The administration of potassium solutions may result in hyperpotassemia during surgery; normal saline solution (NSS) traditionally used in renal transplant may cause hyperchloremic acidosis.
ObjectiveTo compare the safety of Lactated Ringer's (LR) against NSS in renal transplantation.
Search strategyA systematic review was completed on Central Cochrane Registry – controlled trials, Medline, Lilacs, EBSCO and Embase, accessing review articles and contacting expert clinicians. There was no language restriction.
Selection criteriaRandomized controlled trials on adult patients undergoing renal transplantation.
Data collection and analysisIndependent trial selection, quality assessment and data extraction were performed. The mean differentials were estimated with a 95% confidence interval (95% CI). Heterogeneity was evaluated with statistic I-square (I2) and the fixed and random effect models were used.
ResultsFour trials with a total of 237 patients were included. At the end of surgery, the potassium differential was non-significant (means difference (MD: −0.26mEq/L; CI 95%: −0.58 to 0.05 p=0.10; I2=75%); the pH was lower in the NSS group (MD: 0.06; CI 95%: 0.05–0.08; p<0.001; I2=17%). No difference in Creatinine was identified on the third postoperative day (MD: −0.05; CI 95%: −0.59 to 0.48; p=0.85; I2=0%).
ConclusionsThe use of RL vs. NSS during the renal transplantation perioperative period results in lower potassium and chloride levels and a higher pH, with no significant Creatinine changes.
La administración de soluciones con potasio puede causar hiperpotasemia durante cirugía, la Solución Salina Normal (SSN), usada tradicionalmente en trasplante renal, puede generar acidosis hiperclorémica.
ObjetivoComparar la seguridad del Lactato de Ringer (LR) con SSN en trasplante renal.
Estrategia de búsquedaSe realizó una revisión sistemática en el Registro Cochrane Central de ensayos controlados, Medline, Lilacs, EBSCO y Embase, en artículos de revisión y contactando clínicos expertos. No hubo restricción de idioma.
Criterios de selecciónSe incluyeron ensayos controlados aleatorios en pacientes adultos sometidos a trasplante renal.
Recogida y análisis de datosDe forma independiente se realizó selección de estudios, evaluación de la calidad y extracción de datos. Se calculó diferencia de medias con su intervalo de confianza del 95% (IC 95%). Se evaluó la heterogeneidad con el estadístico I-cuadrado (I2). Se usaron los modelos de efectos fijos y aleatorios.
ResultadosSe incluyeron cuatro estudios con un total de 237 pacientes. Al final de cirugía la diferencia de potasio no fue significativa (Diferencia de Medias (DM: −0,26 mEq/L; IC 95%: −0,58 a 0,05 p=0,10; I2=75%), el pH fue menor en el grupo de SSN (DM: 0,06; IC 95%: 0,05 a 0,08; p<0,001; I2:17%). No hubo diferencia en la creatinina al tercer día posoperatorio (DM: −0,05; IC 95%: −0,59 a 0,48; p=0,85; I2=0%).
ConclusionesEl uso de LR comparado con SSN en el perioperatorio de trasplante renal genera menores niveles de potasio y cloro y mayor pH, sin cambios significativos en la creatinina.
Intraoperative fluid management during renal transplantation has traditionally been done with normal saline solution (NSS) because the administration of potassium solutions such as Lactated Ringer's (LR) in large volumes to surgical patients may lead to hyperpotassemia1. Several papers have been published on the topic, showing that the administration of large volumes of NSS, as is usually the case in patients undergoing renal transplantation (RT), is associated with hyperchloremic metabolic acidosis1–4.
According to Steward's theory, fluids usually administered during surgery may alter the acid–base balance and predispose to metabolic acidosis due to a rise in chloride levels5,6. Such acidosis may lead to hyperpotassemia due to the extracellular shifts of potassium ions1–3,7. Hyperchloremia may at the same time result in vasoconstriction of the afferent arteriole and renal graft injury2,3. Other acidosis-related complications may be changes in mental status and abdominal discomfort due to disruptions of the splanchnic vasculature7 and it has even been associated with higher mortality in surgical patients8.
Kidney transplant is the most usual transplantation in our country and around the world9; RT results have improved with the advancement of surgical, immune suppressor, and anesthesia techniques. The presence of hyperpotassemia associated with hyperchrolemic metabolic acidosis may contribute to the graft dysfunction, and hence should be prevented in these patients10.
Several trials have been published comparing the use of LR with NSS but they include few patients2,3,11–14. We did a meta-analysis to assess the effects of LR vs. NSS on the incidence of hyperchloremic metabolic acidosis, hyperpotassemia, volume of fluids infused and kidney graft dysfunction in patients undergoing renal transplant.
MethodologyThis systematic review followed the methodology recommended by the Cochrane Collaboration14. This protocol has not been published and was not registered.
Eligibility criteriaThe search included randomized, clinical controlled trials with no restrictions as to language, date or status of publication, comparing the use of LR against NSS as fluid therapy in renal transplantation patients, over 18 years old. The outcomes evaluated were the level of serum potassium, bicarbonate, chloride, Creatinine and the postoperative pH. The deadline of publication established was July 8, 2013.
Search strategiesIndependently, the three authors did an electronic database search, contacting expert clinicians and searching review articles. No language and date of publication restrictions were applied.
The databases accessed were the Central Cochrane Registry of Controlled Trials, Medline (1966–2013), Lilacs (1982–2013), EBSCO and Embase (1980–2013).
The search terms used were “renal transplant”, “acidosis”, “acidemia”, “hyperpotassemia”, “graft dysfunction”, “Lactate Ringer's” and “saline solution”.
Trial selection and evaluationTwo of the authors independently reviewed all the titles and abstracts identified in the bibliography searched and excluded the irrelevant trials. The remaining assays were evaluated in full text and disagreements were settled with the participation of the third author.
