To determine cumulative incidence of sore throat complaints (STCs) which occur with the insertion of the laryngeal mask (LM) and endotracheal tube (ETT) during the first hour and 24 hours after elective surgery. In addition, to establish risk factors associated with its occurrence.
MethodsIn a cohort study, a total of 451 patients scheduled for elective non-cardiac surgery were included consecutively for 6 months (ASA I-II-III, >18 years old) who underwent LM or ETT airway management for general anesthesia. Through a questionnaire with indirect and direct questions the presence of sore throat, hoarseness, dysphagia and the composite endpoint STCs were assessed one and 24 hours after surgery. Marginal models were used to identify risk factors.
ResultsWe found an incidence of STCs of 26.8% and 13.5% at first and 24 postoperative hours respectively. At first hour, they were classified as sore throat (23.9%), hoarseness (6.7%) and dysphagia (6.4%). Each compound was not mutually exclusive. At 24 hours of follow up, incidence of STCs and its compounds decreases significantly but differently to ETT and LM. STCs were associated with female gender (OR=1.53 95%CI 1.00-2.37, p=0.05), ETT intubation (OR=4.20 95%CI 2.19-8.04, p<0.01) and bloodstain on airway device at extubation (OR=2.00 95%CI 1.18-3.36, p<0.01).
ConclusionsThe incidence of STCs remains important. There are differences in the pattern of reduction between ETT and LM over time and this study confirms risk factors for postoperative STCs like use of ETT, presence of blood during the airway device extraction and female gender.
Los síntomas laringofaríngeos (SLF) son comunes en anestesia. La incidencia de morbilidad laringofaríngea varía en la literatura.
ObjetivosDeterminar la incidencia de SLF al usar máscara laríngea y tubo endotraqueal en la primera y a las 24h posoperatorias y estimar la asociación de factores de riesgo.
MétodosEstudio de cohorte cerrada que incluyó 451 pacientes. Se indagó la presencia deodinofagia, disfonía y disfagia. Se utilizaron modelos marginales para estimar asociación con variables en estudio.
ResultadosLa incidencia de SLF durante la primera y 24h postoperatorias fue del 26 y del 13%, respectivamente. A las 24h, la incidencia disminuyó significativamente.
ConclusionesLa incidencia en un centro hospitalario colombiano de SLF en cirugía ambulatoria es importante. Existen diferencias en la reducción con el tubo endotraqueal y la máscara laríngea en el tiempo.
Many authors and professionals consider these symptoms as minor complications, however, such things do affect the recovery of the patient and are linked to patient dissatisfaction.1,2
The incidence of LPS has been reported at between 5 and 70% and is higher with the use of the endotracheal tube (ETT) than with the laryngeal mask (LM).1 The data on the incidence of laryngo-pharyngeal morbidity vary considerably in the literature; survey methods, as well as the definitions applicable to these symptoms shall be taken into account for analysis and interpretation.3,4
Several authors report the following risk factors for the postoperative presentation of LPS: type of airway device (AW) used, being a female, young age, size and shape of the ETT, use of lubricants, pneumoplug pressure, succinylcholine relaxation, long endotracheal intubation (ETI), history of smoking or preexisting pulmonary disease, presence of blood in the AW device used, having natural teeth and certain types of surgical procedures.1,3,5,6 All the above-mentioned factors and their association with the occurrence of LPS have not been studied in the Colombian population.
The purpose of the study was to establish the incidence of LPSs associated with the insertion of the LM and the EET during the first hour and 24h after elective surgery and additionally estimating the level of association of known risk factors with the occurrence of LPS.
MethodsFollowing the approval by the ethics committee of the Caldas University and the Santa Sofía State Hospital of Manizales, Colombia, the informed consent was obtained from all patients admitted to this observational, prospective, closed-cohort trial.
Patients were included consecutively for six months (May through October 2010), as part of our daily clinical practice. The patients included were over 18 years of age, ASA I, II or III classification and were scheduled for elective surgery under general anesthesia with ETT or LM.
