Hypertension is responsible for a substantial number of deaths due to cardiovascular disease and stroke. A crucial step toward its control is the identification of modifiable predictors of hypertension.
ObjectivesTo estimate the relationship between salt intake, serum uric acid and incident hypertension in a primary care setting.
MethodsRetrospective cohort of the CAMELIA study in which a non-randomized sample of 1098 participants who were ≥ 20 year-old was recruited from a primary care program. Originally, the sample consisted of hypertensive, diabetic and non-diabetic/non-hypertensive subjects. For the analysis, 258 participants with blood pressure (BP) lower than 140/90mm Hg not using antihypertensive drugs and without diabetes mellitus were included. Five years after the first visit, their medical records were reviewed. Patients were divided into two groups according to BP in the first visit: normal BP group (systolic BP ≤ 120mm Hg and diastolic BP ≤ 80mm Hg) and high-normal BP group (systolic BP 121-139mm Hg and/or diastolic BP 81-89mm Hg).
ResultsIn multivariate analysis, high-normal BP, hyperuricemia and salt intake ≥ 6g/day predicted incident hypertension. In participants of thenormal BP group, high salt intake conferred the highest risk. In the high-normal BP group, smoking and serum uric acid were found to be the most important ones.
ConclusionIn a healthy, multiethnic, and normotensive population from an urban primary care program, high-normal BP, hyperuricemia and high salt intake were found to be predictors of incident hypertension.
La hipertensión es responsable de un gran número de muertes debido a cardiopatías e ictus. Un paso esencial para su control es la identificación de factores modificables predictivos de la hipertensión.
ObjetivosCalcular la relación entre ingesta de sal, ácido úrico sérico e hipertensión incidental en un centro de atención primaria.
MétodosCohorte retrospectiva del estudio CAMELIA, en el que se incluyó una muestra no aleatorizada de 1.098 participantes con edades ≥ 20 años, obtenida de un programa de atención primaria. Originalmente, la muestra incluía sujetos hipertensos, diabéticos y no diabéticos/no hipertensos. Para el análisis, se estudiaron 258 participantes con presión arterial (PA) inferior a 140/90mm Hg, sin prescripción de fármacos antihipertensivos, y no diabéticos. Transcurridos cinco años de la primera visita, se revisaron sus historias médicas. Se dividió a los pacientes en dos grupos, con arreglo a su PA en la primera visita: grupo con PA normal (PA sistólica ≤ 120mm Hg y PA diastólica ≤ 80mm Hg), y grupo con PA alta-normal BP (PA sistólica 121-139mm Hg y/o PA diastólica 81-89mm Hg).
ResultadosEn el análisis multivariante, la PA alta-normal, hiperuricemia e ingesta de sal ≥ 6g/día predijeron la hipertensión incidental. En los participantes del grupo de PA normal, la ingesta elevada de sal confirió el mayor riesgo. En el grupo de PA alta-normal, el tabaquismo y el ácido úrico sérico fueron los factores más importantes.
ConclusiónEn una población sana, multiétnica y normotensa, procedente de un programa de atención primaria urbana la PA alta-normal, hiperuricemia e ingesta elevada de sal constituyeron los factores predictivos de la hipertensión incidental.
Cardiovascular diseases are the world leading cause of death1,2. Hypertension is responsible for at least 45% of deaths due to cardiovascular disease and 51% of deaths due to stroke1,2. The most common risk factors for hypertension are age, gender, race, chronic kidney disease, body mass index, unhealthy diet and salt intake, stress, smoking, sedentarism and alcohol1. A crucial step toward preventive strategies would be the identification of modifiable predictors of hypertension.
High salt intake is thought to be one of the most important predictors of incident hypertension (IH)3,4. The majority of the studies supports a role for high salt intake in the development of hypertension 5–7 but the subject is still a matter of controversy. Otsuka et al., for instance, did not observe a relationship between salt intake and IH8.
Hyperuricemia often accompanies metabolic syndrome, hypertension and diabetes9,10. Previous studies have confirmed an important association between uric acid and hypertension11–13 and a recent meta-analysis showed that hyperuricemia may increase the risk of hypertension incidence14.
