Refractory hypoxaemia is a critical, life-threatening condition if not resolved promptly. The flow phenomenon is implicated in its development.
ObjectivesClinical case presentation and non-systematic review of the literature on refractory hypoxaemia and flow phenomenon. Incidence, aetiology and pathophysiology are described.
Materials and methodsClinical case presentation authorised by the Ethics Committee of our institution of a young patient who presented with progressive dyspnoea, reaching functional class IV/IV. A search of the literature was conducted in Pub Med, Scielo and Bireme.
ResultsThere is a growing interest in the physiological flow phenomenon leading to the development of refractory hypoxaemia in the absence of increased pressure in the cardiac cavities. Few reports are found.
ConclusionsFlow phenomenon-related refractory hypoxaemia must be suspected as an exclusion diagnosis in patients with a mediastinal mass. Prone ventilation is proposed as a bridging therapy in order to revert a life-threatening condition.
La hipoxemia refractaria es una condición crítica potencialmente mortal si no se corrige rápidamente. El fenómeno de flujo está implicado en su desarrollo.
ObjetivosPresentación de un caso clínico y revisión no sistemática de la literatura sobre hipoxemia refractaria y el fenómeno de flujo, describimos su incidencia, etiología y fisiopatología.
Material y métodosCon autorización del comité de Ética de nuestra institución, presentamos un caso clínico de un paciente joven quien consulto por disnea progresiva hasta clase funcional IV/IV. La búsqueda bibliográfica se realizó en Pub Med, Scielo y Bireme.
ResultadosSe ha despertado un creciente interés en el fenómeno fisiológico de flujo que conlleva al desarrollo de hipoxemia refractaria en ausencia de aumento de presión en las cavidades cardiacas. Se encuentran escasos reportes.
ConclusionesLa hipoxemia refractaria por el fenómeno de flujo debe ser sospechada como diagnóstico de exclusión en pacientes con masa mediastinal. Proponemos la ventilación en prono como terapia puente para revertir una condición potencialmente mortal.
