The probability of new major cardiovascular events (MCVEs) in patients after an acute myocardial infarction depends on different variables, such as the time elapsed after the first event, the presentation scenario (acute coronary syndrome with/without ST elevation) and the associated risk factors. These events have been related to a persistent proinflammatory response,1 platelet activity and thrombin activity.2 The arrival of medications with stronger and more specific antithrombotic activity at key points in the thrombotic process has permitted the assessment of their effects in the intensive antithrombotic secondary prevention scenario after the first year following a myocardial infarction.3
Various therapeutic strategies have been proposed in this type of prevention, including the extension of dual antiplatelet therapy (DAT),4 beginning additional antiplatelet therapies,5 or using low doses of direct anticoagulants plus aspirin.6 However, the similar and at the same time heterogenous characteristics of this population raise questions regarding when, in whom and how to use this type of therapy.
When?After the first year following a myocardial infarction, continuing DAT may significantly reduce the risk of new MCVEs, with an average follow up of 31 months, regardless of the type of P2Y12 inhibitor (P2Y12I).4 This type of management could be continued and, if it has been discontinued, it could even be restarted and maintain its beneficial effect, since the subanalysis of the PEGASUS (Prevention of Cardiovascular Events in Patients with Prior Heart Attack Using Ticagrelor Compared to Placebo on a Background of Aspirin–Thrombolysis in Myocardial Infarction 54) study showed a greater benefit in patients who continued ticagrelor without interruption or with only a brief interruption (less than one year, ideally less than 30 days).7
Recently, the COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) study evaluated intensive antithrombotic secondary prevention in patients with coronary disease using 2.5mg/12h doses of rivaroxaban plus aspirin. Sixty-eight percent of the patients had had a myocardial infarction prior to randomization and 77% of these infarctions had occurred more than two years prior. In the subgroup analysis, the benefit was consistent in all the groups, according to the time elapsed since the infarction. However, there was a tendency towards a greater effect in the group of more than five years.8 Thus, this strategy seems to be an option for a later time frame and for those who have discontinued DAT for an extended period of time after the infarction.
In Whom?The PEGASUS study included patients over the age of 50 who had had a myocardial infarction one to three years prior to randomization and had at least one of the following risk factors: age greater than 65, diabetes mellitus being treated pharmacologically, a second infarction within the last three years, multivessel coronary disease and chronic kidney disease with a glomerular filtration rate less than 60ml/min. Of these, 83% underwent some form of percutaneous coronary intervention, which in most cases required stent implantation (96.5%). The percentage of patients with peripheral artery disease was low (5.3%). Those with a history of ischemic cerebrovascular accidents (CVA), coagulation disorders, intracerebral hemorrhage, central nervous system tumors or vascular malformations, gastrointestinal bleeding within the last six months and major surgery within the last 30 days were excluded, as these conditions are considered to have a high risk for bleeding.9 In studies such as DAPT (Twelve or 30 months of Dual AntiPlatelet Therapy after drug-eluting stents) the percentage of patients with a prior myocardial infarction was approximately 26%, and they all received different-generation medicated stents.10 Currently, there are some scores that can help determine the need for continuing DAT, balancing the ischemic and bleeding risks (DAPT: Dual AntiPlatelet Therapy score), and others which determine the risk of bleeding to decide whether or not to continue DAT (PRECISE-DAPT: PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual Anti Platelet Therapy score).11,12 One of the important limitations of the DAPT score is that the study on which it is based used a significant percentage of first-generation stents. Thus, it may be more appropriate to use the PEGASUS inclusion criteria.9,13
On the other hand, the COMPASS study included patients over the age of 65 with coronary disease due to myocardial infarction in the last 20 years, or multivessel coronary disease, or prior percutaneous coronary intervention, or multivessel disease submitted to surgical myocardial revascularization. Individuals under the age of 65 were included if they had involvement of vascular territory other than coronary, or two additional risk factors: active smoker, diabetes mellitus, chronic kidney disease with a glomerular filtration rate less than 60ml/min, heart failure or non-lacunar CVA.6 Sixty percent had undergone a percutaneous coronary intervention and 20% had peripheral artery disease. One of the exclusion criteria was a high risk of bleeding, which was the second most frequent cause of exclusion.8 Currently, there are no additive scores to determine the use of the rivaroxaban plus aspirin strategy.
Analyzing these data, treatment with ticagrelor plus aspirin seems to be ideal for those over the age of 50 with at least one risk factor and no history of CVA, evaluating the ischemic and bleeding risk to determine the use of another, less potent, P2Y12I. On the other hand, the strategy with rivaroxaban (COMPASS dose) plus aspirin could be indicated in those over the age of 50 with cardiovascular risk factors but who also have a history of peripheral artery disease and CVA, or both.