We decided to explore the relationship of both, uric acid and salt intake, with incident hypertension in a relatively young and healthy primary care population.
MethodsStudy populationSubjects were recruited from a public primary care program in Niteroi, Rio de Janeiro, Brazil. Thirteen primary care settings were selected by convenience, covering all administrative areas of the city. Inclusion criteria were ≥ 20 years-old and acceptance of written informed consent. Exclusion criteria were pregnancy, immune deficiencies and use of immunosuppressive drugs. The local ethics committee approved the study (CMM/HUAP-220/05).
Study designThis study was a retrospective cohort of the CAMELIA study (Cardiometabolic Renal Family Study). Methodological details of the study protocol are published elsewhere15. Briefly, the CAMELIA study involved 1.098 individuals from primary care settings who were enrolled following an initial selection of index cases. To be accepted as an index case, individuals needed to have a partner who agreed to participate in the study and have at least one child between 12 and 30 years-old who would also enroll. Index cases were divided into four groups based on the presence of hypertension and diabetes mellitus. In this study, only the group without hypertension and diabetes mellitus was included for analysis.
In the initial visit, patients were scheduled to clinical evaluation at the primary care setting andfasting blood samples were taken for analysis. Five years later, participant́s medical records were reviewed and information about clinical evaluation, blood pressure (BP) and blood assays was gathered from the two most recent visits that occurred after the baseline visit. We analyzed BP readings, antihypertensive medication prescription, smoking history, blood analysis, including serum uric acid and salt intake in the whole population. Furthermore, participants were divided into two groups according to BP readings at the first visit (normal BP group and high-normal BP group) to better analyze the factors that could influence the emergence of IH.
Clinical evaluationOffice BP was obtained using an electronic device (model HEM-705CP, Omron Healthcare Inc., Lake Forest, IL, US) and an appropriate sized cuff. Before measurement, patients were seated for 30minutes and refrained from smoking and caffeine ingestion. Three readings, one minute apart, were done, and the average of these measurements was defined as the patient BP. Body mass index (BMI) was calculated as body weight (in kilograms) divided by squared height (in meters). Waist circumference was measured at the midpoint between the lower rib margin and anterior superior iliac crest and was considered high if ≥ 94cm in males and ≥ 80cm in females. Patients were said to be smokers if they smoked more than 100 cigarettes in their life.
ParametersAt baseline, hypertension, normal BP and high-normal BP were defined according to VI Brazilian Guidelines on Hypertension16. Briefly, subjects with BP more than 120/80mm Hg and less than 140/90mm Hg were classified as high-normal BP group and those with BP ≤ 120/80mm Hg as normal BP group. During follow-up, incident hypertension was defined as BP ≥ 140/90mm Hg and/or prescription of antihypertensive medications.High uric acid was defined as serum levels ≥ 6.8mg/dl for males and ≥ 5.4mg/dl for females.High salt intake was defined as ≥ 6g/day. Urinary biochemical parameters were measured in a first morning urine sample and included creatinine (Cr), albumin and sodium. The sodium excretion index (mEq Na/g-creatinine) was estimated by determining the rate of excretion of Na ([Na in mEq/L in a morning urine sample/urinary creatinine in mg/dl]*100) 17. The calculated sodium excretion index was then converted to salt intake, in grams of sodium chloride. Fasting venous blood was collected to measure total cholesterol, triglycerides, high-density lipoprotein cholesterol, glucose, creatinine, and uric acid. The low-density lipoprotein cholesterol level was calculated by the Friedewald formula. The glomerular filtration rate was estimated (eGFR) by CKD-EPI formula18.
Statistical analysisData are presented as mean±standard deviation for continuous variables and absolute and relative frequencies for categorical variables unless otherwise specified. Student t test and chi-square were used to compare, respectively, continuous, and categorical variables. Crude and adjusted relative risks were estimated by single and multiple Poisson regression models. The models were performed using generalized estimating equations (GEE), which are suitable for non-independent observations, since the unit for inclusion in the original study was family. The variables that reached significance of 0.10 in the univariate analysis were included in the multiple models. Values of p<0.05 were considered to be significant at the multiple models. In addition, a log rank test was run to determine cumulative incidence of hypertension. Statistical analyses were performed using SPSS v. 21.0 (Statistical Package for Social Sciences, Chicago, USA).