Hypoxaemia is characterised by a partial oxygen pressure in arterial blood lower than 60mmHg. Under normal conditions, the circulatory and respiratory systems work together to keep it within normal ranges. The absence of homeostasis in those systems to ensure normal oxygen content in arterial blood is known as hypoxaemic respiratory failure and is explained on the basis of five pathophysiological mechanisms, namely: inspired oxygen pressure reduction, development of alveolar hypoventilation, right-to-left shunting, alveolar-capillary exchange membrane diffusion abnormalities, and dead space development. Prompt identification of its aetiology is the best strategy for instituting effective treatment.1–4
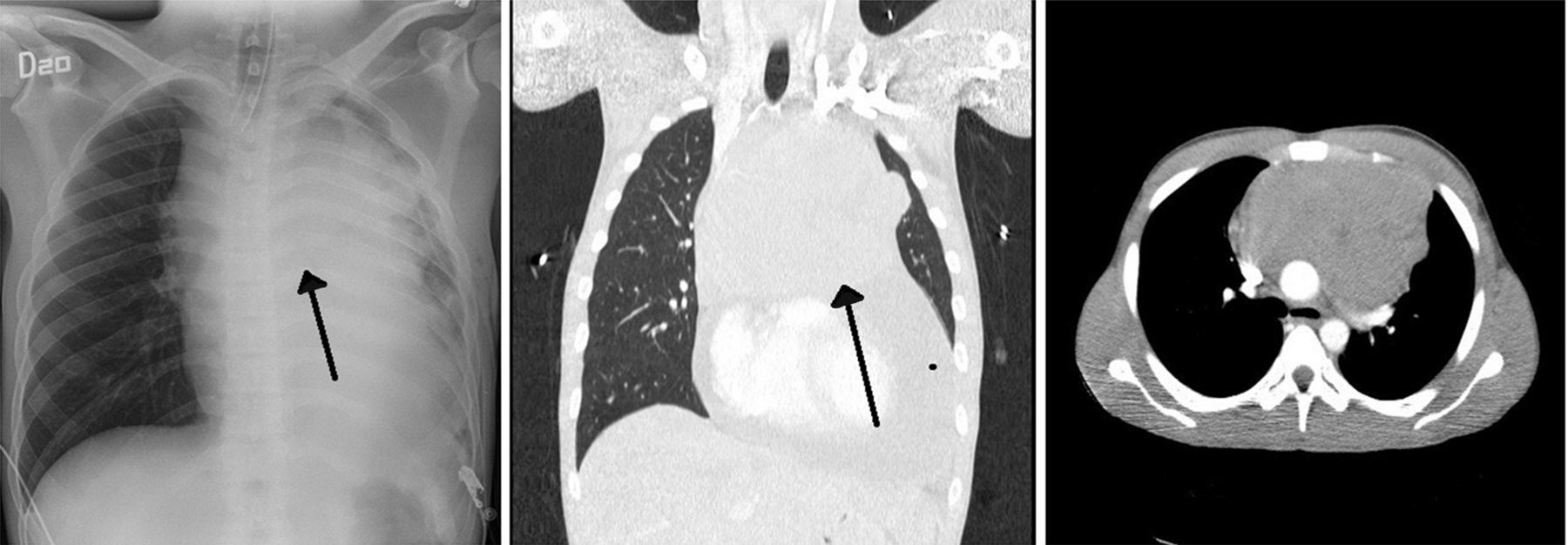
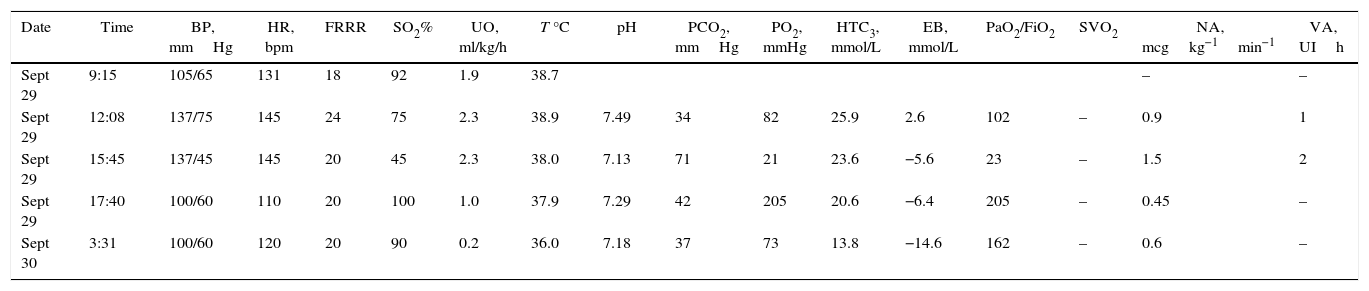
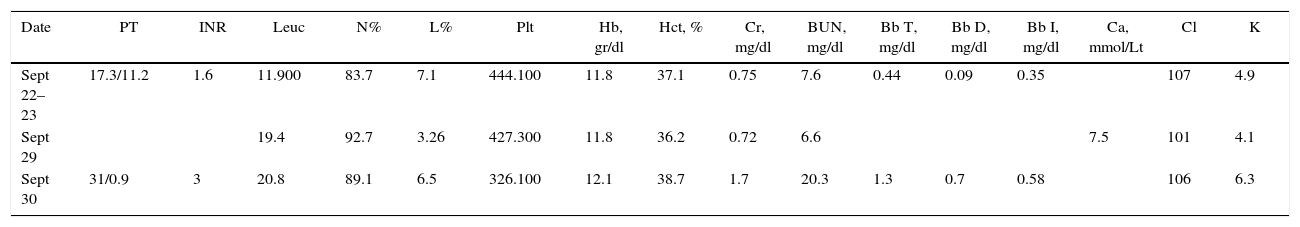
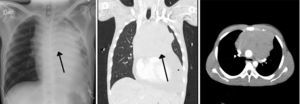
Case descriptionA 23-year old male patient, construction worker of mestizo ethnic origin considered otherwise previously healthy who presented with progressive dyspnoea that had lasted for 30 days, oppressive left chest pain not exacerbated by exercise and weight loss of approximately 2kg over the previous three months. On physical examination, the patient was tachycardic but haemodynamically stable, with evidence of pericardial rub, hepatomegaly, splenomegaly and jugular engorgement grade II. The chest X-ray made in the emergency service revealed a very enlarged cardio-mediastinal silhouette. Echocardiography showed pericardial effusion with normal biventricular function, undilated cavities, no signs of tamponade, and intact septum. A suspected anterior mediastinal mass prompted an axial chest computed tomography scan which confirmed the presence of a 15cm×11cm mass (see Fig. 1). Pericardiocentesis was performed with removal of 750cc of blood, and an ultrasound-guided biopsy was performed. Later, the patient went into rapid clinical decline due to respiratory distress and hypoxaemia, leading to the suspicion of pneumothorax. Clinical confirmation was obtained from the general surgeon, and left thoracostomy was performed. The patient was then transferred to the intensive care unit (ICU) for haemodynamic monitoring. After initial stability, the patient exhibited signs of respiratory distress, hypoxaemia and fever. The decision was made to initiate invasive ventilation support, awake intubation due to the risk of airway collapse from extrinsic compression, blood gases control in acid–base balance with moderate/severe oxygenation disorder. The use of vasopressors was also initiated. Tables 1 and 2 show the haemodynamic profile, blood gases and laboratory results, respectively.
