Although the social and economic consequences of Colombian internal conflicts mainly affected the civilian population, they also had other implications. The ex-combatants, the other side of the conflict, have been the subject of many studies that question their personality structures and antisocial features. Results suggest that ex-combatants usually have characteristics of an antisocial personality disorder (ASPD) that is related with their behaviour.
MethodsQuantitative EEG (qEEG) was used to evaluate differences in cortical activity patterns between an ex-combatants group and a control group. The Psychopathy Checklist-Revised (PCL-R) was used to assess the presence of ASPD in the ex-combatants group, as well as the Diagnostic Interview for Genetic Studies (DIGS) for other mental disorders classified in the DCI-10.
ResultsThere are significant differences in psychopathy levels between groups, as well as in alpha-2 and beta waves, especially in left temporal and frontal areas for alpha-2 waves and left temporal-central regions for beta waves.
ConclusionsqEEG measurements allow spectral resting potential to be differentiated between groups that are related with features typically involved in antisocial personality disorder, and to correlate them with patterns in the questionnaires and clinical interview.
El conflicto armado colombiano, además de secuelas sociales y económicas en la población civil, ha implicado otras consecuencias. Los excombatientes, como la otra cara del conflicto, han sido objeto de interrogación sobre su estructura de personalidad y predisposición a conductas violentas. Se ha encontrado en parte de la población asociada al conflicto armado rasgos característicos del trastorno de personalidad antisocial (TPA) que se relacionarían con los comportamientos en contra de las convenciones sociales.
MétodosMediante registros electrocardiográficos cuantitativos (qEEG), se evaluaron las diferencias en los patrones de actividad cortical entre un grupo de excombatientes, algunos de ellos con diagnóstico de TPA, y un grupo de control ajeno al conflicto armado y sin alteraciones psiquiátricas. Se empleó la Lista de Chequeo de Psicopatía Revisada (PCL-R) para determinar la presencia de criterios diagnósticos de TPA, así como la entrevista diagnóstica para estudios genéticos (DIGS) para clasificar la presencia de otros trastornos mentales incluidos en el CIE-10.
ResultadosSe encontraron diferencias significativas en los niveles de psicopatía evaluados mediante la PCL-R. Con respecto al análisis de la potencia espectral, se observaron diferencias entre grupos en las ondas alfa-2 y beta, en regiones frontal y temporal izquierda y temporocentral izquierda respectivamente.
ConclusionesLa técnica de qEEG permite evidenciar las diferencias entre grupos en el potencial espectral en reposo, las cuales se relacionan con comportamientos desadaptativos característicos del TPA.
Antisocial personality disorder (ASPD) consists of a generalised pattern of disregard and violation of the rights of others.1,2 Individuals with this disorder typically exhibit behaviour such as impulsiveness, irritability, poor expression of emotions, violation of the rights of others, violent responses and breach of social norms.3–5 Although the prevalence of ASPD in the general population is relatively low (3% of males and 1% of females), it is significantly high in the forensic and prison population (from 50% to 80%).6 The clinical characteristics of the disorder do not only affect personal dynamics, they also have an impact on the individuals’ social and family environment, and special importance has therefore been given to studying the mechanisms which influence this condition.2,7,8
Recent studies suggest that subjects with ASPD are more likely to use drugs and become involved in gangs or illegal armed groups; added to the high costs of mental health policies, this also means a great deal of expenditure in response to crime, theft, property damage and security.9,10 At the same time, exposure to violent contexts considerably increases the risk of healthy people suffering from ASPD, sociopathy and sadistic personality disorder.11,12 This phenomenon has been explained as a mechanism of biological and psychological adaptation to stressful conditions of war.13 The Colombian armed conflict has not only caused social, economic and political damage to the country, it has also affected the mental health of the combatants,14,15 with problems such as high levels of aggression, anxiety, depression and ASPD.16,17 The characteristics of this conflict and the large number of demobilised combatants make it a unique scenario for studying the characteristics of ASPD in ex-combatants.