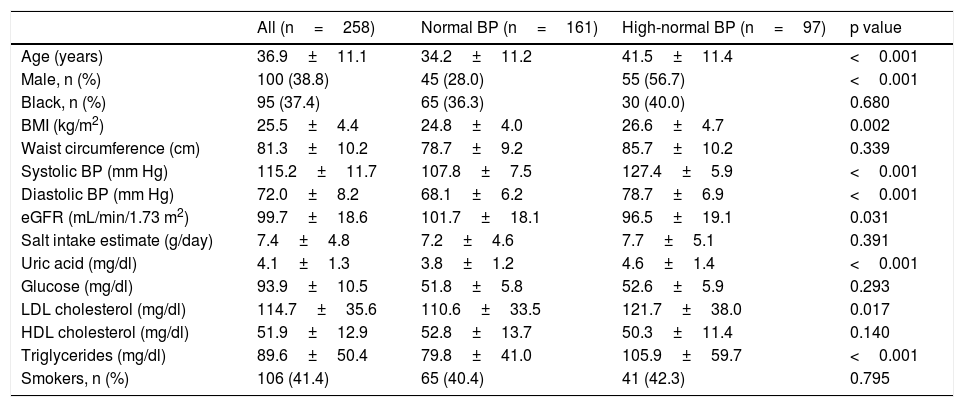
ResultsOf the 1098 CAMELIA's participants, 258met our inclusion criteria. The median time of follow-up was 44 months (7 – 61 months). Baseline characteristics of subjects are in Table 1. There were 161 individuals in normal BP group and 97 in high-normal BP group.
Baseline characteristics of the study participants.
| All (n=258) | Normal BP (n=161) | High-normal BP (n=97) | p value | |
|---|---|---|---|---|
| Age (years) | 36.9±11.1 | 34.2±11.2 | 41.5±11.4 | <0.001 |
| Male, n (%) | 100 (38.8) | 45 (28.0) | 55 (56.7) | <0.001 |
| Black, n (%) | 95 (37.4) | 65 (36.3) | 30 (40.0) | 0.680 |
| BMI (kg/m2) | 25.5±4.4 | 24.8±4.0 | 26.6±4.7 | 0.002 |
| Waist circumference (cm) | 81.3±10.2 | 78.7±9.2 | 85.7±10.2 | 0.339 |
| Systolic BP (mm Hg) | 115.2±11.7 | 107.8±7.5 | 127.4±5.9 | <0.001 |
| Diastolic BP (mm Hg) | 72.0±8.2 | 68.1±6.2 | 78.7±6.9 | <0.001 |
| eGFR (mL/min/1.73 m2) | 99.7±18.6 | 101.7±18.1 | 96.5±19.1 | 0.031 |
| Salt intake estimate (g/day) | 7.4±4.8 | 7.2±4.6 | 7.7±5.1 | 0.391 |
| Uric acid (mg/dl) | 4.1±1.3 | 3.8±1.2 | 4.6±1.4 | <0.001 |
| Glucose (mg/dl) | 93.9±10.5 | 51.8±5.8 | 52.6±5.9 | 0.293 |
| LDL cholesterol (mg/dl) | 114.7±35.6 | 110.6±33.5 | 121.7±38.0 | 0.017 |
| HDL cholesterol (mg/dl) | 51.9±12.9 | 52.8±13.7 | 50.3±11.4 | 0.140 |
