Several remifentanil products are commercialized in Colombia though they have never been compared in a clinical setting.
ObjectiveThe aim of this study was to investigate the pharmacodynamic profile of the branded remifentanil molecule (group O: Glaxo SmithKline Manufacturing S.P.A.) and two unbranded molecules (group A: Laboratorios Chalver de Colombia S.A. and group B: Instituto Biológico Contemporáneo, Argentina) registered in Colombia.
MethodsWe carried out a double-blind, randomized, controlled trial. The branded molecule of remifentanil (group O, n=29) was compared with the two unbranded molecules (group A, n=29; group B, n=32) during anesthetic induction and tracheal intubation in adult patients ASA I without predictors for difficult airway. The target controlled infusion (TCI) doses evaluated were 6, 8 and 10ng/ml with the Minto model. Induction was complemented with propofol 5mcg/ml (TCI) with the Schneider model and rocuronium 0.6mg/kg. The primary outcome was the difference between preintubation (TCI equilirium) and postintubation (maximum measurement within 5min) mean arterial pressure and heart rate.
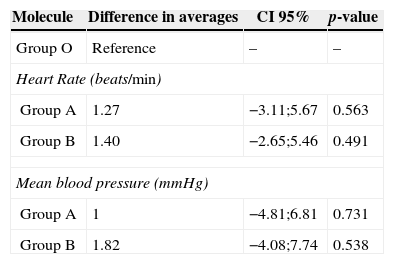
ResultsA similar pharmacodynamic profile was observed in all of the studied remifentanil molecules. The differences in the change in heart rate were 1.27 (95% CI −3.11;5.67) with molecule A and 1.40 (95% CI −2.65;5.46) with molecule B compared to molecule O (beats/min). The differences in the change in mean arterial pressure were 1 (95% CI -4.81;6.81) with molecule A and 1.82 (95% CI −4.08;7.74) with molecule B compared to molecule O (mmHg). There was one case of arterial hypotension in each group.
ConclusionThe results suggest that, from a pharmacodynamic point of view, branded and unbranded remifentanil molecules are similar for laryngoscopy/intubation with TCI doses 6, 8 and 10ng/ml.
En Colombia se comercializan diferentes moléculas de Remifentanil que nunca han sido comparadas en un entorno clínico.
ObjetivoEl objetivo de este estudio fue investigar el perfil farmacodinámico de la molécula innovadora de Remifentanil (grupo O: Glaxo SmithKline Manufacturing S.P.A.) y dos moléculas genéricas (grupo A: Laboratorios Chalver de Colombia S.A. y grupo B: Instituto Biológico Contemporáneo, Argentina) registradas en Colombia.
MétodosSe llevó a cabo un experimento clínico doble ciego, aleatorizado, controlado. Se comparó la molécula original de Remifentanil (grupo O, n=29) frente a las dos moléculas genéricas (grupo A, n=29; grupo B, n=32) durante la inducción anestésica e intubación orotraqueal de pacientes adultos ASA I sin predictores de vía aérea difícil. Se evaluaron las dosis 6, 8 y 10ng/ml (TCI, Target Controlled Infusion) con el modelo de Minto. La inducción se complementó con Propofol 5 mcg/ml (TCI) con modelo de Schneider y Rocuronio 0.6mg/kg. El desenlace primario se evaluó como las diferencias en la presión arterial media y en la frecuencia cardiaca preintubación (momento en que se alcanza la concentración objetivo en sitio efecto) y posintubación (máximo valor alcanzado en 5 minutos).
ResultadosSe observó similitud en el perfil farmacodinámico de las moléculas de Remifentanil estudiadas. Las diferencias en el cambio de frecuencia cardiaca fue de 1.27 (IC 95% −3.11;5.67) con la molécula A y 1.40 (IC 95% −2.65;5.46) con la molécula B frente a la molécula O (latidos/minuto). Las diferencias en el cambio de presión arterial media fue de 1 (CI 95% −4.81;6.81) para la molécula A y 1.82 (IC 95% −4.08;7.74) para la molécula B frente a la molécula O (mmHg). Hubo un caso de hipotensión arterial en cada grupo.
ConclusiónLos resultados sugieren que desde un punto de vista farmacodinámico las moléculas innovadora y genéricas de Remifentanil son similares para la laringoscopia/intubación con dosis TCI de 6, 8 y 10ng/ml.