How?The ticagrelor dose of 60mg every 12hours plus aspirin was proven to significantly reduce MCVEs compared with aspirin (7.7% vs. 9.9%; p 0.004), with an increase in major bleeding according to the TIMI (Thrombolysis in Myocardial Infarction) scale (2.3% vs. 1.06%, p <0.001), but with no significant difference in fatal bleeds or intracerebral hemorrhage (0.71% vs. 0.60%, p 0.47).7 The rivaroxaban dose of 2.5mg every 12hours plus aspirin, compared with aspirin, also showed a significant reduction in MCVEs (4.0% vs. 6.0%; p <0.0001), with an increase in major bleeding events according to the ISTH (International Society of Thrombosis and Hemostasis) scale (2% vs. 1%; p <0.0001), with no significant difference in fatal bleeds or intracerebral hemorrhages (< 1% vs. <1%; p 0.54).8
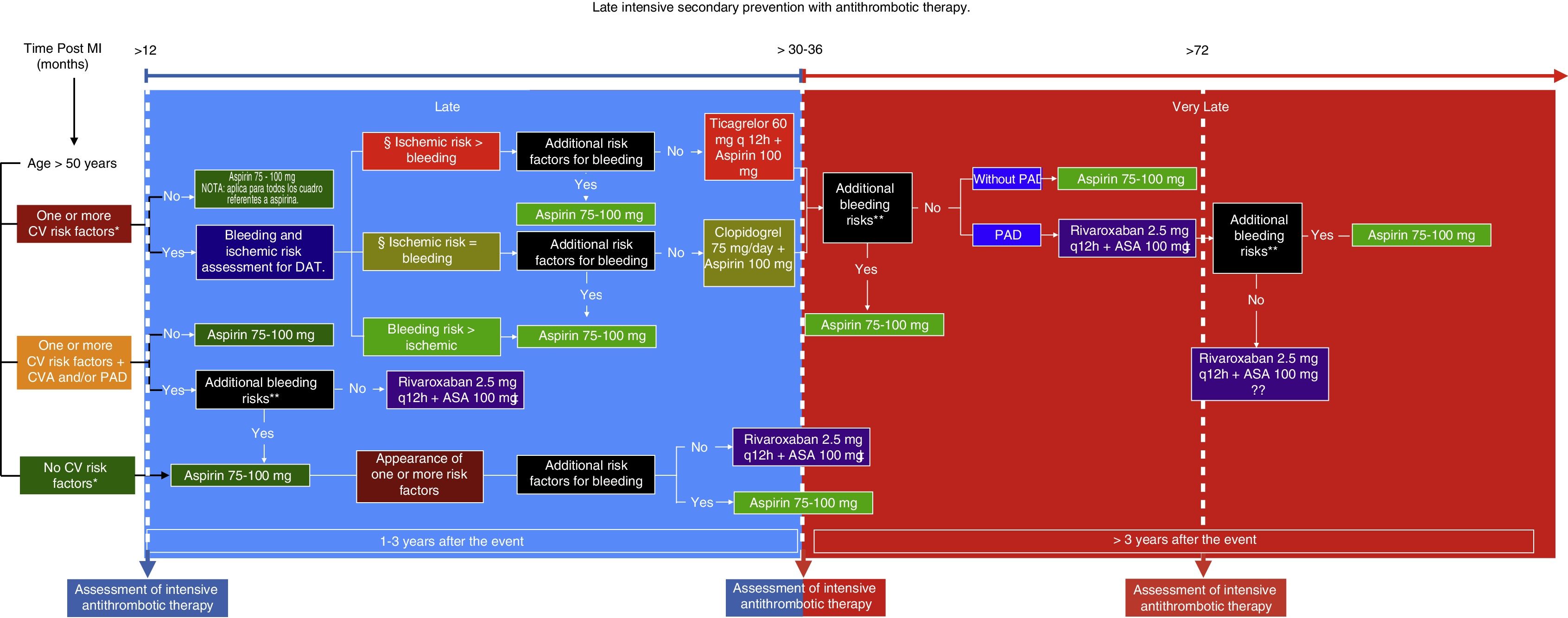
Having answered these questions and analyzed the data, a route is proposed to determine both the type as well as the time of initiation of late intensive antithrombotic secondary prevention in patients who have suffered a myocardial infarction (Fig. 1). This route includes an additional assessment of bleeding risk based on the studieś exclusion criteria and the patientś own circumstances, since, over time, the reassessment of these factors could be key in deciding whether or not to use this therapy. Likewise, it is extremely important to evaluate the goals of some of the cardiovascular risk factors since, despite that fact that they are not described precisely in the studies, the success of the treatment ultimately depends on the sum of the control of all these conditions. In addition, certain cardiovascular anatomy considerations which increase the patientś ischemic risk could be analyzed. This type of treatment is undoubtedly beneficial for secondary prevention of new thrombotic effects a year after having suffered a myocardial infarction, and thus its use is recommended. However, both patient and treatment selection must be individualized.
Late intensive secondary prevention with antithrombotic therapy. MI: Myocardial infarction. CV: Cardiovascular. CVA: Ischemic cerebrovascular accident. PAD: Peripheral artery disease. DAT: Dual antiplatelety therapy.
CV risk factors: Diabetes mellitus being treated pharmacologically; Arterial hypertension; Dyslipidemia; Chronic kidney disease with glomerular filtration rate (GFR) <60mL/min, more than one prior MI, multivessel disease.
Review optimal CV risk factor control and GFR.
Anatomical considerations: Stent >60 mm, total chronic stent occlusion, complex stenting in bifurcations.
Additional bleeding risks: Coagulation disorders, central nervous system tumors or vascular malformations, bleeding in the last six months, fragility/old age >75 years, active cancer.
Not recommended for chronic kidney disease GFR <30mL/min, Ejection fraction <30%, or history of non-lacunar or hemorrhagic CVA.
It is not currently known how long this therapy should continue after two years.
Many questions remain, such as the duration of this type of therapy, whether control of inflammation or the risk factors will determine this duration, cost-effectiveness according to the country or region, and how long aspirin will be part of secondary prevention.
For now, the idea is to provide some clarity regarding the type of patients who should receive this type of prevention, which has been termed “intensive” due to the additional antithrombotic potency these medications give to aspirin, and which we call “late” since it is given after the first year following a myocardial infarction.
Conflicts of interestNone.