| Triglycerides (mg/dl) | 89.6±50.4 | 79.8±41.0 | 105.9±59.7 | <0.001 |
| Smokers, n (%) | 106 (41.4) | 65 (40.4) | 41 (42.3) | 0.795 |
Mean±S.D. unless otherwise specified; BMI, body mass index; BP, blood pressure; Cr, creatinine; eGFR, estimated glomerular filtration rate by CKD-EPI equation; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
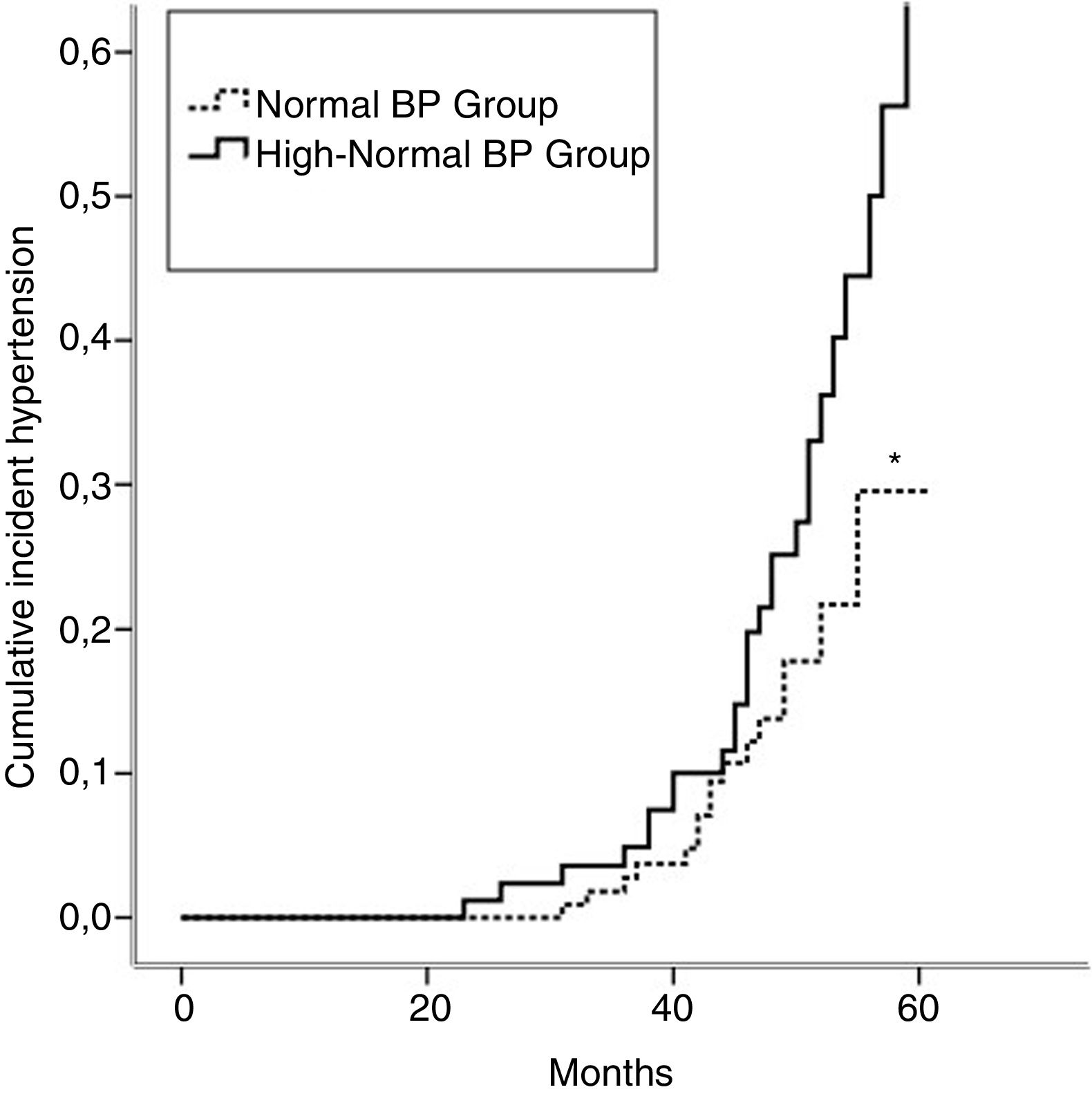
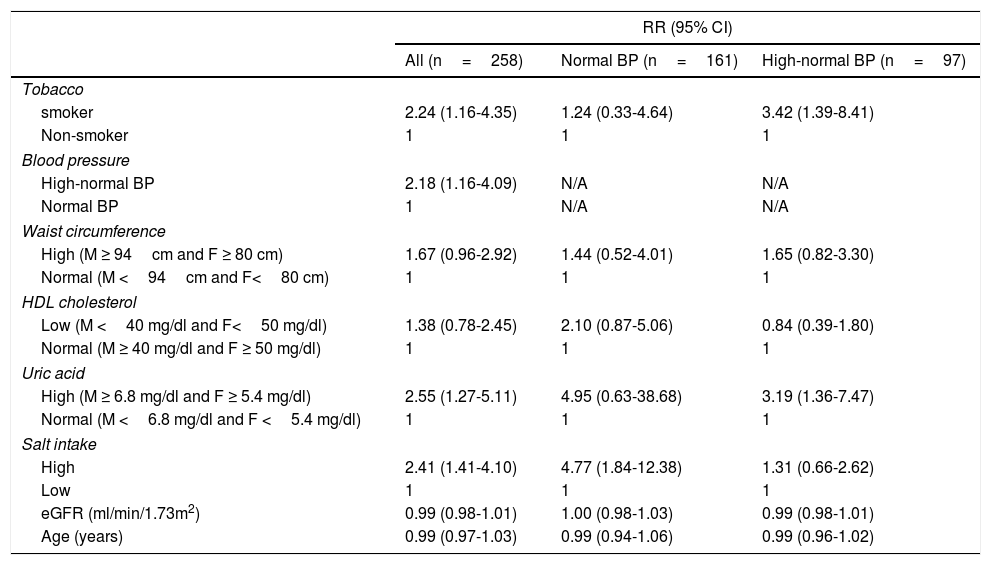
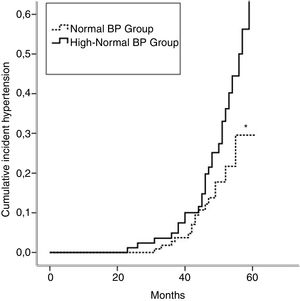
Incident hypertension occurred in 42 (16.3%) patients and was similar in men and women (15.0% vs. 17.1%, p=0.731). Cumulative incidence rates of hypertension in normal BP and high-normal BP groups are shown in Figure 1. In a crude analysis for the whole sample, IH was associated with age, smoking, high-normal BP, BMI, waist circumference, HDL-cholesterol, uric acid, and salt intake. In the multivariate analysis, smoking, high-normal BP, hyperuricemia, and high salt intake were kept aspredictors of IH (Table 2). When the analysis was performed separating the two groups, high salt intake was found to be the only predictor of IH in the normal BP group. In contrast, smoking and hyperuricemia were the factors that predict IH in the high-normal BP group (Table 2).
Adjusted* relative risks of incident hypertension.
| RR (95% CI) | |||