Haemodynamic profile, blood gases and vasopressor support.
| Date | Time | BP, mmHg | HR, bpm | FRRR | SO2% | UO, ml/kg/h | T °C | pH | PCO2, mmHg | PO2, mmHg | HTC3, mmol/L | EB, mmol/L | PaO2/FiO2 | SVO2 | NA, mcgkg−1min−1 | VA, UIh |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sept 29 | 9:15 | 105/65 | 131 | 18 | 92 | 1.9 | 38.7 | – | – | |||||||
| Sept 29 | 12:08 | 137/75 | 145 | 24 | 75 | 2.3 | 38.9 | 7.49 | 34 | 82 | 25.9 | 2.6 | 102 | – | 0.9 | 1 |
| Sept 29 | 15:45 | 137/45 | 145 | 20 | 45 | 2.3 | 38.0 | 7.13 | 71 | 21 | 23.6 | −5.6 | 23 | – | 1.5 | 2 |
| Sept 29 | 17:40 | 100/60 | 110 | 20 | 100 | 1.0 | 37.9 | 7.29 | 42 | 205 | 20.6 | −6.4 | 205 | – | 0.45 | – |
| Sept 30 | 3:31 | 100/60 | 120 | 20 | 90 | 0.2 | 36.0 | 7.18 | 37 | 73 | 13.8 | −14.6 | 162 | – | 0.6 | – |
BP, blood pressure; HR, heart rate; UO, urinary output; SO2, arterial oxygen saturation; T, temperature; EB, excess base; SVO2, venous oxygen saturation; NA, noradrenaline; VA, vasopressin; N/A, not available.
Source: authors.
Laboratory tests.
| Date | PT | INR | Leuc | N% | L% | Plt | Hb, gr/dl | Hct, % | Cr, mg/dl | BUN, mg/dl | Bb T, mg/dl | Bb D, mg/dl | Bb I, mg/dl | Ca, mmol/Lt | Cl | K |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sept 22–23 | 17.3/11.2 | 1.6 | 11.900 | 83.7 | 7.1 | 444.100 | 11.8 | 37.1 | 0.75 | 7.6 | 0.44 | 0.09 | 0.35 | 107 | 4.9 | |
| Sept 29 | 19.4 | 92.7 | 3.26 | 427.300 | 11.8 | 36.2 | 0.72 | 6.6 | 7.5 | 101 | 4.1 | |||||
| Sept 30 | 31/0.9 | 3 | 20.8 | 89.1 | 6.5 | 326.100 | 12.1 | 38.7 | 1.7 | 20.3 | 1.3 | 0.7 | 0.58 | 106 | 6.3 |
| Date | AST U/L | ALT U/L | Alkaline phosphatase | Na, mmol/L | Lactate, mmol/L | Phosphorus | Magnesium | Uric acid |
|---|---|---|---|---|---|---|---|---|
| Sept 22–23 | 22 | 13 | 51 | 135 | 1.2 | |||
| Sept 29 | 132 | 6.5 | 2.0 | |||||
| Sept 30 | 134 | 6.5 | 5.9 |
PT, prothrombin time; INR, International Normalised Ratio; Leu, leukocytes; N, neutrophils; L, lymphocytes; Plt, platelets; Proc, procalcitonin; Hb, haemoglobin; Hct, hematocrit; Cr, creatinine; BUN, blood urea nitrogen; Bb, bilirubin; T, total; D, direct; I, indirect; AST, aspartate aminotransferase; ALT, alanine aminotransferase; Na, sodium; K, potassium; Cl, chlorine; Ca, calcium; P, phosphorus.