All patients admitted for emergency surgery, head and neck surgery, patients previously using oro/naso-gastric catheter or during the procedure, requirement for extended intubation (>24h) and compromised mental function that could limit the evaluation of the results, were excluded.
Every patient received general anesthesia in accordance with the criteria of the treating anesthesiologist. Inhaled anesthetics were used (sevofluorane, isofluorane), intravenous induction agents (propofol, pentothal, etomidate and ketamine), opioids (remifentanil, fentanyl), benzodiazepines (midazolam) and muscle relaxants (succinylcholine, rocuronium, vecuronium and cisatracurium).
The AW used and its size were determined based on the surgical procedure, the patient's condition and the anthropometric characteristics. The people responsible for managing the AW were: the anesthesiologist in charge, the anesthesia resident, medical students doing anesthesia training or surgery general practitioners. All the trainees were under the supervision of the anesthesiology specialist at all times.
The method for evaluating the pneumoplug pressure and the use of the local anesthetic agent (lidocaine in gel) were determined by the anesthesiologist in charge prior to starting the induction. Following the induction the patient was placed in supine, prone or lateral decubitus position depending on the requirements of the procedure. The anesthesia specialist established the extubation technique (profound or awake) and the need for aspiration at the end of the procedure. When AW aspiration was needed, a 40cmH2O negative pressure Nelaton catheter was used. The length of time was determined from the start of anesthesia up to the removal of the AW device.
All patients were followed for 24h postoperatively. At the post anesthesia care unit (PACU) patients were interviewed either by the anesthesiologist in charge of the Unit, the anesthesia resident or a nurse trained by the trial researchers. All interviewers in the PACU were unaware of the anesthetic management received by the patients. Two interviews were held. The first one was 1h after being admitted to the PACU; the second interview was conducted by the trained nurse only, 24h post surgery, either directly if the patient was still hospitalized, or on the phone in case of out-patient.
A questionnaire was developed to assess the presence of LPS. The questions were designed keeping in mind prior studies reported in the literature.1,3,7 We also considered the opinion of anesthesiologists in validating the questions asked to establish the likelihood of LPS. Odynophagia was defined as sore throat whether associated or not with swallowing; dysphonia referred to changes in the voice characteristics or pain when speaking and dysphagia made reference to difficulty in swallowing.3
The first two questions indirectly probed for the presence of LPSs: “How did you feel after surgery?” “Have you experienced any discomfort other than at the surgical site?” The third question was approached directly: “Have you experienced any throat soreness, swallowing discomfort or problems to speak or swallow?” A positive answer to any of the last three items was indicative of LPS.
Statistical analysisContinuous variables were presented as means and standard deviation (SD) or median and interquartile range (25–75%) when asymmetry was present.
The primary outcome in the trial was the accumulated incidence of LPS. It was estimated as the number of patients with any of the three components (odynophagia, dysphonia and dysphagia) divides into the total number of patients at risk. Measurements were taken after the first and twenty-four hours postoperatively. Each outcome component did not exclude the others and the results are presented individually. The McNemar test was used to contrast the change in LPS incidence.
For the multivariate analysis a marginal generalized equations estimate (GEE) model was used to explore the association between co-variables studied and the incidence of LPS in the first hour and 24h after surgery, taking into account the longitudinal repeated approach.8 There were no missed values during the valuation of the repeated measurements at two points in time. The variables included in the model were selected on the basis of a conceptual and clinical framework.
The statistical model included as co-variables: age, gender, smoking, ASA status, type of procedure, position during surgery, device used for AW management, number of attempts when inserting the device, use of local anesthetic agents with the device, staff responsible for AW management, device pressure measurement, pharyngeal aspiration at the end of the procedure, anesthetic condition of the patient at the time of extraction of the device, presence of blood in the AW device and duration of surgery. Dummy variables were developed for the categorical variables with more than two categories. The standard error was estimated using the robust estimation method. The results are shown in terms of odds ratio (OR), confidence intervals (95% CI) and p values.