The anesthetic act involves the administration of medications with specific pharmacological actions directed towards controlling the systemic response to harmful stimuli, including laryngoscopy and intubation1. Several drugs and techniques have been investigated for their ability to modulate this response and the release of catecholamines with these stimuli2,3. Opioid medications are highly effective in the prevention of this type of responses, and, therefore, they are employed both in balanced anesthesia and total intravenous anesthesia4,5. Many opioids, like remifentanil, fulfill an important role in the control of changes in hemodynamic variables as a response to orotracheal intubation2,6,7.
Remifentanil is a potent synthetic opioid of the anilidopiperidine family with unique pharmacological characteristics that make it ideal for laryngoscopy/intubation, mainly its rapid onset and finalization of action when administered in continuous infusion8,9. However, when manual infusion is used, over- or underdosing of the medication is common, which increases the risk of collateral effects. With administration through TCI (Target Controlled Infusion), it is possible to reach target concentrations in the effect-site, optimizing the contribution of the medication and, therefore, the safety profile as well10. In addition, given that TCI systems allow researchers to obtain concentrations of the medication in question in steady state that are very close to the target, thereby controlling pharmacokinetic aspects in experiments, the pharmacodynamic observations are very meaningful for anesthetic clinical pharmacology11.
Frequently, in the clinical setting it is necessary to replace original medications with generic molecules because of costs12,13. These molecules become available for use once the protection that patents offer to original compounds expires. Nevertheless, there is literature that suggests that there are differences in the manufacturing processes that may influence pharmacological behavior, so larger doses are often required to achieve the desired effect14,15.
In Colombia, different molecules of remifentanil are sold, though they have never been compared in a clinical setting. In our hospital there were reports of increases in the amount of remifentanil required for general anesthesia after the introduction of two generic molecules. The hypothesis arose that the observed clinical effects of remifentanil could be the result of differences between the branded molecule and the unbranded molecules of the medication. The objective of this study was to investigate the pharmacodynamic profile of the original, branded remifentanil molecule (group O: Glaxo SmithKline Manufacturing S.P.A.) and of two generic molecules (group A: Laboratorios Chalver de Colombia S.A. and group B: Instituto Biológico Contemporáneo, Argentina) registered in Colombia.
MethodsStudy designThis double-blind, randomized, and controlled clinical experiment was carried out in Hospital de San José (Bogotá) between February 2012 and November 2013. The study was approved by the Ethics Commitee for Research with Human Subjects of the Fundación Universitaria de Ciencias de la Salud and signed informed consent was obtained from each patient before randomization (Clinicaltrials.gov registration NCT02048293).
ParticipantsThe target population consisted of men and women between 18 and 50 years of age, ASA I, that underwent any surgical procedure under general anesthesia with orotracheal intubation. Patients with known hypersensitivity to any drug necessary for total intravenous anesthesia, and those who reported chronic opioid use, were excluded, since this can be associated with a greater amount of anesthetic required for the induction of anesthesia. Also, pregnant women were excluded because of the known pharmacokinetic differences associated with these medications. Patients with predictors of difficult airway could require a procedure other than orotracheal intubation with direct laryngoscopy view and were therefore also excluded.
InterventionsThe branded remifentanil molecule (group O, n=29) was compared with two unbranded molecules (group A, n=29; group B, n32) during induction of anesthesia and orotracheal intubation. The pharmacy unit provided a mix that contained a dilution of one of the three molecules of remifentanil at a concentration of 20 mcg/ml in a volume of 50ml for administration with total intravenous anesthesia. For the anesthesia, total intravenous anesthesia pumps (TIVA ORCHESTRA—BASE PRIMEA, FRESENIUS KABI) were used with the Minto pharmacokinetic model for the administration of remifentantil at TCI concentrations of 6, 8, or 10ng/ml and with the Schneider model for the administration of propofol at a TCI concentration of 5 mcg/mL16. The anesthetic act was performed in a conventional manner: pre-oxygenation until obtaining SO2 of 99%, initiation of propofol infusion until obtaining a target concentration in the effect-site of 5 mcg/mL, initiation of remifentanil infusion until reaching the target concentration in the effect-site in accordance with the allotment corresponding to each case, and administration of rocuronium at a dosage of 0.6mg/kg with previous calibration of the TOF-WATCH monitor. Once the corneal reflex was lost, with jaw relaxation, and a TOF value equal to 0 measured in the adductor of the thumb, the anesthesiologist performed the laryngoscopy/intubation.