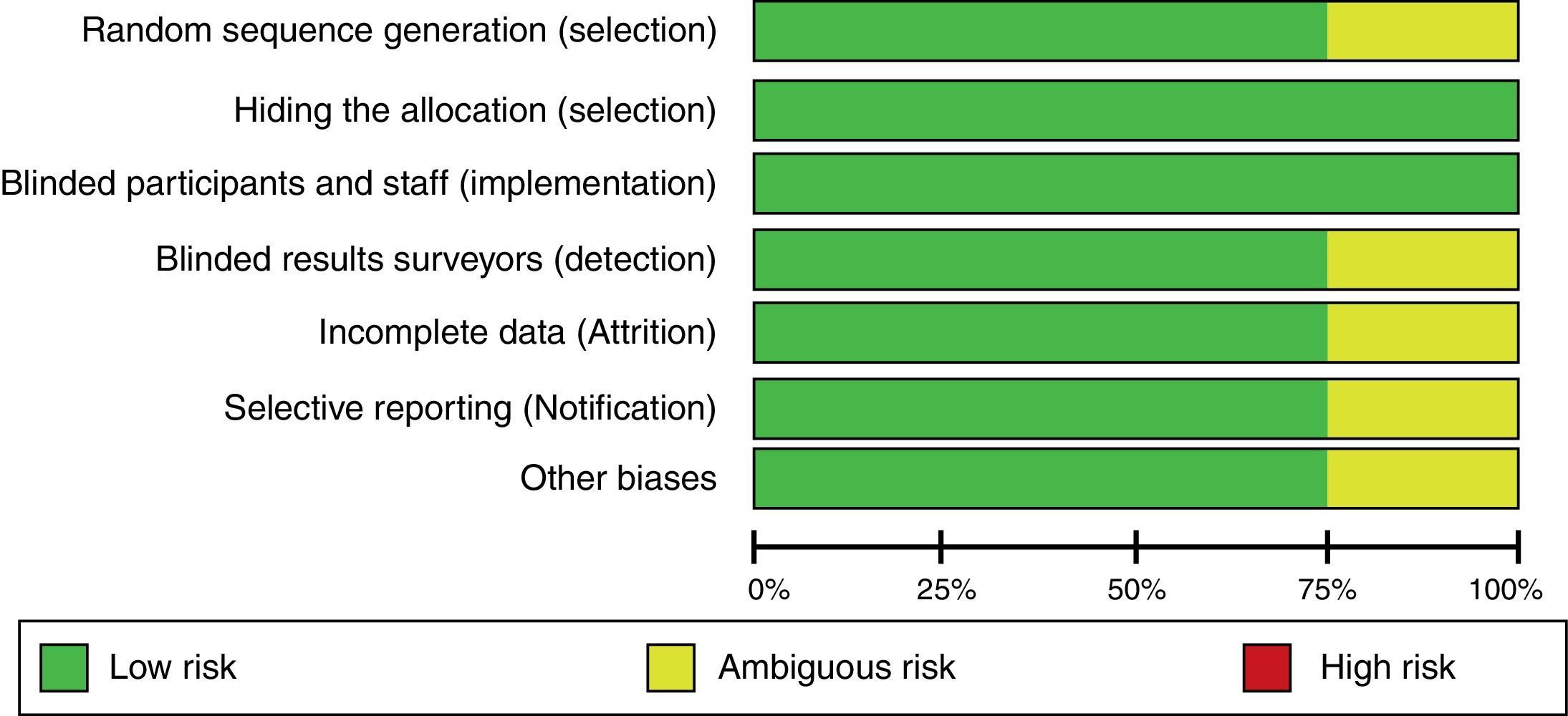
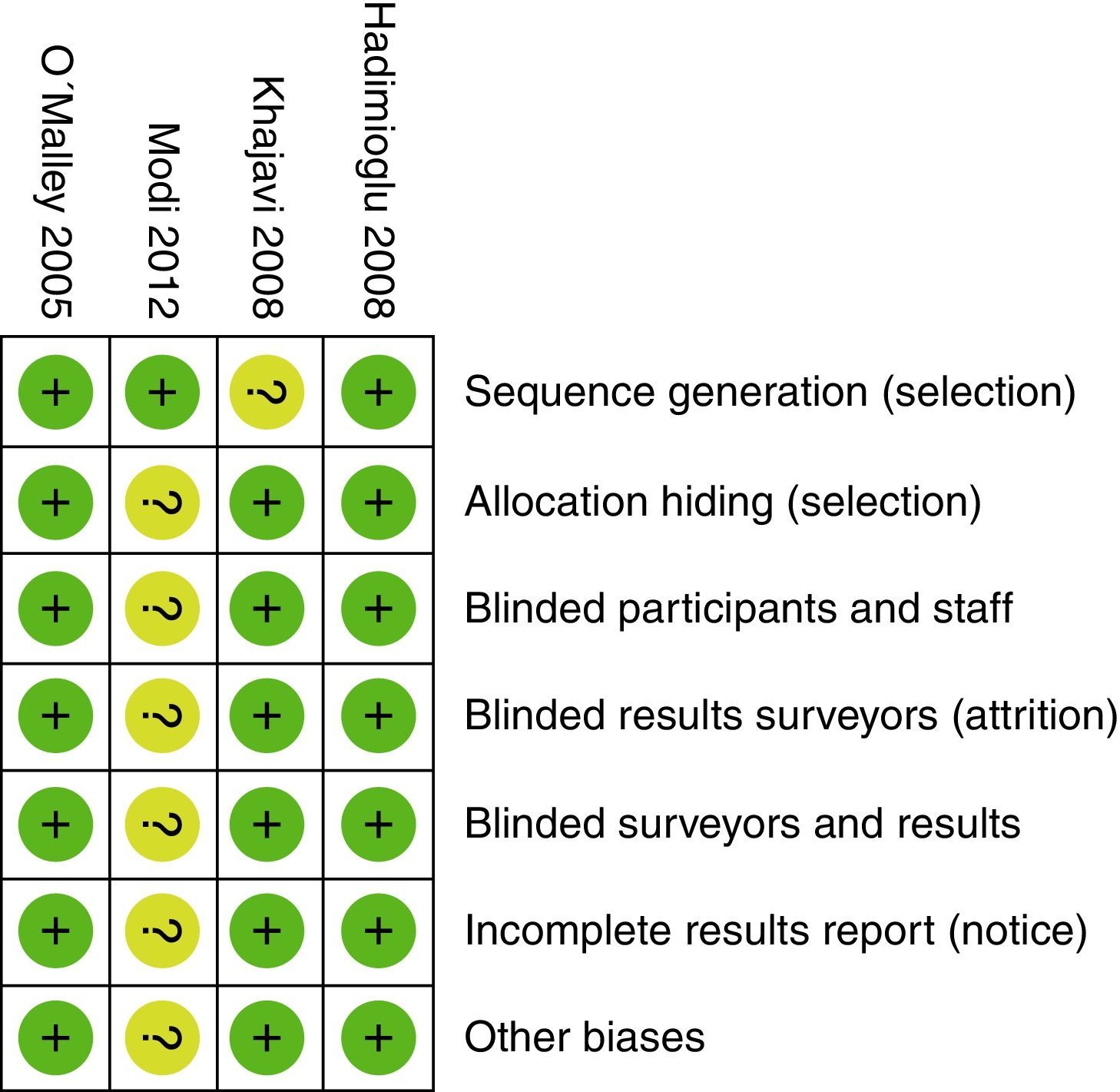
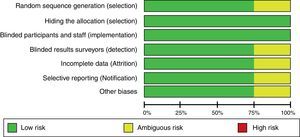
All three researchers – in accordance with the Cochrane Collaboration guide – independently assessed the validity of the trials selected15. The random sequence generation, the sequence hiding, blinding, information gathering, losses to follow-up during the trial, inclusion of incomplete data, selective outcome reporting, and other biases were all evaluated. Based on this methodology, the risk of biases was classified into high, uncertain, and low.
Data collectionBased on the recommendations from Cochrane Consumers and Communication Review data extraction template14 the analysis of the information extracted was done using an information extraction table. The information that was required but not available after reading the articles was requested to the authors directly. The article with the largest sample was written in Farsi13 and only the abstract was available in English. There were failed attempts to contact the authors in order to obtain the complete data. An investigator extracted the information from the trials, following the table closely. To ensure the accuracy of the data, a second investigator then reviewed the information collected.
