the study, 11 (55%) required pharmacological reversal of the neuromuscular block.
Results of starting times, duration and complete recovery from neuromuscular relaxation.
| Characteristic | n | Mean | IQR | Min–Max |
| Lag time | 20 | 32.5 | 25–54.5 | 10–90 |
| Latency | 20 | 240 | 217–284 | 175–360 |
| Clinical duration 25 | 20 | 43.5 | 35–60.5 | 18–98 |
| Clinical duration 50 | 18 | 57.5 | 40–79 | 27–154 |
| Clinical duration 75 | 12 | 58.5 | 36.5–81.5 | 32–120 |
| Clinical duration 100 | 9 | 55 | 42–74 | 38–186 |
| Complete recovery | 18 | 87 | 59–157 | 39–218 |
IQR: inter quartile range.
Lag time: time in seconds between the starting flow of the relaxant into the circulation and a 5% loss of neuromuscular activity.
Latency time: corresponds to the time in seconds between the administration of the relaxant and the maximum block.
Clinical duration 25, 50, 70 and 100: time in minutes between the administration of the relaxant and recovery of 25%, 50%, 75% and 100% respectively, of the first response in the 4 T1 series.
Complete recovery: time elapsed between the administration of the relaxant and total recovery.
Overall, a considerable variability is observed from the data collected. A limitation of the study was the completion of the surgical procedure before complete recovery was achieved; this results in the need for pharmacological reversal and hence, some duration variables failed to be measured in 100% of the patients, particularly those related to clinical duration 75 and 100.
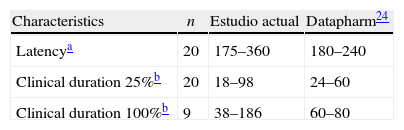
Table 3 provides a comparison of some of the data provided by this study versus the existing literature. For instance, the clinical durations found exceed those reported in the literature – a fact that could be due to pharmacogenetic type of factors, yet to be established, but which are specific for the population studied and become increasingly relevant in the light of the current knowledge, as factors responsible for such pharmacodynamic differences.
These results may be considered as contrary to the inclusion of vecuronium5,12–15,22 in the group of intermediate action neuromuscular relaxants, as has been traditionally accepted in the classic anesthesiology books, supported by trials with a limited number of patients. Hence, in accordance with the action times recorded herein, the agent tends to behave rather as a long-acting relaxant.
In addition to the abovementioned pharmacogenetics, suggested as a potential causal factor of the data obtained in this study, there are other contributing factors such as insufficient sample size, racial genetic variability, using a quantitative neuromuscular function monitor (mechanomyography and acceleromyography)23 that gives more precise measurements, and finally, another factor to consider is the change in the practice of anesthesia, particularly during the last two decades due to the increasingly stringent criteria to consider the neuromuscular function recovered following the use of myorelaxant agents.
In conclusion, the behavior of vecuronium according to the results obtained brings it closer to the so-called long-acting neuromuscular relaxants, with considerable individual variability in its clinical effect and high unpredictability in terms of duration of action, bearing in mind that we had patients who recovered in 230min. Thus we suggest using a neuromuscular function monitor to assess complete recovery following the administration of neuromuscular blocking agents, and hence prevent the risk of residual relaxation.
FundingVitalis Pharmaceutical Laboratories provided the TOF WATCH SX® monitor for the study. All other funds were provided by the authors.
Conflicts of interestNone declared.
Please cite this article as: Reyes L, et al. Variabilidad clínica del vecuronio. Experiencia en una institución en Colombia. Rev Colomb Anestesiol. 2012;40:251–5.