OutcomesThe measurements of mean blood pressure (mmHg) and heart rate (beats/min) were employed as the main pharmacodynamic measurements. Blood pressure and heart rate are well known as the most widely used hemodynamic parameters during anesthesia and surgery for evaluating cardiovascular status, especially in moments of the transoperative period with intentional injury, such as orotracheal intubation or the first surgical incision17. Although there are other parameters, like stroke volume, cardiac index, and systemic vascular resistance, that could be more accurate for this evaluation, they have a limited use in the daily practice of anesthesia. Therefore, blood pressure and heart rate are keys for advanced cardiovascular evaluation during anesthesia.
These variables were measured with conventional, non-invasive, monitoring equipment. They were recorded 3 times before intubation (admission to the surgical unity, initiation of monitoring in the operating room, with administration of a balanced dose of TCI remifentanil) and 6 times during (laryngoscopy) and after orotracheal intubation (minutes 1, 2, 3, 4, and 5). The pharmacodynamic outcome was calculated as the difference between base values (moment in which the target concentration in the effect-site for the remifentanil was reached), and the maximum value reached in the subsequent measurements (until minute 5).
As a secondary outcome, the intubation conditions were evaluated with the Cooper scale. This scale qualifies the conditions of orotracheal intubation as “excellent”, “good”, “fair”, or “poor”. It evaluates the relaxation of the jaw, the condition of the vocal cords, and response to intubation18. Finally, each patient's age, sex, weight, and height were also recorded.
Sample sizePrevious information relating to calculating sample size was not available. Taking into account our patient recruitment capacity, a sample of 30 patients per remifentanil molecule was predefined. With this sample size, we would be able to detect a difference of 5 beats/min between the original molecule and generic molecules with a power of 80% and a level of significance of 0.05 if a standard deviation of 7 was assumed for the outcome of heart rate.