Source: authors.
The ventilation mode used was controlled-assisted with 50% fraction of inspired oxygen and a positive end-expiratory pressure (PEEP) of 8. There was initial improvement with a saturation greater than 90%. One hour later the patient went again into progressive hypoxaemia with no improvement despite FiO2 titration at 100%. Blood gases showed a pH of 7.31, PaO2 of 21mmHg, PaCO2 of 71mmHg and base excess of less than 5, with a PaO2/FiO2 ratio of 23. On chest X-ray there was partial atelectasis of the upper lobe that did not explain the current picture. Lung recruitment with PEEP titration was tried with no improvement. A suspected acute pulmonary embolism prompted performance of an angio-CT which was not available at the time, but given the urgency, a pulmonary angiography was performed instead, and was normal. Bacteraemia was suspected considering the presence of fever and clinical decline, and antibiotic therapy was initiated.
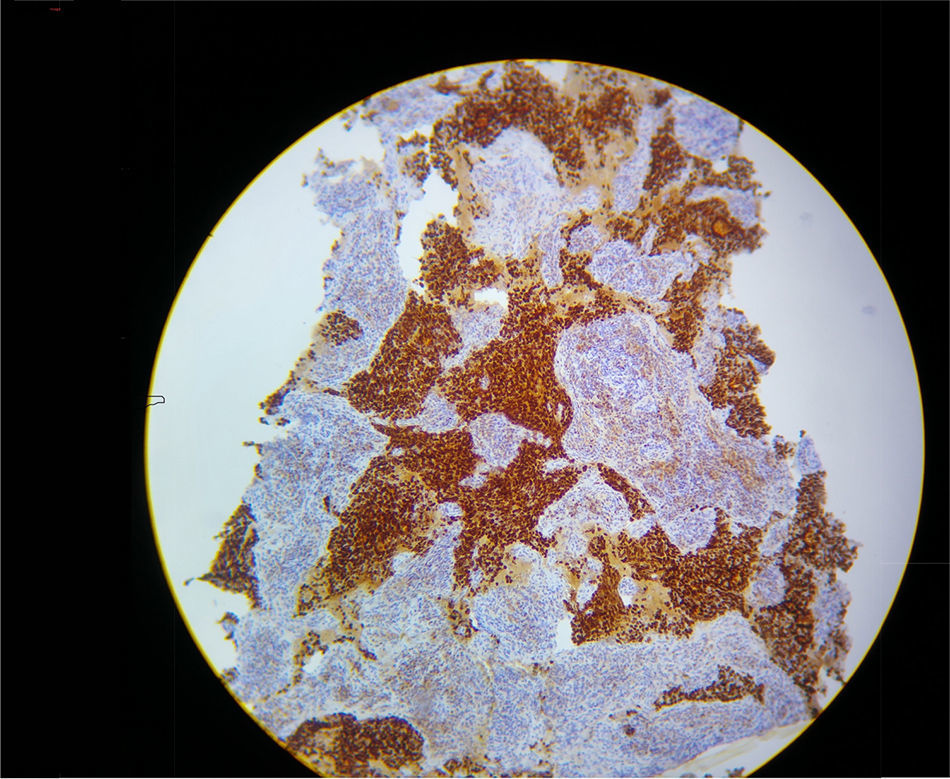
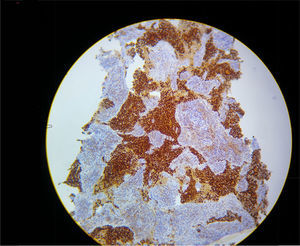
In view of refractory hypoxaemia, and having tried all the ventilation resources described, with adequate sedation and thinking about a mechanical cause, the patient was put on prone ventilation. This resulted in almost immediate recovery of oxygenation, with the following blood gases: pH 7.29, PaO2 205mmHg, PaCO2 42mm Hg and excess base of – 6.9 with a PaO2/FiO2 ratio of 205. Severe hypoxaemia was estimated to have lasted 2h. Later, hyperkalemia, hypercalcemia and hyperphosphatemia were documented, constituting a tumour lysis syndrome with progressive shock that did not respond to management, resulting in death due to multiple organ dysfunction. The final pathology results confirmed the presence of an adenocarcinoma of the thymus (see Fig. 2).