Results analyzedThe results analyzed included the average serum potassium in mEq/L during the postoperative period, serum Creatinine in mg/dL three days after surgery, pH immediately after surgery, the volume of infused solution in liters and bicarbonate and chloride in the arterial blood expressed in mEq/L following surgery.
Statistical analysisThis meta-analysis estimated the mean difference with its respective 95% confidence interval (95% CI) for the variables considered, using Review Manager software, version 5.1. An analysis using the fixed or random effects model was completed, based on the existence of statistical heterogeneity. The statistical heterogeneity was evaluated using the Q Cochrane test and the I2 statistic. When I2 was less than 40%, we used the fixed effects model and if I2 was above 40%, the random effects model was used. The sensitivity analysis was completed based on the methodological quality of the trials, removing some trials and re-analyzing the data. Similarly, a sub-group analysis was done, based on the patients’ characteristics and the mode of intervention used.
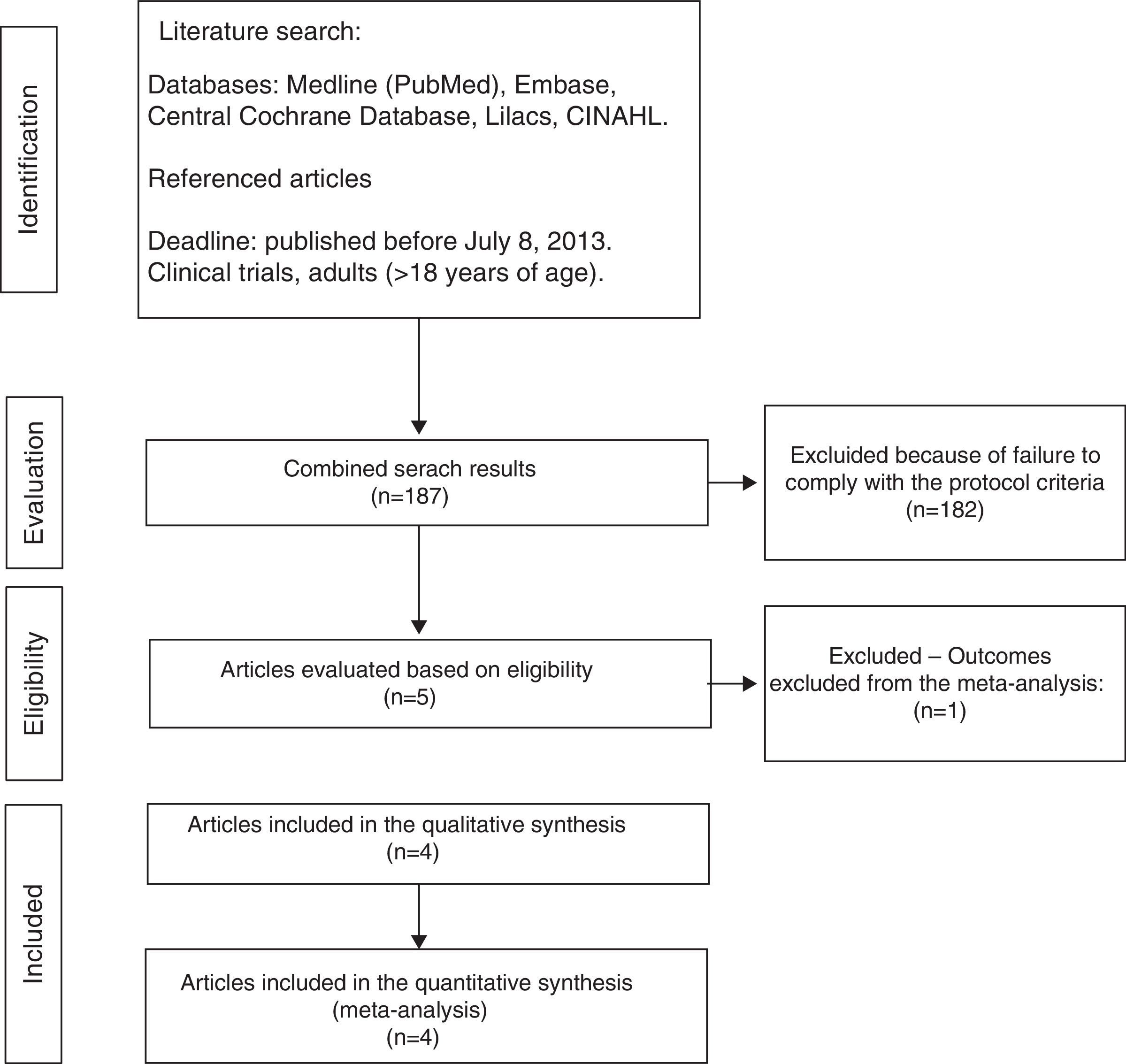
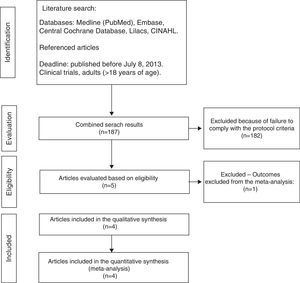
ResultsTrial selection processThe initial search identified 187 articles, of which 182 were ruled out due to failure to meet the eligibility criteria (Fig. 1).