Randomization and blindingThe randomization plan was created using the randomization website randomization.com (http://www.randomization.com) created by Gerald E. Dallal, PhD, on July 16, 2008. A simple randomization of the three molecules was made for the three commonly used doses of remifentanil in our hospital (6, 8, and 10ng/ml).
The patient, the anesthesiologist, and the researchers were unaware of the producer of the molecule of remifentanil used for induction of anesthesia. The type of molecule was only known to the Pharmacy Unit of Hospital de San José. The Pharmacy Unit personnel did not participate in the study design, in the administration of the medication, data collection, or in the data analysis.
Statistical analysisThe measures of central tendency and dispersion were employed for the quantitative variables, and absolute values and percentages were used for the categorical values. For comparisons between the base and maximum changes in heart rate and blood pressure between the original and generic remifentanil molecules, Student's t-test was used, since it is considered that these physiological variables present a normal distribution in ASA I populations. Differences were considered to be statistically significant with p-values <0.05.
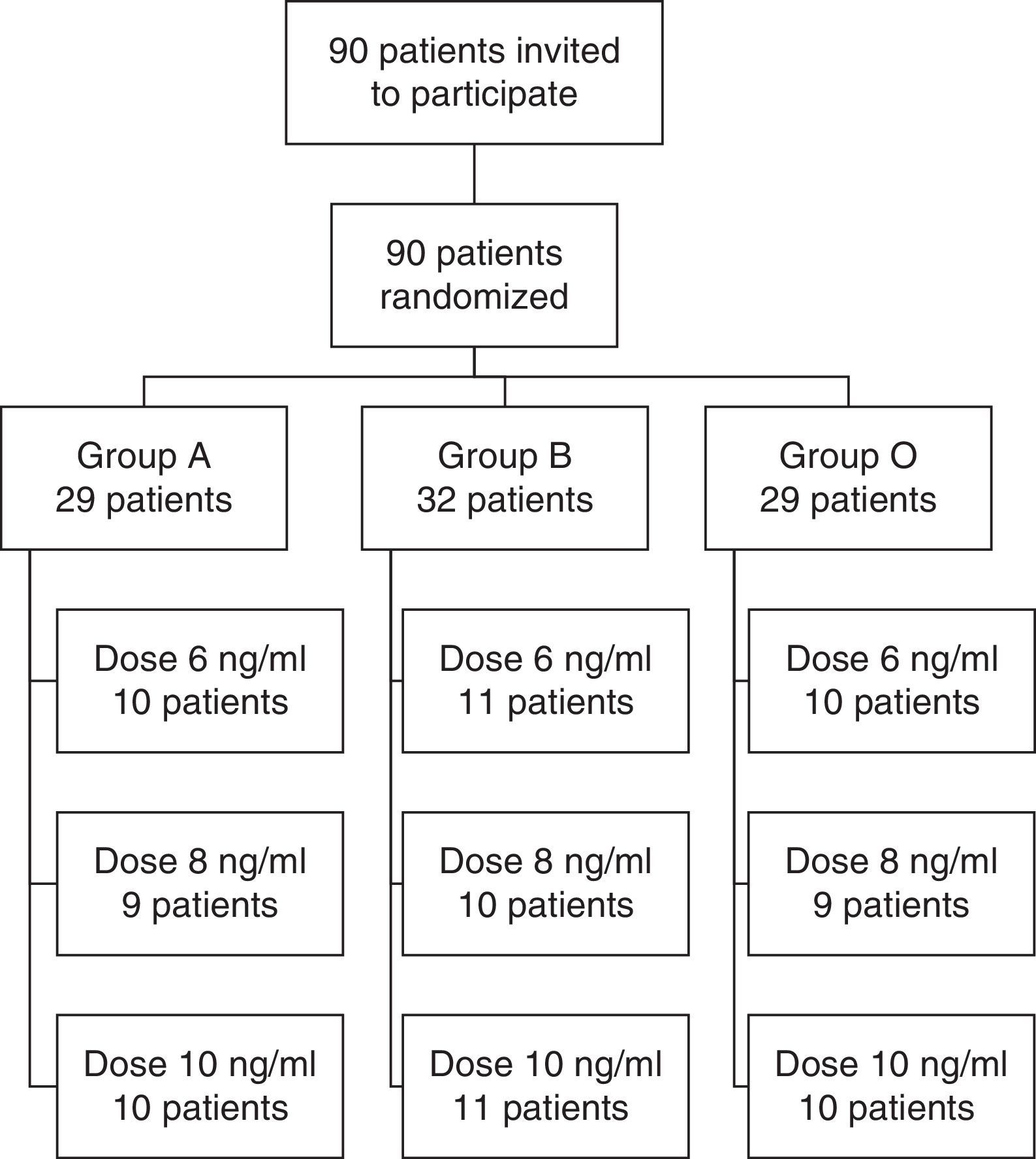
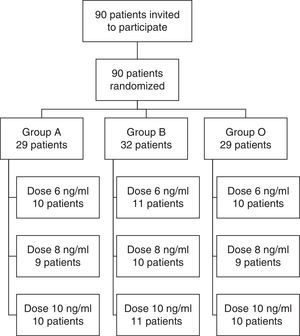
ResultsA total of 90 patients were randomized between March and August 2013. Fig. 1 shows a flow chart of the participants and the results of the randomization. All patients invited to the study signed an informed consent form and were included in the analysis.
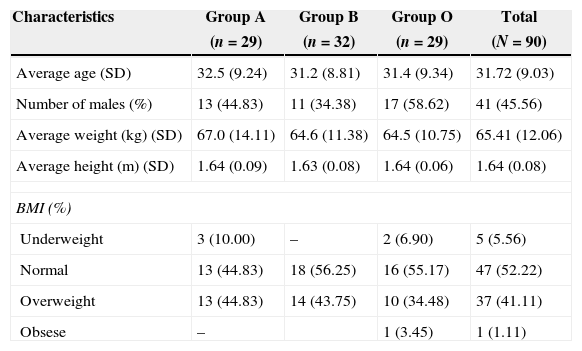
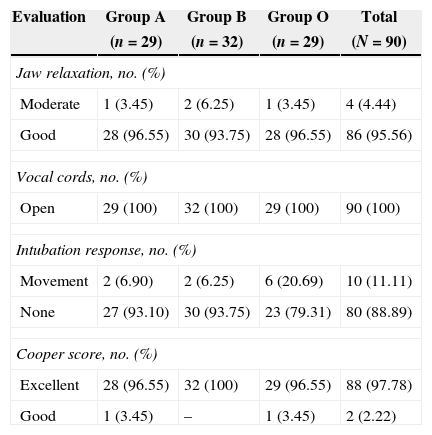
Table 1 shows the characteristics of the patients by their random assignment. On average, the patients were middle aged, the male:female ratio was approximately 1:1.1. The majority of the patients had a body mass index between normal and overweight. Table 2 shows the results of the intubation conditions (Cooper score). The intubation conditions were satisfactory in all patients, regardless of the medication or dosage used. In each treatment group, one episode of low blood pressure occurred. In addition, an episode of psychomotor agitation was observed in group A, and an episode of postoperative hyperalgesia was observed in group B.3 and 4
Patient characteristics.