All the analyses were done using SAS version 9.2 software (SAS Institute, Cary, NC).9 Statistical modeling was done with PROC GENMOD a non-structured correlation matrix was used for repeated measurements. Interaction terms among the co-variables were tested. The statistical significance was set at α<0.05 for all the analyses.
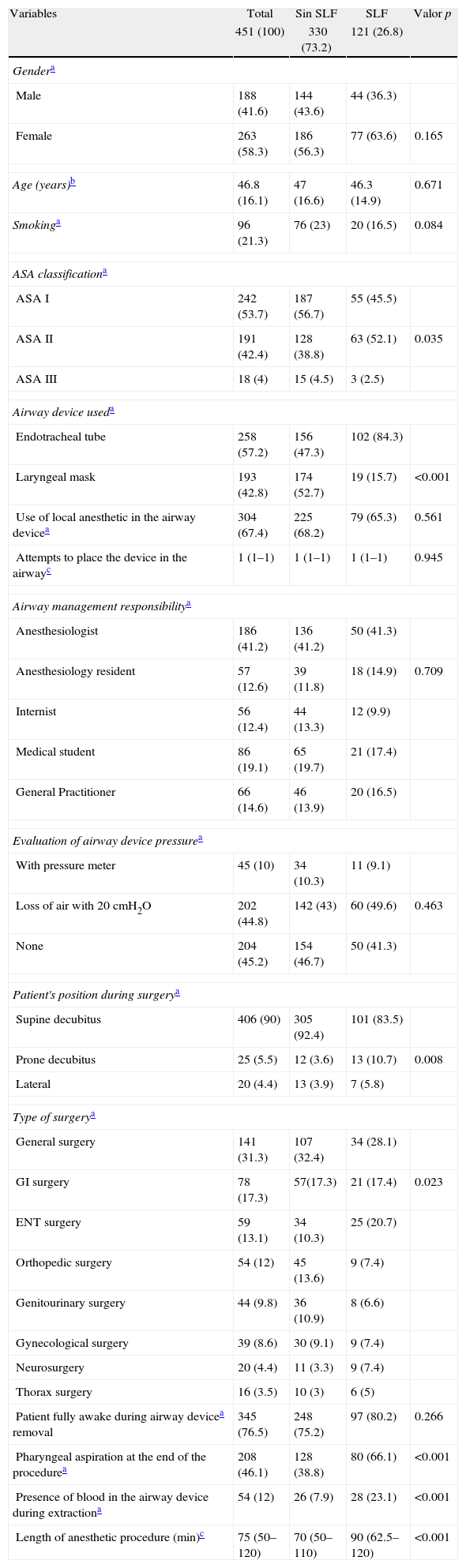
ResultsDuring the 6 months of the study, 451 patients were recruited. Most of them were healthy females ASA 1 classification and an age average of 47 years. The ETT was used in 57.2% (258 patients) and anesthesiologists performed most of the procedures. The general characteristics of the patients included are shown in Table 1.
Characteristics of patients with LPSs in the first postoperative hour (n=451).