Among the classic tools for the study of ASPD, there are different neuropsychological tests and psychiatric interviews that seek to assess the patient according to social profiles. One of the instruments which, for its sensitivity to disturbances in affective and social functions, has become the reference standard for assessing psychopathy (the highest expression of antisocial personality disorder) is the Psychopathy Checklist-Revised (PCL-R).18 As a complement to pencil and paper instruments, in recent years the use of neurophysiology techniques, such as the recording of cortical electrical activity (electroencephalography [EEG]) and functional magnetic resonance imaging, has contributed to the study of these types of disorders by enabling greater discrimination of cognitive processes and better temporal and spatial resolution of their neurobiological mechanisms.2,7,19
Among the studies using EEG, few have worked with ASPD and they have focused on event-related potentials. Compared to normal people, the population with ASPD has been found to have changes in the P300 wave when executing attention tasks,20,21 in the negativity of the MMN wave in visual and auditory discrimination tasks22 and in the N170 wave for face perception.23 A number of studies have quantified the EEG signals (quantitative EEG [qEEG]) and found decreases in alpha band activity and increases in the theta and gamma bands in frontal-temporal and temporal-occipital regions, which have been shown to be related to items in the PCL-R interview.2,7,9,19
Specifically in ex-combatants of the Colombian armed conflict, only one study has worked with EEG; relating levels of empathy with brain activity. When presented with stimuli with affective content, ex-combatants were found to have changes in the LPP wave, which indicates changes in late stages of emotional processing.13 However, this study does not provide information on possible personality disorders present in the population and their relationship with the EEG measurements. In view of the above, the aim of this study was to further our understanding of the neurophysiology of ex-combatants of the Colombian armed conflict with ASPD, and to characterise the qEEG of a group from this population in comparison to a control group. Differences are expected in the EEG measurements of the population with ASPD versus the controls which correlate with the PCL-R scores.
MethodsPopulationThe initial sample consisted of 20 men randomly selected from among a group of ex-combatants belonging to the Reintegration Programme run by the Presidency of the Republic of Colombia's High Council for Reintegration, Antioquia region. The participants were aged over 18 and were able to read and write. In addition, 20 control subjects were selected matched by age, gender and schooling, with no history of belonging to either an illegal or legal armed group. The exclusion criteria for both groups were having a history of psychiatric or neurological disease and auditory or visual impairment.
After applying the PCL-R test and analysing compliance with criteria for personality disorder according to the tenth version of the International Classification of Diseases (ICD-10), ASPD was identified in 13 of the ex-combatant subjects, who became the ExASPD group (average age, 37.30±8.44 years). In the control group, nobody met ASPD criteria, and only one subject was rejected because he had been in an urban gang in his adolescence, resulting in a total of 19 subjects in this group (average age, 35.75±6.97 years).
The informed consent form was approved by the bioethics committee of the University Research Headquarters at the Universidad de Antioquia. The project database was generated in accordance with the ethical standards expressed in the Declaration of Helsinki. The participants were informed about the benefits and possible risks of the research, which was classified as non-invasive with minimal risk. The information obtained was only accessible to the principal investigator and his/her co-investigators, as mentioned in Resolution No. 008430 of 1993 of the Colombian Ministry of Health. The ethical aspects relating to human research were taken into account, as expressed in articles 49–56 of Law 1090 of 2006 of the Ministry of Social Protection.
Psychiatric assessmentPsychopathy Checklist-RevisedThe PCL-R is a 20-item scale for measuring psychopathy designed by Robert Hare. Each item can be classified as: does not apply at all (rating 0); applies somewhat (rating 1); and definitely applies (rating 2), and it can have a total score from 0 to 40.24 The scale has already been validated for the Colombian population, demonstrating that the psychometric properties are repeatable.25
The participants were assessed in a clinic behind closed doors, where they were told that they were going to be asked some questions about their experiences at different stages of their lives. They were asked to give their permission for the interview to be recorded on a voice recorder. The test lasted approximately 45–60min, during which time the interviewer confirmed the confidentiality of the data provided and the option of not answering questions or even stopping the interview voluntarily at any time.