| Characteristics | Group A | Group B | Group O | Total |
|---|---|---|---|---|
| (n=29) | (n=32) | (n=29) | (N=90) | |
| Average age (SD) | 32.5 (9.24) | 31.2 (8.81) | 31.4 (9.34) | 31.72 (9.03) |
| Number of males (%) | 13 (44.83) | 11 (34.38) | 17 (58.62) | 41 (45.56) |
| Average weight (kg) (SD) | 67.0 (14.11) | 64.6 (11.38) | 64.5 (10.75) | 65.41 (12.06) |
| Average height (m) (SD) | 1.64 (0.09) | 1.63 (0.08) | 1.64 (0.06) | 1.64 (0.08) |
| BMI (%) | ||||
| Underweight | 3 (10.00) | – | 2 (6.90) | 5 (5.56) |
| Normal | 13 (44.83) | 18 (56.25) | 16 (55.17) | 47 (52.22) |
| Overweight | 13 (44.83) | 14 (43.75) | 10 (34.48) | 37 (41.11) |
| Obsese | – | 1 (3.45) | 1 (1.11) | |
SD: standard deviation; kg: kilograms; m: meters; BMI: body mass index.
Source: Authors.
Intubation conditions (Cooper scale).
| Evaluation | Group A | Group B | Group O | Total |
|---|---|---|---|---|
| (n=29) | (n=32) | (n=29) | (N=90) | |
| Jaw relaxation, no. (%) | ||||
| Moderate | 1 (3.45) | 2 (6.25) | 1 (3.45) | 4 (4.44) |
| Good | 28 (96.55) | 30 (93.75) | 28 (96.55) | 86 (95.56) |
| Vocal cords, no. (%) | ||||
| Open | 29 (100) | 32 (100) | 29 (100) | 90 (100) |
| Intubation response, no. (%) | ||||