| Variables | Total | Sin SLF | SLF | Valor p |
| 451 (100) | 330 (73.2) | 121 (26.8) | ||
| Gendera | ||||
| Male | 188 (41.6) | 144 (43.6) | 44 (36.3) | |
| Female | 263 (58.3) | 186 (56.3) | 77 (63.6) | 0.165 |
| Age (years)b | 46.8 (16.1) | 47 (16.6) | 46.3 (14.9) | 0.671 |
| Smokinga | 96 (21.3) | 76 (23) | 20 (16.5) | 0.084 |
| ASA classificationa | ||||
| ASA I | 242 (53.7) | 187 (56.7) | 55 (45.5) | |
| ASA II | 191 (42.4) | 128 (38.8) | 63 (52.1) | 0.035 |
| ASA III | 18 (4) | 15 (4.5) | 3 (2.5) | |
| Airway device useda | ||||
| Endotracheal tube | 258 (57.2) | 156 (47.3) | 102 (84.3) | |
| Laryngeal mask | 193 (42.8) | 174 (52.7) | 19 (15.7) | <0.001 |
| Use of local anesthetic in the airway devicea | 304 (67.4) | 225 (68.2) | 79 (65.3) | 0.561 |
| Attempts to place the device in the airwayc | 1 (1–1) | 1 (1–1) | 1 (1–1) | 0.945 |
| Airway management responsibilitya | ||||
| Anesthesiologist | 186 (41.2) | 136 (41.2) | 50 (41.3) | |
| Anesthesiology resident | 57 (12.6) | 39 (11.8) | 18 (14.9) | 0.709 |
| Internist | 56 (12.4) | 44 (13.3) | 12 (9.9) | |
| Medical student | 86 (19.1) | 65 (19.7) | 21 (17.4) | |
| General Practitioner | 66 (14.6) | 46 (13.9) | 20 (16.5) | |
| Evaluation of airway device pressurea | ||||
| With pressure meter | 45 (10) | 34 (10.3) | 11 (9.1) | |
| Loss of air with 20cmH2O | 202 (44.8) | 142 (43) | 60 (49.6) | 0.463 |
| None | 204 (45.2) | 154 (46.7) | 50 (41.3) | |
| Patient's position during surgerya | ||||
| Supine decubitus | 406 (90) | 305 (92.4) | 101 (83.5) | |