After qualifying each item, the participants were classified according to the total score of the PCL-R into: normal subjects, with PCL-R ≤20; having traits suggestive of ASPD, with PCL-R 21 to ≤30; and psychopaths, with PCL-R ≥31. This classification is in line with that made by Abalos Riquelme et al. in 2004.25
We also applied the Diagnostic Interview for Genetic Studies (DIGS), which enabled us to make a categorical diagnosis of the mental disorders included in the ICD-10.26 This interview was conducted to establish whether or not the participants met diagnostic criteria for antisocial personality disorder and compare with the PCL-R classification.
EEG recordingThe EEG signals were recorded with a Neuroscan amplifier (Neuroscan Medical System, Neurosoft Inc., Sterling, Virginia, United States) (0.1±200Hz bandpass) with 64 channels positioned according to the international 10–10 system. We used an electrode placed on the midline, between the Cz and CPz channels as reference (later recalculated to the average of all the channels) and a sampling frequency of 1000Hz. Simultaneously, the horizontal and vertical eye movement signals were recorded using a bipolar montage (0.1±100Hz passband).
The recordings were made in a Faraday cage, and the participants were instructed to keep still for 5min, sitting comfortably. We monitored the participants during the recordings to make sure they did not fall asleep.
The signal was segmented into one-second periods (1000 samples). The periods with ocular, muscular or other artefacts were removed by a computerised automated procedure based on linear trend, joint probability and kurtosis approach.27 The analysis of independent components was then used to identify signals of ocular, muscular and exogenous origin (visually detected by an expert [C.T.]). These artefactual sources from the original signal were corrected. Lastly, the automated period removal procedure was repeated to subtract those with residual artefact.
Calculation of the spectral energyFor the quantitative analysis of the signal, we used the Fourier transform with the Welch methodology for the calculation of the relative spectral power in 6 frequency bands: delta (0.5–4.0Hz), theta (4.0–8.0Hz), alpha-1 (8.0–10.0Hz), alpha-2 (10.0–13.0Hz), beta (13.0–25.0Hz) and gamma (25.0–50.0Hz). The power measurements were normalised by dividing the power in one band by the sum of the power in all the bands. We selected 50 periods in each recording at random for the power calculation. Subsequently, due to the non-normal distribution of the data, the power values were log-normalised. As a result, an array of power values of dimension 64×6×50 was obtained for each recording, with 64 being the number of channels; 6, the frequency bands and 50, the number of periods selected. The original matrix was reduced to 64×6 by averaging the spectral power in the 50 periods.
Statistical analysisStatistical significance was established at 5% for all the tests. The criterion of normality for all parameters was verified using the Jarque–Bera test28 with critical values calculated by Monte Carlo simulations. For measurements with normal distribution, the unpaired two-sample t-test was used to compare the spectral characteristics of the two groups (ExASPD and controls) in a specific band and region. When the assumption of normality was not met, the nonparametric Mann–Whitney–Wilcoxon test (U test) was used. For the regions and bands where differences were found between the groups, the relationship between the spectral values and the total PCL-R score and the score for each item was calculated using the Pearson correlation analysis.