|---|---|---|---|
| All (n=258) | Normal BP (n=161) | High-normal BP (n=97) | |
| Tobacco | |||
| smoker | 2.24 (1.16-4.35) | 1.24 (0.33-4.64) | 3.42 (1.39-8.41) |
| Non-smoker | 1 | 1 | 1 |
| Blood pressure | |||
| High-normal BP | 2.18 (1.16-4.09) | N/A | N/A |
| Normal BP | 1 | N/A | N/A |
| Waist circumference | |||
| High (M ≥ 94cm and F ≥ 80 cm) | 1.67 (0.96-2.92) | 1.44 (0.52-4.01) | 1.65 (0.82-3.30) |
| Normal (M <94cm and F<80 cm) | 1 | 1 | 1 |
| HDL cholesterol | |||
| Low (M <40 mg/dl and F<50 mg/dl) | 1.38 (0.78-2.45) | 2.10 (0.87-5.06) | 0.84 (0.39-1.80) |
| Normal (M ≥ 40 mg/dl and F ≥ 50 mg/dl) | 1 | 1 | 1 |
| Uric acid | |||
| High (M ≥ 6.8 mg/dl and F ≥ 5.4 mg/dl) | 2.55 (1.27-5.11) | 4.95 (0.63-38.68) | 3.19 (1.36-7.47) |
| Normal (M <6.8 mg/dl and F <5.4 mg/dl) | 1 | 1 | 1 |
| Salt intake | |||
| High | 2.41 (1.41-4.10) | 4.77 (1.84-12.38) | 1.31 (0.66-2.62) |
| Low | 1 | 1 | 1 |
| eGFR (ml/min/1.73m2) | 0.99 (0.98-1.01) | 1.00 (0.98-1.03) | 0.99 (0.98-1.01) |
| Age (years) | 0.99 (0.97-1.03) | 0.99 (0.94-1.06) | 0.99 (0.96-1.02) |
*Estimated by Generalized Estimating Equations, Poisson log linear regression. BP, blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate by CKD-EPI; F, female; HDL, high density lipoprotein; M, male; N/A, not applicable; SBP, systolic blood pressure.