| Movement | 2 (6.90) | 2 (6.25) | 6 (20.69) | 10 (11.11) |
| None | 27 (93.10) | 30 (93.75) | 23 (79.31) | 80 (88.89) |
| Cooper score, no. (%) | ||||
| Excellent | 28 (96.55) | 32 (100) | 29 (96.55) | 88 (97.78) |
| Good | 1 (3.45) | – | 1 (3.45) | 2 (2.22) |
Source: Authors.
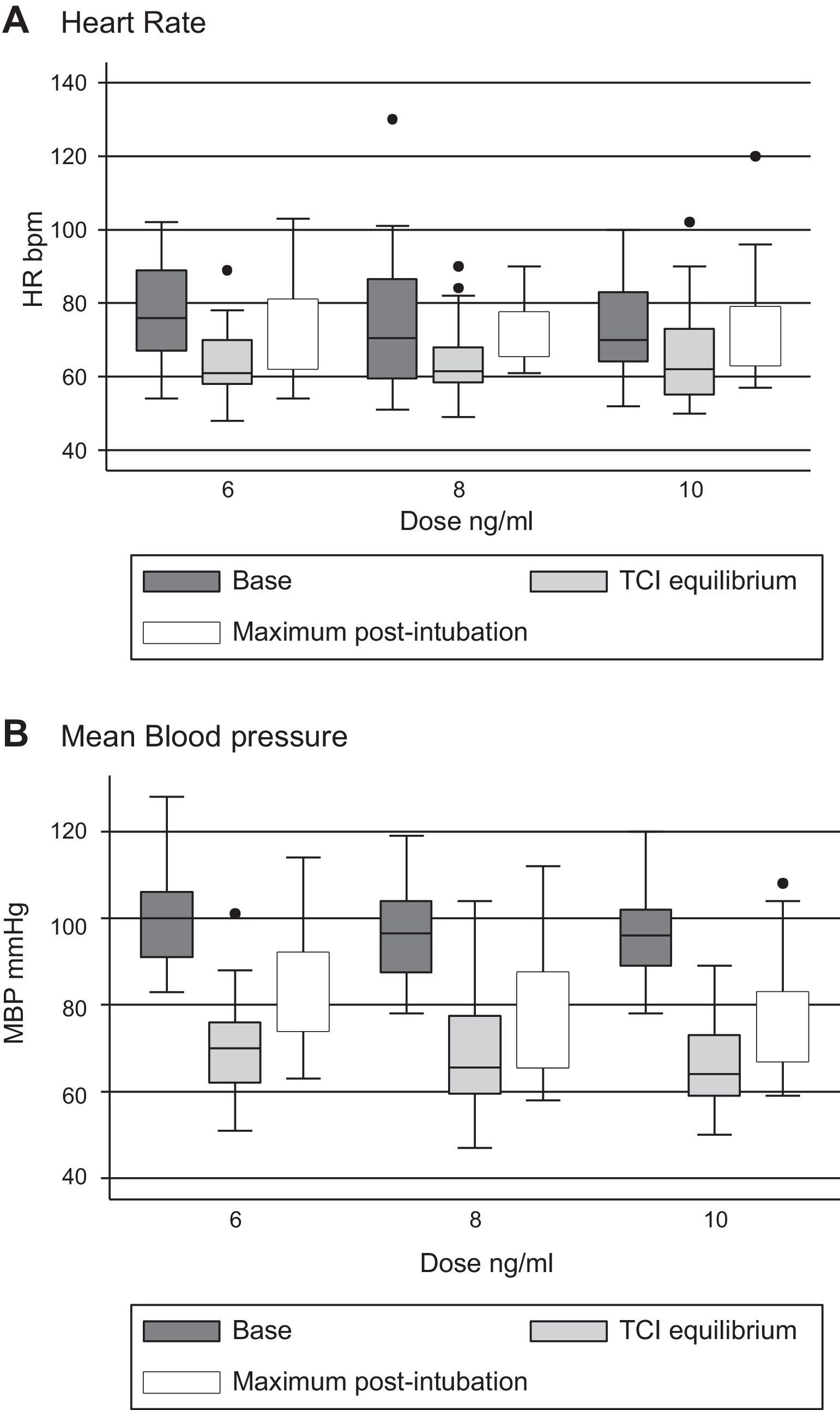
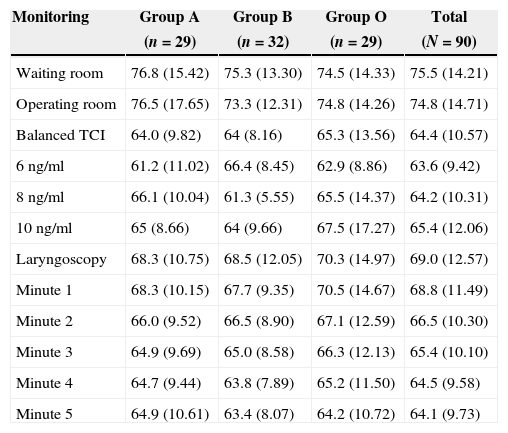
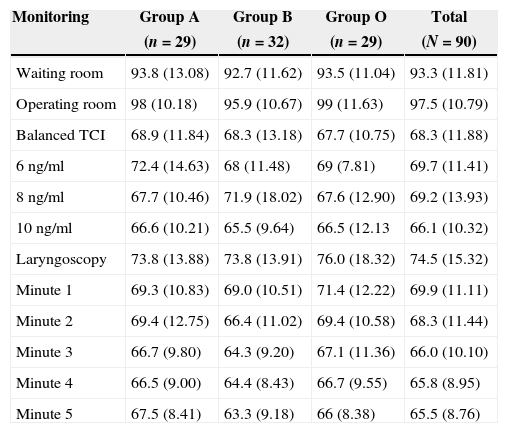
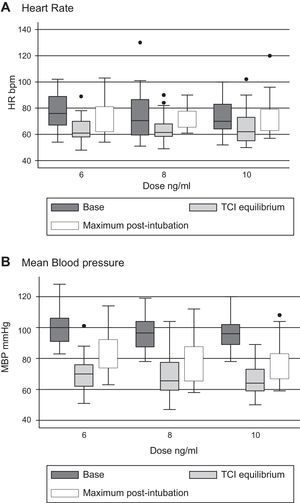
Tables 3 and 4 show the behavior of heart rate and average blood pressure, respectively. In all three treatment groups, a progressive reduction of these hemodynamic variables during the monitoring time is evident. Nevertheless, it was not necessary to reduce the dosage of remifentanil in any of the three groups, since these reductions amounted to 0.5% for heart rate and 4% for mean blood pressure with respect to the base values (moment of TCI equilibrium). In the same way, though the event of interest—laryngoscopy/intubation—can be associated with an increase in the values of heart rate and mean blood pressure, these increases amounted to 7% and 9% respectively with respect to the base values. As such, increased remifentanil was not required (Fig. 2).