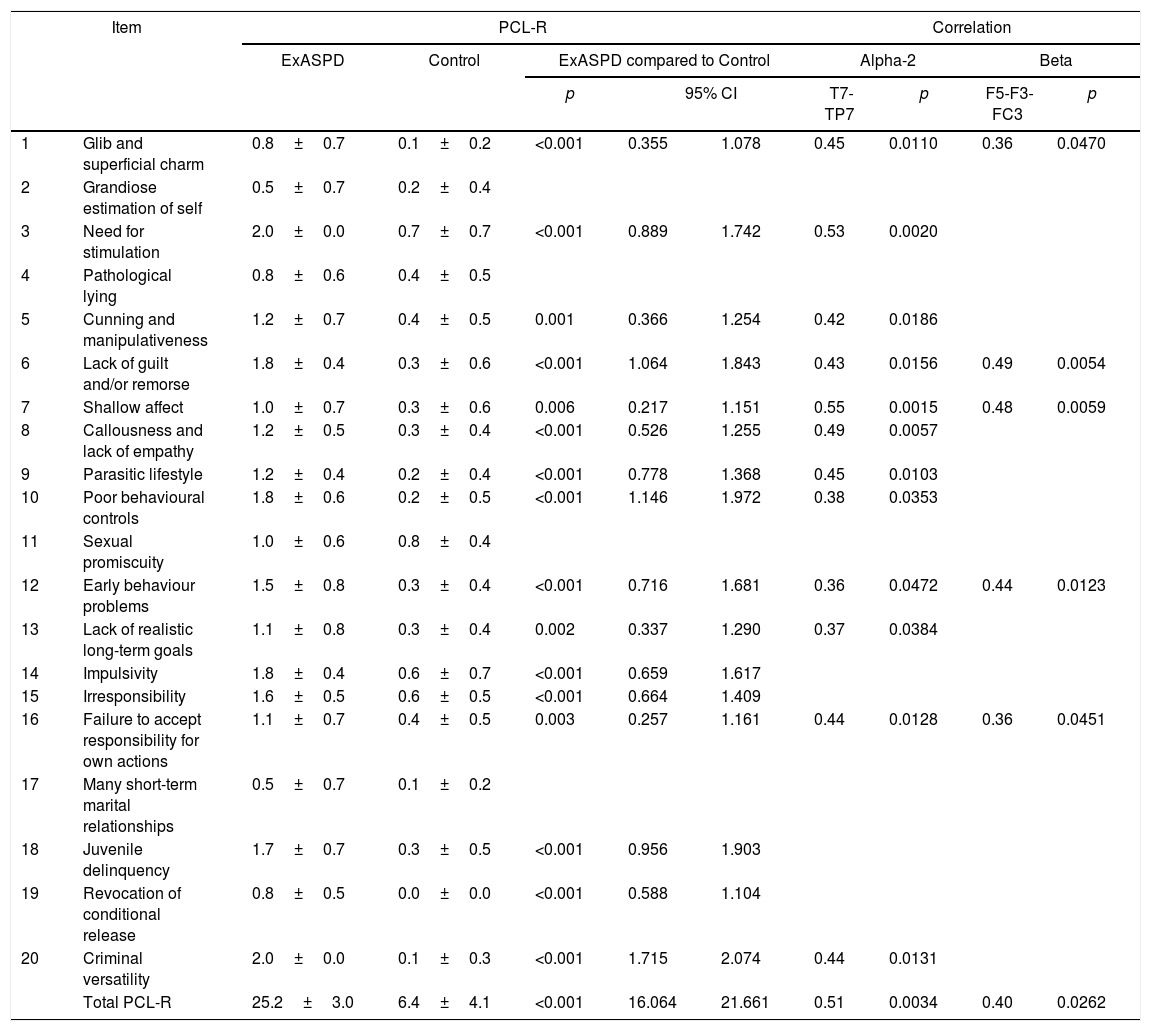
ResultsPCL-R and DIGSWhen the demographic information was compared, no differences were found in age (p=0.5679; t=−0.568; 95% confidence interval [95% CI], −7.06 to 3.95) or schooling (p=0.3120; t=−1.028; 95% CI, −3.134 to 1.034) between the ExASPD and control groups. As expected, there were significant differences in the levels of psychopathy according to the results of the PCL-R when comparing the two groups (Table 1). The differences were significant in all the items except “grandiose estimation of self”, “pathological lying”, “sexual promiscuity” and “many short-term marital relationships”.