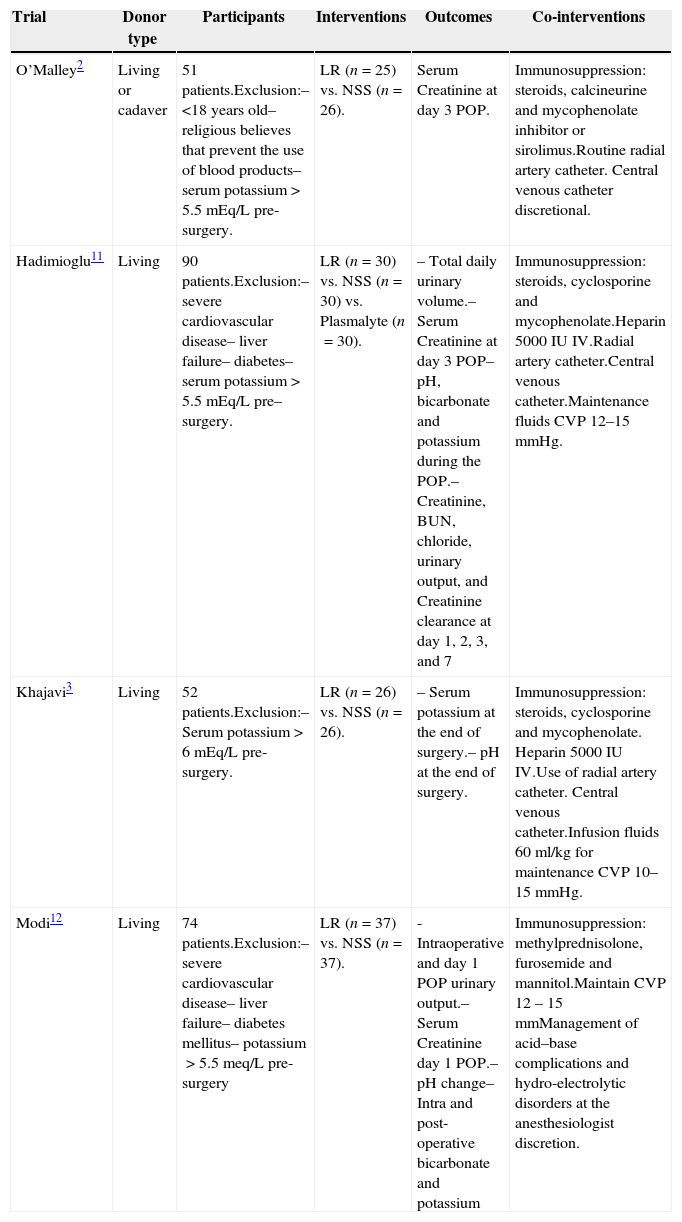
Characteristics of the trials includedFour randomized controlled trials were identified with 237 participants that met the inclusion criteria. All of the trials were double blind. The main characteristics of these trials are shown in Table 1. Lactated Ringer's was used as the “experimental” intervention, while NSS was used as the “control”; observation times and follow-up after renal transplantation were variable for the various trials, and hence the results of the measurements at similar time intervals were used to make them comparable.
Characteristics of the trials selected for the meta-analysis.
| Trial | Donor type | Participants | Interventions | Outcomes | Co-interventions |
|---|---|---|---|---|---|
| O’Malley2 | Living or cadaver | 51 patients.Exclusion:– <18 years old– religious believes that prevent the use of blood products– serum potassium>5.5mEq/L pre-surgery. | LR (n=25) vs. NSS (n=26). | Serum Creatinine at day 3 POP. | Immunosuppression: steroids, calcineurine and mycophenolate inhibitor or sirolimus.Routine radial artery catheter. Central venous catheter discretional. |
| Hadimioglu11 | Living | 90 patients.Exclusion:– severe cardiovascular disease– liver failure– diabetes– serum potassium>5.5 mEq/L pre– surgery. | LR (n=30) vs. NSS (n=30) vs. Plasmalyte (n=30). | – Total daily urinary volume.– Serum Creatinine at day 3 POP– pH, bicarbonate and potassium during the POP.– Creatinine, BUN, chloride, urinary output, and Creatinine clearance at day 1, 2, 3, and 7 | Immunosuppression: steroids, cyclosporine and mycophenolate.Heparin 5000IU IV.Radial artery catheter.Central venous catheter.Maintenance fluids CVP 12–15mmHg. |
| Khajavi3 | Living | 52 patients.Exclusion:– Serum potassium>6mEq/L pre-surgery. | LR (n=26) vs. NSS (n=26). | – Serum potassium at the end of surgery.– pH at the end of surgery. | Immunosuppression: steroids, cyclosporine and mycophenolate. Heparin 5000IU IV.Use of radial artery catheter. Central venous catheter.Infusion fluids 60ml/kg for maintenance CVP 10–15mmHg. |
| Modi12 | Living | 74 patients.Exclusion:– severe cardiovascular disease– liver failure– diabetes mellitus– potassium>5.5meq/L pre-surgery | LR (n=37) vs. NSS (n=37). | -Intraoperative and day 1 POP urinary output.– Serum Creatinine day 1 POP.– pH change– Intra and post-operative bicarbonate and potassium | Immunosuppression: methylprednisolone, furosemide and mannitol.Maintain CVP 12 – 15mmManagement of acid–base complications and hydro-electrolytic disorders at the anesthesiologist discretion. |
LR: Lactated Ringer's. NSS: Normal saline solution. POP: postoperative. BUN: Blood urea nitrogen. CVP: central venous pressure.