Heart rate monitoring.
| Monitoring | Group A | Group B | Group O | Total |
|---|---|---|---|---|
| (n=29) | (n=32) | (n=29) | (N=90) | |
| Waiting room | 76.8 (15.42) | 75.3 (13.30) | 74.5 (14.33) | 75.5 (14.21) |
| Operating room | 76.5 (17.65) | 73.3 (12.31) | 74.8 (14.26) | 74.8 (14.71) |
| Balanced TCI | 64.0 (9.82) | 64 (8.16) | 65.3 (13.56) | 64.4 (10.57) |
| 6ng/ml | 61.2 (11.02) | 66.4 (8.45) | 62.9 (8.86) | 63.6 (9.42) |
| 8ng/ml | 66.1 (10.04) | 61.3 (5.55) | 65.5 (14.37) | 64.2 (10.31) |
| 10ng/ml | 65 (8.66) | 64 (9.66) | 67.5 (17.27) | 65.4 (12.06) |
| Laryngoscopy | 68.3 (10.75) | 68.5 (12.05) | 70.3 (14.97) | 69.0 (12.57) |
| Minute 1 | 68.3 (10.15) | 67.7 (9.35) | 70.5 (14.67) | 68.8 (11.49) |
| Minute 2 | 66.0 (9.52) | 66.5 (8.90) | 67.1 (12.59) | 66.5 (10.30) |
| Minute 3 | 64.9 (9.69) | 65.0 (8.58) | 66.3 (12.13) | 65.4 (10.10) |
| Minute 4 | 64.7 (9.44) | 63.8 (7.89) | 65.2 (11.50) | 64.5 (9.58) |
| Minute 5 | 64.9 (10.61) | 63.4 (8.07) | 64.2 (10.72) | 64.1 (9.73) |
TCI: target controlled infusion.
Source: Authors.
Monitoring of mean blood pressure.
| Monitoring | Group A | Group B | Group O | Total |
|---|---|---|---|---|
| (n=29) | (n=32) | (n=29) | (N=90) | |
| Waiting room | 93.8 (13.08) | 92.7 (11.62) | 93.5 (11.04) | 93.3 (11.81) |
| Operating room | 98 (10.18) | 95.9 (10.67) | 99 (11.63) | 97.5 (10.79) |
| Balanced TCI | 68.9 (11.84) | 68.3 (13.18) | 67.7 (10.75) | 68.3 (11.88) |
| 6ng/ml | 72.4 (14.63) | 68 (11.48) | 69 (7.81) | 69.7 (11.41) |
| 8ng/ml | 67.7 (10.46) | 71.9 (18.02) | 67.6 (12.90) | 69.2 (13.93) |
| 10ng/ml | 66.6 (10.21) | 65.5 (9.64) | 66.5 (12.13 | 66.1 (10.32) |
| Laryngoscopy | 73.8 (13.88) | 73.8 (13.91) | 76.0 (18.32) | 74.5 (15.32) |
| Minute 1 | 69.3 (10.83) | 69.0 (10.51) | 71.4 (12.22) | 69.9 (11.11) |
| Minute 2 | 69.4 (12.75) | 66.4 (11.02) | 69.4 (10.58) | 68.3 (11.44) |
| Minute 3 | 66.7 (9.80) | 64.3 (9.20) | 67.1 (11.36) | 66.0 (10.10) |
| Minute 4 | 66.5 (9.00) | 64.4 (8.43) | 66.7 (9.55) | 65.8 (8.95) |
| Minute 5 | 67.5 (8.41) | 63.3 (9.18) | 66 (8.38) | 65.5 (8.76) |
TCI: target controlled infusion.
Source: Authors.
The results of the hypothesis tests are shown in Table 5. No statistically significant differences were found in the pharmacodynamic profiles of the molecules of remifentanil studied. Nevertheless, the generic molecules presented a small increase in the records of the studied variables compared to the original molecule.