| Prone decubitus | 25 (5.5) | 12 (3.6) | 13 (10.7) | 0.008 |
| Lateral | 20 (4.4) | 13 (3.9) | 7 (5.8) | |
| Type of surgerya | ||||
| General surgery | 141 (31.3) | 107 (32.4) | 34 (28.1) | |
| GI surgery | 78 (17.3) | 57(17.3) | 21 (17.4) | 0.023 |
| ENT surgery | 59 (13.1) | 34 (10.3) | 25 (20.7) | |
| Orthopedic surgery | 54 (12) | 45 (13.6) | 9 (7.4) | |
| Genitourinary surgery | 44 (9.8) | 36 (10.9) | 8 (6.6) | |
| Gynecological surgery | 39 (8.6) | 30 (9.1) | 9 (7.4) | |
| Neurosurgery | 20 (4.4) | 11 (3.3) | 9 (7.4) | |
| Thorax surgery | 16 (3.5) | 10 (3) | 6 (5) | |
| Patient fully awake during airway devicea removal | 345 (76.5) | 248 (75.2) | 97 (80.2) | 0.266 |
| Pharyngeal aspiration at the end of the procedurea | 208 (46.1) | 128 (38.8) | 80 (66.1) | <0.001 |
| Presence of blood in the airway device during extractiona | 54 (12) | 26 (7.9) | 28 (23.1) | <0.001 |
| Length of anesthetic procedure (min)c | 75 (50–120) | 70 (50–110) | 90 (62.5–120) | <0.001 |
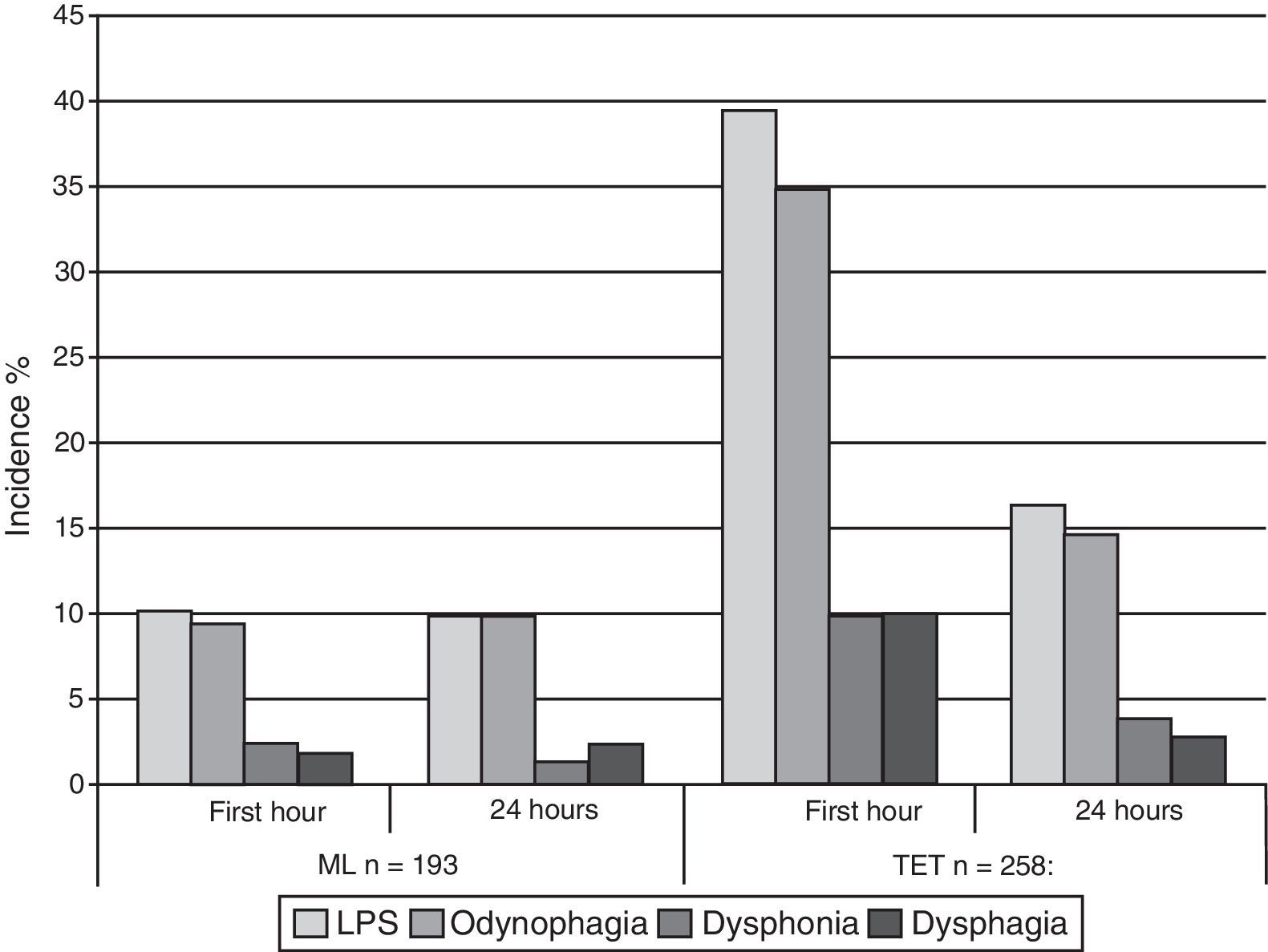
The global LPS incidence was 26.8% (121 patients), discriminated as follows: odynophagia was present in 23.9%, dysphonia 6.7%, and dysphagia 6.4%. After 24h of follow-up, the global incidence dropped to 13.5% (61 patients) (p<0.001) and each component was classified as: odynophagia (23.9–12.6%, p<0.001), dysphonia (6.7–2.7%, p=0.002) and dysphagia (6.4–2.4%, p=0.003). Table 2 and Fig. 1 present the overall stratified incidence of the AW device used in the first hour and 24h after surgery.