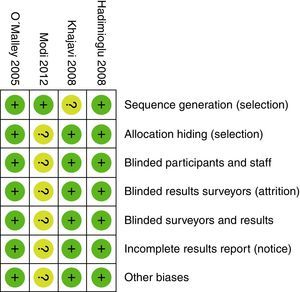
When evaluating the risk of biases in the articles included, we found that most of them used computerized software for randomization. However, the Khajavi et al3. trial is the exception as it fails to indicate how the randomization process was done. All of them used opaque envelopes to hide the allocation and a proper masking method was used in every case. The Modi et al12. trial was classified as “ambiguous risk” due to missing information to rule out any detection, attrition and notification risks. Figs. 2 and 3 illustrate the bias risks.
The results considered for the analysis included the laboratory measurements during the perioperative period that the authors rated as most significant. The potassium difference was not significant at the end of surgery (mean difference (MD): −0.26mEq/L; 95% CI: −0.58 to 0.05; p=0.10; I2=75%) although it was done through fixed effects; RL resulted in a lower value (Fig. 4).
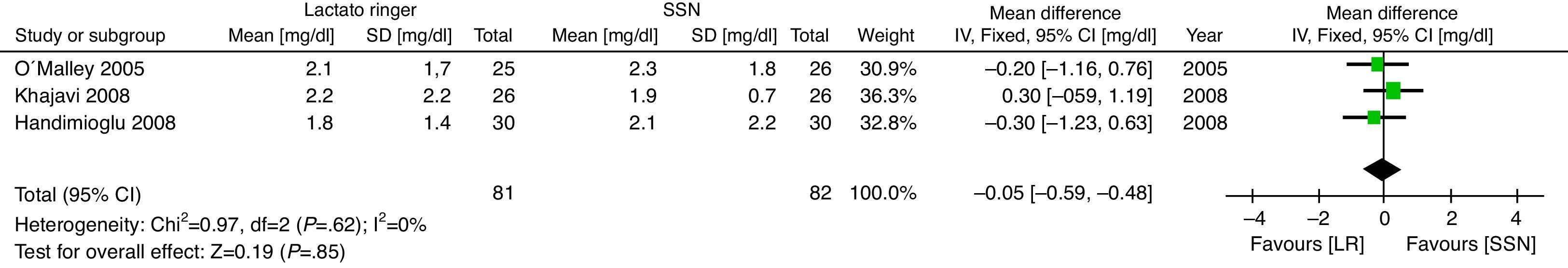
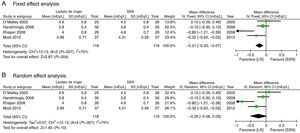
The secondary results considered show that there is no difference in the Creatinine value expressed in mg/dL on the third postoperative day (MD: −0.05; 95% CI: −0.59 to 0.48; p=0.85; I2=0%) (Fig. 5).
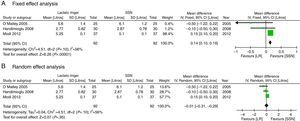
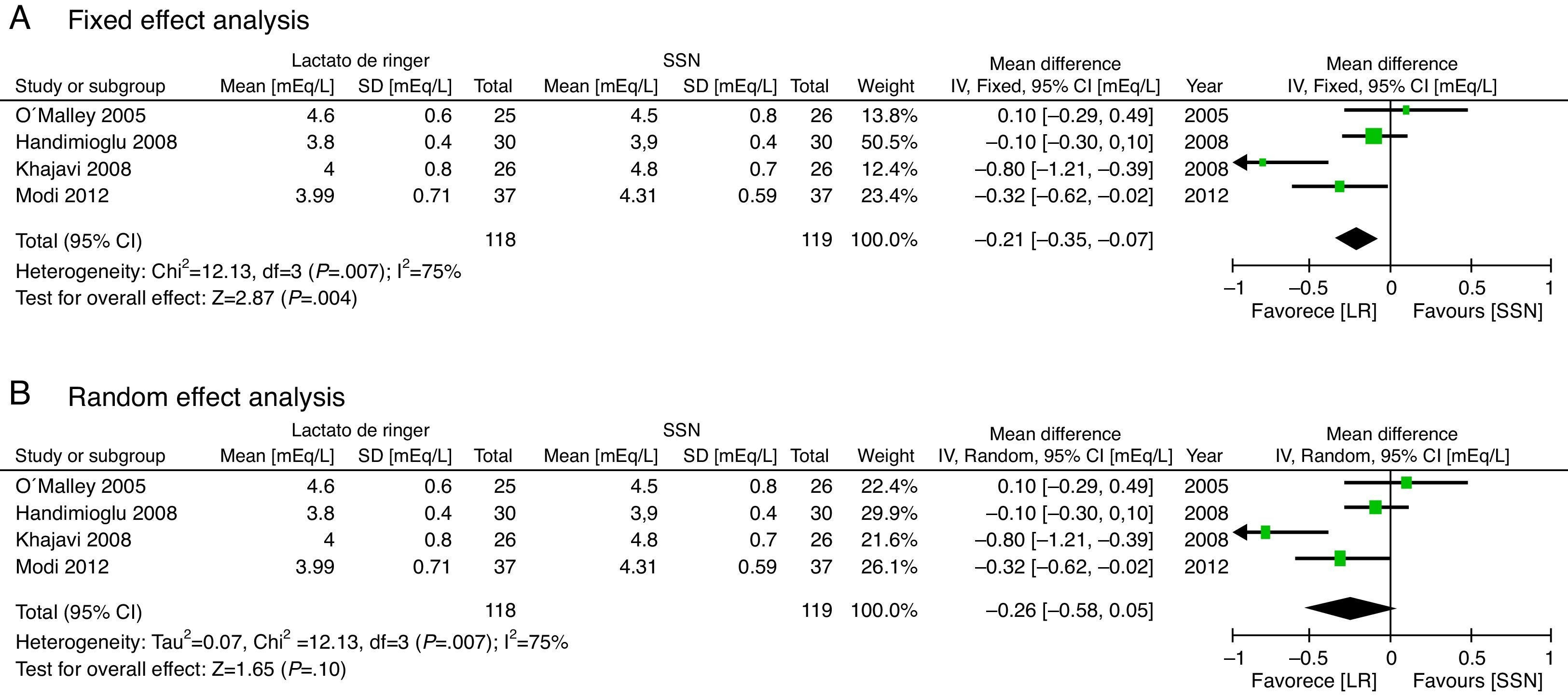
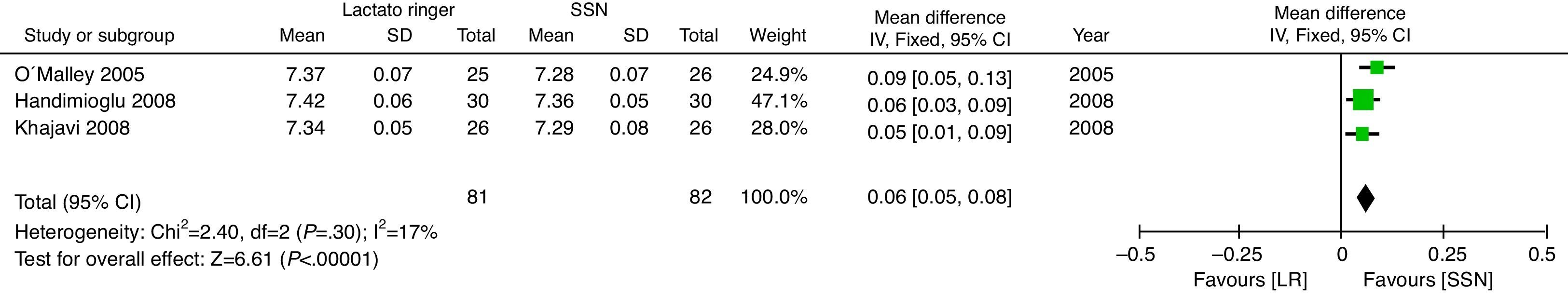
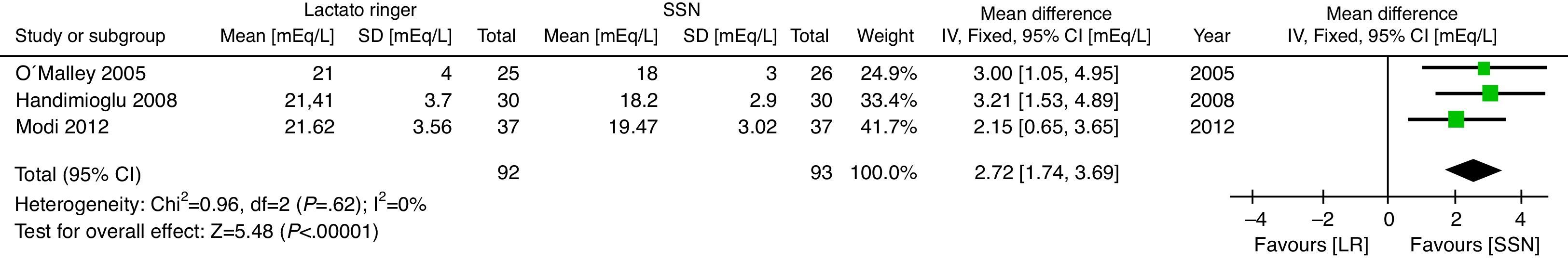
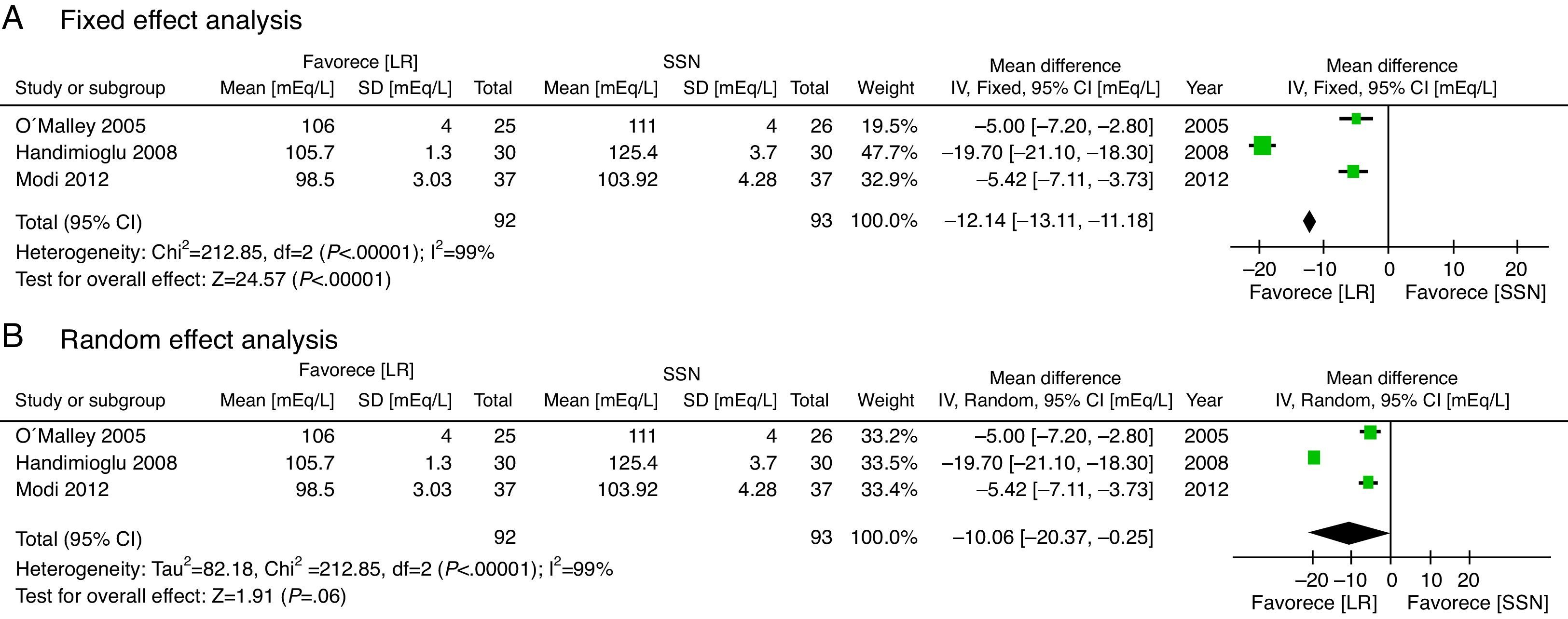
