This work is an approach to the article by Douketis et al.1
Around 10% of patients with atrial fibrillation must be operated on each year, which offers a perioperative challenge for the surgical team. Traditionally, bridging anticoagulation therapies have been used with heparin based on the argument that suspending the anticoagulation for long periods of time increases the risk of thromboembolic events. However, there is no consensus regarding this management strategy. Currently, the American College of Chest Physicians2 sets a recommended grade 2C for bridging therapy, which makes it evident that there are insufficient good quality studies to make a clear recommendation. Siegal et al.3 published a meta-analysis in which bridging therapy was associated with a greater risk of bleeding, with an odds ratio (OR) 5.4 for any type of bleeding and 3.6 for major bleeding. Thromboembolic events showed no difference between bridging therapy and no therapy, with an OR of 0.8, due to the discrepancy as to what ought to be the proper conduct. Current publications suggest three scenarios. The first scenario corresponds to patients who can be operated on without suspending anticoagulation therapy, such as those undergoing cataract surgery. The second scenario is that of patients to be operated who require coagulation at close to normal levels and who have a low risk of thromboembolic events; these patients can be taken off anticoagulants. The third scenario corresponds to other possibilities in which bridging therapy should be the option of choice.4
The objective of the studyThe clinical experiment put forward by Douketis et al. aims to determine if, in the case of those patients who are taken to surgery or invasive procedures and who present atrial fibrillation under treatment with warfarin, not using bridging therapy with low molecular weight heparin does not generate greater embolic events as compared to those patients who are administered bridging therapy, and if the presentation of major bleeding would occur more in this case.
Study designTo answer this question, a randomized clinical trial was devised with 1884 patient participants over the age of 18 who presented chronic atrial fibrillation and who had been receiving treatment with warfarin over the course of three months prior to the surgery or invasive procedure. Patients with an INR of between 2 and 3, and who complied with at least one of the criteria of the CHA2DS2-VASc, were included. 934 patients were assigned to receive dalteparin twice at 100 UI/kg while another 950 patients were chosen to receive a placebo. The warfarin was suspended five days prior to surgery and was reinitiated the night following the surgery. Meanwhile, bridging therapy and the placebo were initiated three days before the surgery and suspended 24 prior to it. Placebo and bridging therapy were later reinitiated 24 to 72h after the surgery until an INR of 2 was reached. The patients were monitored for between 30 and 37 days following the surgery to assess for possible arterial thromboembolic events, including stroke, TIA, and systemic embolism. As a safety outcome, bleeding was evaluated.
ResultsThe incidence of arterial thromboembolism among the patients who did not receive bridging therapy was 0.4% while for those who did receive it the incidence was 0.3%. This represents absolute differences of 0.1% with a CI 95% (−0.6 to 0.8), a p-value of 0.01 for non-inferiority and a p-value of 0.73 for superiority.
As for the outcome of major bleeding, it occurred in 1.3% of the patients who did not receive bridging therapy, while in the group with bridging therapy it occurred in 3.2%. This means that there was a relative risk of bleeding without bridging therapy of 0.41; CI 95% (0.20–0.78); p-value=0.005.
Comments from reviewersOne of the methodological aspects of this study that is worth analyzing is the approach used to show the noninferiority of bridging therapy as compared to the placebo. This is precisely one the indicators that, for ethical reasons, there is no justification for showing superiority over the placebo. In this study, the sample size was based on the following presumptions:
- 1.
A 1%5 incidence of thromboembolic events in the bridging therapy group and a 1% incidence as well in patients without bridging therapy according to a systematic review and meta-analysis by Dunn, AS.6
- 2.
A margin of non-inferiority of 1.0%.
- 3.
With a margin of differences of 1%, a power of 80%, and two-tailed alpha of 0.05, the required sample size was 1641 patients per arm.
Moreover, the calculations for the sample size tending to determine the differences in major bleeding were based on presumptions of an incidence of 3% for patients with bridging therapy versus an incidence of 1% of major bleeding in patients without bridging therapy. With a sample size of 1641, this would be sufficient to find superiority with a power of 98%. Finally, and after two interim analyses, noninferiority with the outcome of arterial thromboembolism and superiority with the outcome of bleeding were found.