Comparison of maximum changes in heart rate and blood pressure with the original and generic molecules of remifentanil.
| Molecule | Difference in averages | CI 95% | p-value |
|---|---|---|---|
| Group O | Reference | – | – |
| Heart Rate (beats/min) | |||
| Group A | 1.27 | −3.11;5.67 | 0.563 |
| Group B | 1.40 | −2.65;5.46 | 0.491 |
| Mean blood pressure (mmHg) | |||
| Group A | 1 | −4.81;6.81 | 0.731 |
| Group B | 1.82 | −4.08;7.74 | 0.538 |
Source: Authors.
In this study, the branded molecule and the unbranded molecules of remifentanil evaluated produced a reduction in blood pressure and heart rate during the induction of anesthesia and controlled the hemodynamic response to laryngoscopy and intubation. These results indicate that the generic molecules available on the market in Colombia and compared here have a pharmacodynamic profile that is similar to the original, branded molecule, contrary to our hypothesis based on reports of problems of effectiveness that preceded the study.
Opioids are useful, and widely used by anesthesiologists, as adjuvants prior to laryngoscopy and orotracheal intubation to prevent increases in blood pressure and heart rate17. In previous studies, there are reports that, in some cases, the synergistic effect of remifentanil with other drugs used in the induction of anesthesia produces important reductions in hemodynamic response, and, for this reason, adjustments of dosage or of volume support or vasoactive therapy were required19. In this study, no differences were observed in the use of the three different doses of the medication through the total intravenous anesthesia technique with TCI. We believe that this lack of difference may be due to the fact that the patients included in the experiment were young, relatively healthy, without major comorbidities, and with an adequate cardiac reserve.
Several studies suggested that the maximum hemodynamic response occurred between 1 and 5minutes after orotracheal intubation3,20. Therefore, we measured blood pressure and heart rate starting at admission to the surgical unit until 5minutes after intubation. However, we did not find a specific point in time of maximum response. In addition, we found no exaggerated response due to the fact that there was adequate suppression, even with the lowest target concentrations used (6ng/ml TCI). We believe that this may be due to the differences between the technique with TCI and other conventional methods of drug administration. The peak effect of remifentanil is achieved at 1.6minutes8. It is only after this period that an adequate concentration in the effect-site can be guaranteed. And, if laryngoscopy/intubation is performed early, the target of the opioid administration is not reached.
Among the limitations of this study are that heart rate and blood pressure are subject to large variability between patients. This may have affected the power of the study to detect statistically significant differences between the groups studied. Taking into account that the standard deviation observed was greater, we estimate that the power of the study to detect changes of 5 beats/min was reduced to 50% Furthermore, the patients presented variability with respect to the time needed to reach a value of 0 in the TOF monitor, which was a requirement for performing the laryngoscopy. This could have modified the time between the moment in which the target concentration of the medication was reached and the laryngoscopy/intubation. As such, some patients were exposed to high doses of propofol and remifentanil for more time, which would excessively modulate the hemodynamic response.
ConclusionThe results show, for the first time in a randomized clinical trial, a pharmacodynamic similarity between the original remifentanil molecule and two generic molecules sold in Colombia. Differences in effect-site concentrations employed with the Minto model had no apparent clinical impact. The high degree of similarity in the behavior of the variables of mean blood pressure and heart rate suggest the possibility of comparing the results of clinical studies carried out with any of these three molecules. Nevertheless, monitoring of future reports of failure in the modulation of hemodynamic response to intubation with generic molecules could lead to clear hypotheses with respect to the causes of these failures (e.g. defects in specific batches of medication).
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThe present study was funded with resources from the Department of Anesthesiology, San José Hospital, Bogotá, Colombia.
Conflict of interestThe authors have no conflicts of interest to declare.
The authors wish to thank the Department of Pharmacy for their participation in the randomization and blinding process.
In the Research Group DEORUMOPUS.
Please cite this article as: Muñoz LA, Reyes LE, Niño CG, Gomez WG, Diaz WR, Romero JC, et al. Comparación de los Perfiles Farmacodinámicos de Tres Moléculas de Remifentanilo en cuanto a su Respuesta Hemodinámica a las Maniobras de Laringoscopia e Intubación Traqueal. Rev Colomb Anestesiol. 2015;43:186–193.