With regard to the acid–base status, the NSS group exhibited higher acidosis, the pH was higher in the LR group (MD: 0.06; 95% CI: 0.05–0.08; p<0.001; I2=17%) (Fig. 6) and bicarbonate was also higher for the LR group (MD: 2.72; 95% CI: 1.74–3.69; p<0.001; I2=0%) (Fig. 7). Chloride was lower during the postoperative period, though based on the random analysis method the value is not statistically significant (MD: 10.06; 95% CI: −20.37 to 0.25; p=0.06; I2=99%) (Fig. 8).
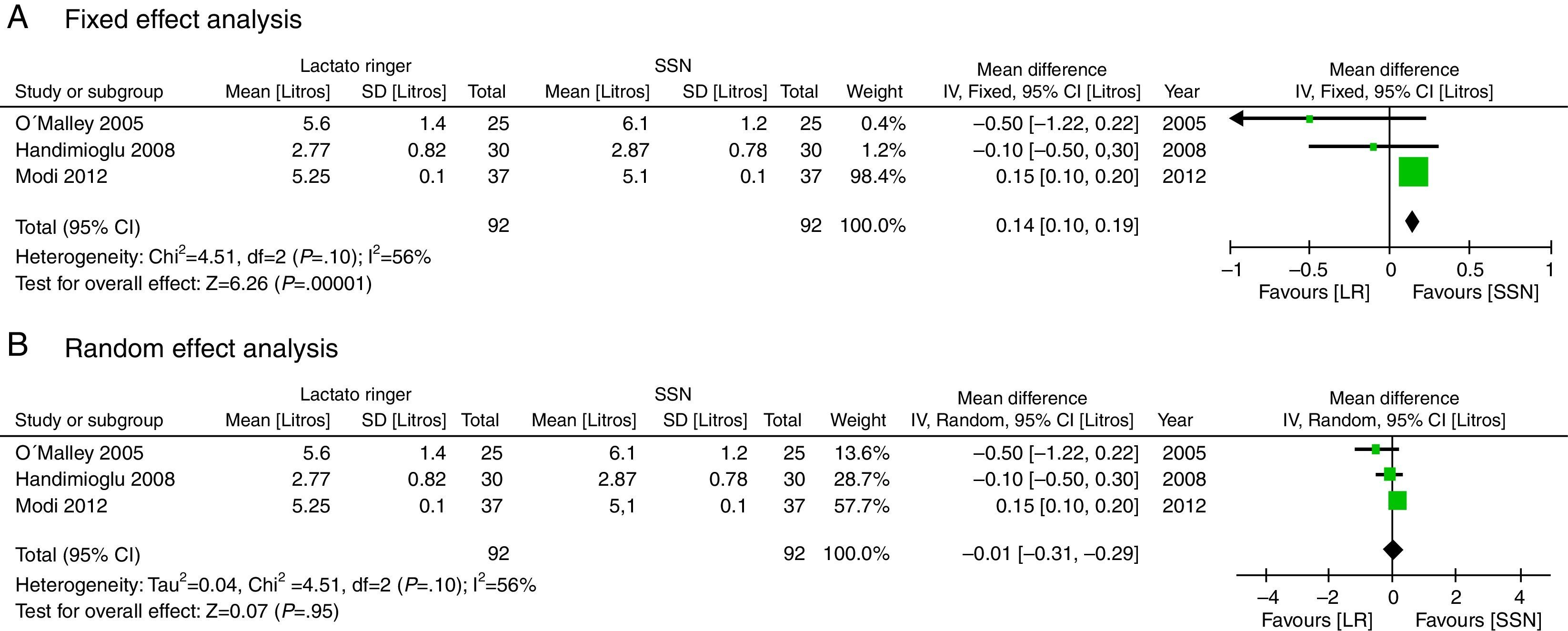
There was no significant statistical difference in the fluid volume infused in liters (MD: −0.01; 95% CI: −0.31 to 0.29; p=0.95; I2=56%), though according to fixed methods, the LR group is higher (Fig. 9).