DiscussionLife-threatening severe hypoxaemia requires prompt differential diagnosis in order to institute effective, live-saving treatment.1,2 If pathophysiological causes are considered at first, including low oxygen barometric pressure and development of alveolar hypoventilation, they are not consistent with the clinical context described. The patient was in an external environment with adequate fraction of inspired oxygen (with no history of immersion or drowning). Moreover, it is not consistent with hypoventilation because the problem persisted even after starting mechanical ventilation with adequate minute volume as evidenced by normal initial PCO2 values.3,4 (See Table 1.) There are another three pathophysiological causes to consider, namely, dead space, exchange membrane diffusion abnormalities, and right-to-left shunting. As part of the diagnostic approach, the chest X-ray found partial left upper lobe atelectasis, ruled out the presence of alveolar infiltrates and, given the patient's history and absence of fibrosis, exchange membrane diffusion abnormality is improbable.5,6 An acute dead space was considered but diseases that cause it are few, and in the context of the patient, given the history of a tumour in progression, the only related cause is pulmonary thromboembolism,7,8 which was ruled out by pulmonary angiography. The most probable exclusion diagnosis is right-to-left shunting, characterised physiologically precisely by absence of response to treatment with elevated FiO2. But how could this patient have an acute shunt if the prior echocardiogram was normal? Since 2000, it has been described in the literature that high right heart pressures are not needed for a shunt to develop.9,10 In fact, the so called “flow phenomenon” has been described in which a persistent atrial Eustachian valve remnant may direct blood flow from the inferior vena cava preferentially towards the persistent foramen ovale. In the presence of physical conditions that change the relative spatial position of the heart in the mediastinum, “stretching” of the foramen ovale may occur, allowing for partial opening and giving rise to the flow phenomenon.11,12 The appearance of a pneumothorax, the placement of a thoracic tube and the generation of atelectasis were mechanical phenomena that probably contributed to the genesis of the phenomenon. This is also supported by the finding that the problem was solved only with changes in patient position; when placed in prone ventilation, hypoxaemia was reverted almost immediately, reaching a PaO2/FiO2 of 205.13–15 Therefore, this case is a report supporting the description of this flow phenomenon in the literature. We were unable to demonstrate the presence of a physical shunt, but we believe that the diagnostic approach is sufficiently broad as to consider it as an exclusion diagnosis. It is noteworthy that the simple therapeutic intervention of prone ventilation may improve the critical oxygenation disorder, and it is proposed as a live-saving bridging therapy before surgical management.16,17
ConclusionsFlow phenomenon-related refractory hypoxaemia must be suspected as an exclusion diagnosis in patients with a mediastinal mass. Ventilation in prone position is proposed as a bridging therapy to revert a life-threatening condition.
Patient perspectiveThe patient's family reported to have received adequate medical attention.
Ethical responsibilityProtection of humans and animal subjectsThe authors declare not having performed any experiments in humans or animals for this research.
Confidentiality of dataThe authors declare having followed the protocols of their institutions regarding patient data disclosure.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects to whom the paper refers. The corresponding author is in possession of this document.
FundingThe authors did not receive any form of sponsorship in preparing this paper.
Conflict of interestThe authors declare having no conflict of interest.
Please cite this article as: Laverde-Sabogal CE, Espinosa-Almanza CJ. Fenómeno de flujo, diagnóstico diferencial de la hipoxemia refractaria en pacientes con masa mediastinal anterior. Reporte de caso. Rev Colomb Anestesiol. 2017;45:66–70.