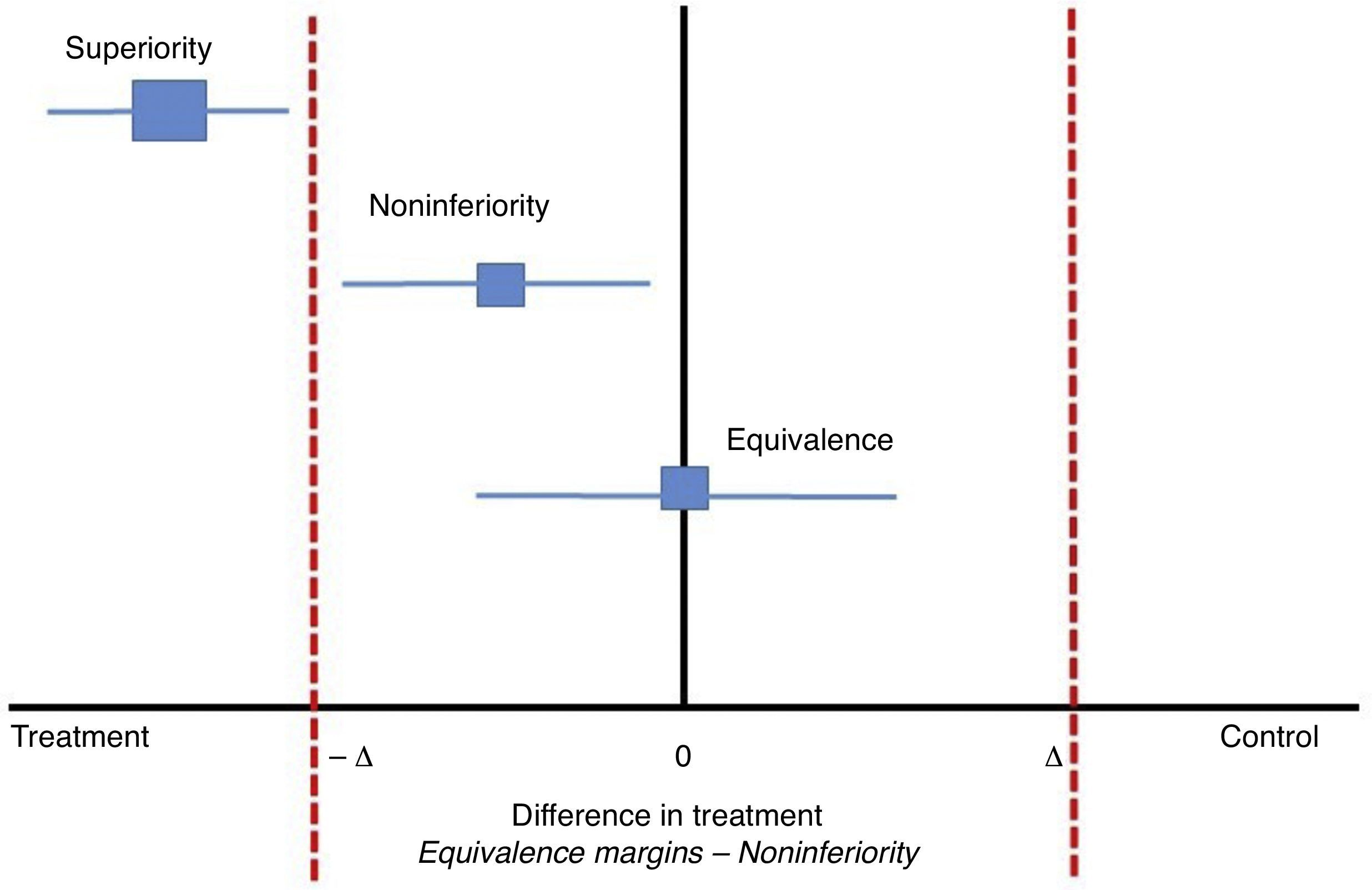
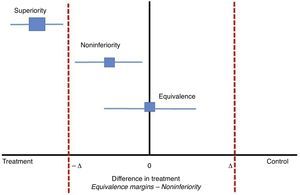
Classically, clinical experiments on parallel groups attempt to find differences between two interventions with respect to an outcome. These outcomes are proposed previously so that the null hypothesis may be rejected if the differences are found. Now, in studies of noninferiority, the proposal is that the new treatment should not be worse than the conventional treatment or, said in a different way, that the new treatment is at least as efficient as the previous one within a range between a negative difference value and zero (−Δ to 0). In classical clinical experiments, this value can be a negative difference if the new intervention is worse than the standard or positive if the new treatment is better than the standard; zero would indicate no difference between the new and standard treatments (−Δ a+Δ). In noninferiority studies, the choice of the differences between the two treatments with respect to the effect of the outcome is crucial (see Fig. 1). Some authors propose that these differences could be half of the measure of the effect.7 Nonetheless, the clinical relevance will take priority over the purely statistical concept.
In studies of noninferiority, the margin of differences would be between −Δ and zero. Adapted from Hahn, S., Understanding noninferiority trials.8
Another important aspect is that, usually, clinical experiments that aim to demonstrate noninferiority test a one-tailed hypothesis. However, this limits the finding of superiority (if there is one). Therefore, some authors recommend testing two-tailed hypotheses.
In some cases, in a clinical experiment, the decision is made to make a change from superiority to noninferiority. In general, this does not lead to problems because it is expected that the confidence interval will exclude the noninferiority margin as well as the zero that reflects no difference. However, in the opposite situation, it would not be valid unless the noninferiority margins were previously defined. Choosing the inferiority margin is one of the most critical aspects in clinical trials. In this analysis, two interim analyses based on the superiority of the two main outcomes were planned. That said, the study ended when noninferiority in the outcome of arterial thromboembolism was shown.
Finally, we can state that, in recent publications, including this one, it is suggested that bridging therapy worsens results in terms of bleeding in surgery while not reducing the risk of embolic events. Thus it is not recommended.1,9
Level of evidenceGrade I B.10
Recommended readingDouketis, J.D et al.11
Hahn, S.8
FundingThe authors did not receive sponsorship to undertake this article
Conflict of interestsThe authors have no conflicts of interest to declare.
Please cite this article as: Oliveros-Rodríguez H, Ruiz-Ávila HA. Comentarios sobre: “Perioperative Bridging Anticoagulation in Patients with Atrial Fibrillation”. Rev Colomb Anestesiol. 2015;43:340–342.