LPS outcome analysis stratified according to airway device, one and 24h after surgery.
| Laryngeal mask (n=193) | Endotracheal tube (n=258) | |||||
| First hour | 24h | p value* | First hour | 24h | p value | |
| LPSs | 19 (9.8) | 19 (9.8) | 1.000 | 102 (39.5) | 42 (16.3) | <0.001 |
| Odynophagia | 18 (9.3) | 19 (9.8) | 1.000 | 90 (34.9) | 38 (14.7) | <0.001 |
| Dysphonia | 4 (2.1) | 2 (1) | 0.625 | 26 (10.1) | 10 (3.9) | 0.003 |
| Dysphagia | 3 (1.6) | 4 (2.1) | 1.000 | 26 (10.1) | 7 (2.7) | <0.001 |
During the first hour, the incidence of LPS was 39.5% and 15.7% for ETT and LM, respectively (p<0.001).
The multivariate analysis showed tree independently associated co-variables in the presence of LPS in the first hour and after 24h of follow-up, taking into account its longitudinal and repeated pattern. These were as follows: (OR=1.53 95% CI 1.00–2.37, p=0.050 GEE), use of ETT (OR=4.20 95% CI 2.19–8.04, p<0.001 GEE) and the presence of blood in the AW device during removal (OR=2.00 95% CI 1.18–3.36, p=0.009 GEE) (Table 3). Interaction among variables was not documented.
Adjusted co-variable association with LPS (n=451).
| Variablesa | OR adjusted | 95% CI | p value |
| Female | 1.53 | 1.00–2.73 | 0.050 |
| Age (years) | 0.99 | 0.97–1.00 | 0.138 |
| Smoking | 0.82 | 0.49–1.36 | 0.425 |
| ASAbclassification | |||
| ASA II | 1.15 | 0.72–1.84 | 0.397 |
| ASA III | 0.58 | 0.17–2.01 | 0.595 |
| Use of endotracheal tube | 4.20 | 2.19–8.04 | <0.001 |
| Use of local anesthetic agent in airway device | 1.28 | 0.82–2.01 | 0.262 |
| Attempts to place the device in the airway | 0.81 | 0.50–1.30 | 0.391 |
| Responsible for airway managementc | |||
| Anesthesiologist | 0.60 | 0.35–1.04 | 0.072 |
| Anesthesia resident | 0.75 | 0.35–1.62 | 0.476 |
| Internist | 0.58 | 0.29–1.18 | 0.139 |
| Medical student | 0.54 | 0.28–1.04 | 0.067 |
| Pressure evaluation of airway deviced | |||
| With pressure meter | 1.02 | 0.48–2.12 | 0.957 |
| Air loss with 20cmH2O | 1.01 | 0.65–1.57 | 0.942 |
| Patient's position during surgerye | |||
| Supine decubitus | 1.29 | 0.35–4.78 | 0.697 |
| Prone decubitus | 1.63 | 0.36–7.39 | 0.524 |
| Type of surgeryf | |||
| General surgery | 1.63 | 0.72–3.68 | 0.239 |
| GI surgery | 0.90 | 0.39–2.05 | 0.808 |
| ENT surgery | 1.61 | 0.67–3.88 | 0.281 |
| Genitourinary surgery | 1.64 | 0.60–4.46 | 0.332 |
| Gynecologic surgery | 1.20 | 0.44–3.26 | 0.710 |
| Neurosurgery | 1.36 | 0.43–4.26 | 0.595 |
| Thorax surgery | 2.99 | 0.68–13.01 | 0.143 |
| Patient totally awake during airway device removal | 1.27 | 0.77–2.09 | 0.334 |
| Pharyngeal aspiration at the end of the procedure | 0.81 | 0.50–1.32 | 0.408 |
| Presence of blood in the airway device during removal | 2.00 | 1.18–3.36 | 0.009 |
| Duration of the anesthetic procedure (min) | 1.00 | 0.99–1.00 | 0.528 |
LPSs are a frequent postoperative problem. This outcome, considered by many authors and professionals as a minor discomfort, represents an area of potential improvement in our clinical practice. However, we still lack the strategies to fully prevent LPSs, probably due to a poor pathophysiological understanding.10
The incidence of postoperative sore throat has been reported ranging from 14.4% to 70% with ETT4,11–14 and from 5.8% to 34% with LM.6,12,15,16 Unfortunately, the incidences reported depend on the method used for the evaluation and classification of the outcome. Higher incidences have been shown when the questions regarding symptoms are direct.3 In this trial, direct and indirect dichotomy questioning was used, obtained from scientific publications and validated by experts. Additionally, the people who evaluated the outcome were blinded to the anesthetic interventions. With this approach for the evaluation of outcomes, our incidence of LPS and its components is consistent with the literature.1,3,5,6
We find that the incidence of LPS using ETT is higher than with LM in the first hour and 24h following surgery. This finding is consistent with prior publications.3,5 Interestingly enough, after 24h of follow-up, the incidence of LPS decreases over 50% for ETT but does not drop in patients where the LM was used. Rieger et al.3 documented a similar patter of decreased LPS incidence.