In this retrospective cohort, we evaluated the relationship between uric acid, salt intake and incident hypertension. For this, we studied a sample derived from a population assisted by a primary care program, originallyenrolled in the CAMELIA study, five years after the first visit. For the present study, only healthy and young participants were selected bypassing metabolic syndrome and/or chronic kidney disease as confounders that could distort the relationship between hypertension, salt intake and uric acid. Consistent with previous studies11,12,14,19,20, we showed that smoking, high-normal BP, hyperuricemia, and high salt intake were predictors of IH.
High salt intake may promote hypertension through volume expansion, stimulation of sympathetic nervous system, modifying renin secretion and even promoting inflammation and endothelial dysfunction19. Uric acid is associated to hypertension and may promote high blood pressure by reducing pressure dependent natriuresis, activation of the renin-angiotensin-aldosterone system and suppression of nitric oxide9. Finally, nicotine activates sympathetic nervous system and elevates blood pressure.
When analyzing the whole population, smoking, hyperuricemia, high salt intake and high-normal BP were found to be predictors of IH. As recently reviewed 21, the issue of predictors of IH was the subject of a number of studies, but only a few of them enrolled truly healthy participants.In support to our findings, the Framingham Offspring Studywhose participants were similar to ours regarding age and BP, showed that age, sex (female), BP, BMI, smoking and parental history of hypertension were predictors of IH22. However, they did not evaluate either salt intake or uric acid. In a cohort study involving 118,920 healthy subjects, serum uric acid, age, systolic BP and BMI were also found to be risk factors for IH 12. In addition, higher salt intake was associated with increases in uric acid and higher salt intake was an independent risk factor for development of hypertension in individuals with higher uric acidin the PREVEND study 19.
In the present study, IH predictors were different in patients with normal and high-normal BP. As far as we could know only one study resorted to a similar strategy comparing predictive factors in normal and high-normal BP participants 23. They did not address the salt intake issue but in some way similar to our results, they reported that age, BP, BMI, serum glucose and uric acid were predictors of IH in patients with high-normal BP23. On the other hand, only age, BMI and BP were predictors of IH in normal BP patients. Participants were older and a non-negligible fraction of them was diabetic, factors that can account for the minor differences between studies.
We could not find a definite explanation concerning the differences in the predictive factors of IH for the normal BP group and the high-normal BP group. If hyperuricemia indeed pave the way for future hypertension in some population groups, our finding of its predictive value in the high-normal BP group could simply be a reflex of an ongoing process.
This study has some limitations. At first, sample size was not very large for this kind of study. Secondly, follow up data was collected from medical records and we could not rule out measurement bias. However, most patients considered hypertensive in follow up visits were under antihypertensive medication, so the probability of misdiagnosis was minimized. Some authors also consider spot urine specimen as a limitation to salt intake estimation. However, 24-hour urinary measurement is not practical for large-scale studies and there are many previous studies validating salt intake estimation from spot urine specimen17,24.
ConclusionsRecognition of modifiable incident hypertension predictors is an important step toward preventive strategies to reduce blood pressure and hence cardiovascular mortality. In the present study, smoking, high-normal BP, hyperuricemia and high salt intake were the most important predictors of IH. Salt intake was the most important predictor in individuals from the normal BP group. On the other hand, smokers and individuals with elevated serum uric acid had the highest risk of IH in the high-normal BP group. More studies are needed to confirm differences in IH predictive factors between individuals with normal BP and high-normal BP.
Conflicts of interestNone.
Acknowledgement of grant supportNone.