DiscussionRenal transplant has actually become one additional option for the treatment of chronic renal disease15. The 5-year survival is 70%, while the survival for patients that continue on dialysis is only 30%15–19. Patients undergoing renal transplant have multiple comorbidities, including cardiovascular19, hypertension, dyslipidemia, hyperphosphatemia and hyperhomocysteinemia20–23, in addition to pulmonary hypertension24. This represents an anesthetic challenge for the perioperative period25. According to the government data there were 2693 renal transplants in 2008, and 3691 in 2010; this shows a growing number of transplant procedures in the country26,27. General anesthesia is currently the technique of choice; however, regional techniques have proven to be beneficial, particularly in terms of postoperative pain28,29. An in-depth knowledge of the various surgical steps is critical to optimize the surgical conditions30–32.
The use of large volumes of fluids during the intraoperative period has typically reported improved graft function10,25,32–39.
Fluid therapy is a critical element in the intraoperative management of a patient undergoing renal transplantation10,40, particularly because the multiple physiological and pathological variables increase the complexity of the procedure41. Classically, the administration of large volumes of potassium solutions, such as LR, may lead to hyperpotassemia and hence NSS1 has been used instead; several studies indicate that NSS continues to be the choice for this procedure2. Recent studies, however, suggest that more balanced approaches, such as LR, may prevent hyperchloremic metabolic acidosis3–5, and this is not the case if large NSS volumes are used, as has been shown in other types of patients4,8,42–45.
There is some controversy about the best type of crystalloids to use in a RT patient46–48. The use of colloids in these patients is limited48,34 and it is not recommended because of adverse events, including renal failure49–52.
This meta-analysis showed that the administration of LR may be an option for fluid management therapy in renal transplantation since contrary to old beliefs, this solution did not elicit higher hyperpotassemia or higher rates of graft dysfunction as shown by the fact that no differences were identified in the Creatinine values three days after surgery. The potassium difference was not significant at the end of surgery, though when fixed effects were used, LR showed a lower value (Fig. 4). Further analysis of this variable indicated that although there is significant heterogeneity in the results, such heterogeneity decreases upon removing the Khajavi et al.3 trial; the explanation could be the difference in renal ischemia time that was loner in the NSS group. The presence of hyperpotassemia in the NSS group could be mainly explained because potassium acts as a buffer in the presence of acidosis. And, as mentioned above, the administration of large volumes of NSS causes hyperchloremic metabolic acidosis4,8,42–45.
The meta-analysis confirms that the NSS causes metabolic acidosis, probably as a result of hyperchloremia, as illustrated in Figs. 6–8. The patients who received NSS had lower pH values and lover serum bicarbonate, and the data were not heterogeneous for the various trials. Serum chloride was higher in the NSS group, as compared against the patients receiving LR, though there is significant heterogeneity with this particular variable. It should be mentioned however that other anions such as sulfates and phosphates, inter alia, may accumulate in patients with chronic renal disease; nevertheless, crystalloids do not affect the concentration and chloride could be the key factor in the development of metabolic acidosis52. To this date, several trials show that hyperchloremia per se could be the cause for an unfavorable outcome in renal function44,45,52–54. The success in preventing perioperative complications includes proper patient identification and optimization, with an anesthetic plan that integrates the various variables affecting the evolution of the renal transplant. It should be highlighted however, that no significant differences were identified between the 2 groups in terms of amount of infused solution in the three trials analyzed; this certainly deserves some consideration since classically hyperchloremic metabolic acidosis has been associated with the infusion of large volumes of fluids. Further trials with larger numbers of patients and long-term follow-up are needed, in order to obtain a better understanding of the clinical implications of hyperchloremic metabolic acidosis.
The clinically relevant result, Creatinine levels at day 3, showed no differences between the two groups. This indicates that the administration of LR is safe for patients undergoing renal transplantation surgery. The heterogeneity of the trials is low in terms of this variable, making the result even stronger. No adverse effects were described in any of the trials using Lactated Ringer's therapy, so no conclusions can be made on this particular point.
This meta-analysis exhibits a number of limitations including the small number of trials and a small number of patients, in addition to differences in follow-up times and in the variables evaluated. The observation and follow-up times after renal transplantation varied among the various trials, but the results of the measurements used were from similar time intervals to make them comparable. The outcome that evaluates renal function using 3rd postoperative day Creatinine was only reported in three trials. This limits the interpretation of this variable, because the number of patients is further reduced. The heterogeneity of some of the variables was important; however, it is impossible to avoid heterogeneity in this type of trials, considering the differences in the populations evaluated, the respective treatment protocols and the duration of the trials. The Nuraei et al13. trial was excluded because it was written in Farsi and despite numerous attempts to contact the authors, no replies were received. The exclusion criteria were strict and hence some patients with cardiovascular complications could have been excluded preventing a definite conclusion about this group of patients.
Nevertheless, this paper may still be the best available evidence to approach the issue of identifying the best fluid therapy option for patients undergoing kidney transplantation procedures.
ConclusionThe use of LR in the perioperative period of renal transplant procedures results in similar potassium levels during the postoperative period, higher pH and bicarbonate levels, and lower chloride, with no significant changes on the 3rd day postoperative Creatinine values, despite using a similar infusion volume as compared to NSS.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
FundingThe authors did not receive sponsorship to undertake this article.
Please cite this article as: Trujillo-Zea JA, Aristizabal-Henao N, Fonseca-Ruiz N. Lactato de Ringer vs. Solución salina normal para trasplante renal. Revisión sistemática y metaanálisis. Rev Colomb Anestesiol. 2015;43:194–203.






![Post-surgical Arterial blood bicarbonate levels [mEq/L]. Post-surgical Arterial blood bicarbonate levels [mEq/L].](https://static.elsevier.es/multimedia/22562087/0000004300000003/v3_201512070814/S2256208715000322/v3_201512070814/en/main.assets/thumbnail/gr7.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
![Post-surgical chloride levels [mEq/L]. (A) Fixed effect analysis. (B) Random effect analysis. Post-surgical chloride levels [mEq/L]. (A) Fixed effect analysis. (B) Random effect analysis.](https://static.elsevier.es/multimedia/22562087/0000004300000003/v3_201512070814/S2256208715000322/v3_201512070814/en/main.assets/thumbnail/gr8.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)