Several authors report the severity of each component in the LPSs.5 Such severity may affect the frequency of presentation and it is worth asking what is worst for the patient: having mild but frequent LPSs or more severe but infrequent LPSs? This question should be kept in mind for future research work.
LPSs have been associated to trauma, inflammation and laryngopharyngeal manipulation. The area of direct contact with these anatomic structures is larger for the LM than for the ETT. As a result of such structural differences, the pathophysiological origin of the LPS may be different for the LM versus the ETT. Considering the inflated position of the LM in the hypopharynx and the constant pressure over the mucosa, the LM could cause more severe damage resulting in a longer LPS.17 This could be a tentative explanation for the negligible reduction in LPSs 24h after the use of the LM. Furthermore, the passage of inspired gasses and the direct contact with the vocal folds may further contribute to extent the LPSs with the use of the LM.3
We present an analysis for repeated advanced categorical evaluations (longitudinal). Our modeling takes into account the repeated nature of the outcome evaluations at various points in time and their correlation with each patient.8 We identified three variables significantly associated with the presence of postoperative PLSs: use of ETT, female gender and the presence of blood in the AW device during extubation. Other authors had already reported those risk factors but no one used repeated measurement analysis.1,3,5 We did not find any other associated co-variables.
The differences found in the incidence of PLSs in our trial may be accounted for by the following facts: our anesthetic techniques were not standardized for all patients. Furthermore, our trial took place at a University hospital where many professionals are undergoing training and exhibit varying levels of expertise. A potential selection bias could have been introduced because the participating professionals were aware of the trial and could have been more careful in managing the airway.
ETI is a standard technique in the practice of anesthesia, with multiple advantages in selected patients.5 While the selection of the device for AW management is based on the clinical characteristics typical to the patient, practice and experience in airway management facilitates placement of the devices with less manipulation and physical trauma. Furthermore, several interventions to avoid or eliminate this adverse event are currently being studied with promising results. Some of them are: the use of benzydamine hydrochloride spray over the ETT balloon or over the oral mucosa18,19; the use of Amyl-m-cresol tablets by mouth20 or inhaled fluticasone propionate.21 Moreover, only a comprehensive pathophysiological understanding of LPSs may enhance our ability to overcome them. It should be stressed that sore throat is a generic name for a range of conditions that probably are of different origin and require diverse management strategies.
In conclusion, the incidence of LPSs in outpatient surgery at a Colombian hospital is relevant and consistent with worldwide publications. There are differences in the patterns to reduce the incidence of ETT and LM-related LPS at various time points and our study confirms the risk factors for the occurrence of such LPSs, including ETT, being a female and the presence of blood in the AW device during its removal.
FundingThis research was part of the “Young Researcher Project” of Colciencias.
Conflicts of interestThe authors have no conflicts of interest to declare.
Our acknowledgments to the Hospital Santa Sofia de Manizales surgery staff and to the nurse Dora Nancy for her collaboration.
Please cite this article as: Ríos AM, Calvache JA, Gómez JC, Gómez LM, Aguirre OD, Delgado-Noguera MF, et al. Síntomas laringo faringeos postoperatorios en cirugía electiva. Incidencia y factores asociados. Rev Colomb Anestesiol. 2014;42:9–15.