PCL-R scores for the two groups and correlation with the qEEG.
| Item | PCL-R | Correlation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ExASPD | Control | ExASPD compared to Control | Alpha-2 | Beta | ||||||
| p | 95% CI | T7-TP7 | p | F5-F3-FC3 | p | |||||
| 1 | Glib and superficial charm | 0.8±0.7 | 0.1±0.2 | <0.001 | 0.355 | 1.078 | 0.45 | 0.0110 | 0.36 | 0.0470 |
| 2 | Grandiose estimation of self | 0.5±0.7 | 0.2±0.4 | |||||||
| 3 | Need for stimulation | 2.0±0.0 | 0.7±0.7 | <0.001 | 0.889 | 1.742 | 0.53 | 0.0020 | ||
| 4 | Pathological lying | 0.8±0.6 | 0.4±0.5 | |||||||
| 5 | Cunning and manipulativeness | 1.2±0.7 | 0.4±0.5 | 0.001 | 0.366 | 1.254 | 0.42 | 0.0186 | ||
| 6 | Lack of guilt and/or remorse | 1.8±0.4 | 0.3±0.6 | <0.001 | 1.064 | 1.843 | 0.43 | 0.0156 | 0.49 | 0.0054 |
| 7 | Shallow affect | 1.0±0.7 | 0.3±0.6 | 0.006 | 0.217 | 1.151 | 0.55 | 0.0015 | 0.48 | 0.0059 |
| 8 | Callousness and lack of empathy | 1.2±0.5 | 0.3±0.4 | <0.001 | 0.526 | 1.255 | 0.49 | 0.0057 | ||
| 9 | Parasitic lifestyle | 1.2±0.4 | 0.2±0.4 | <0.001 | 0.778 | 1.368 | 0.45 | 0.0103 | ||
| 10 | Poor behavioural controls | 1.8±0.6 | 0.2±0.5 | <0.001 | 1.146 | 1.972 | 0.38 | 0.0353 | ||
| 11 | Sexual promiscuity | 1.0±0.6 | 0.8±0.4 | |||||||
| 12 | Early behaviour problems | 1.5±0.8 | 0.3±0.4 | <0.001 | 0.716 | 1.681 | 0.36 | 0.0472 | 0.44 | 0.0123 |
| 13 | Lack of realistic long-term goals | 1.1±0.8 | 0.3±0.4 | 0.002 | 0.337 | 1.290 | 0.37 | 0.0384 | ||
| 14 | Impulsivity | 1.8±0.4 | 0.6±0.7 | <0.001 | 0.659 | 1.617 | ||||
| 15 | Irresponsibility | 1.6±0.5 | 0.6±0.5 | <0.001 | 0.664 | 1.409 | ||||
| 16 | Failure to accept responsibility for own actions | 1.1±0.7 | 0.4±0.5 | 0.003 | 0.257 | 1.161 | 0.44 | 0.0128 | 0.36 | 0.0451 |
| 17 | Many short-term marital relationships | 0.5±0.7 | 0.1±0.2 | |||||||
| 18 | Juvenile delinquency | 1.7±0.7 | 0.3±0.5 | <0.001 | 0.956 | 1.903 | ||||
| 19 | Revocation of conditional release | 0.8±0.5 | 0.0±0.0 | <0.001 | 0.588 | 1.104 | ||||
| 20 | Criminal versatility | 2.0±0.0 | 0.1±0.3 | <0.001 | 1.715 | 2.074 | 0.44 | 0.0131 | ||
| Total PCL-R | 25.2±3.0 | 6.4±4.1 | <0.001 | 16.064 | 21.661 | 0.51 | 0.0034 | 0.40 | 0.0262 | |
Only values with statistical significance are shown.
With regard to DIGS, none of the participants had psychiatric disorders such as major depression, mania or hypomania, dysthymia or cyclothymia, psychosis or anxiety disorders. The interview revealed that 13 of the 20 ex-combatants assessed had dissocial or antisocial personality disorder according to ICD-10 criteria, which coincided with the PCL-R scores established for ASPD.
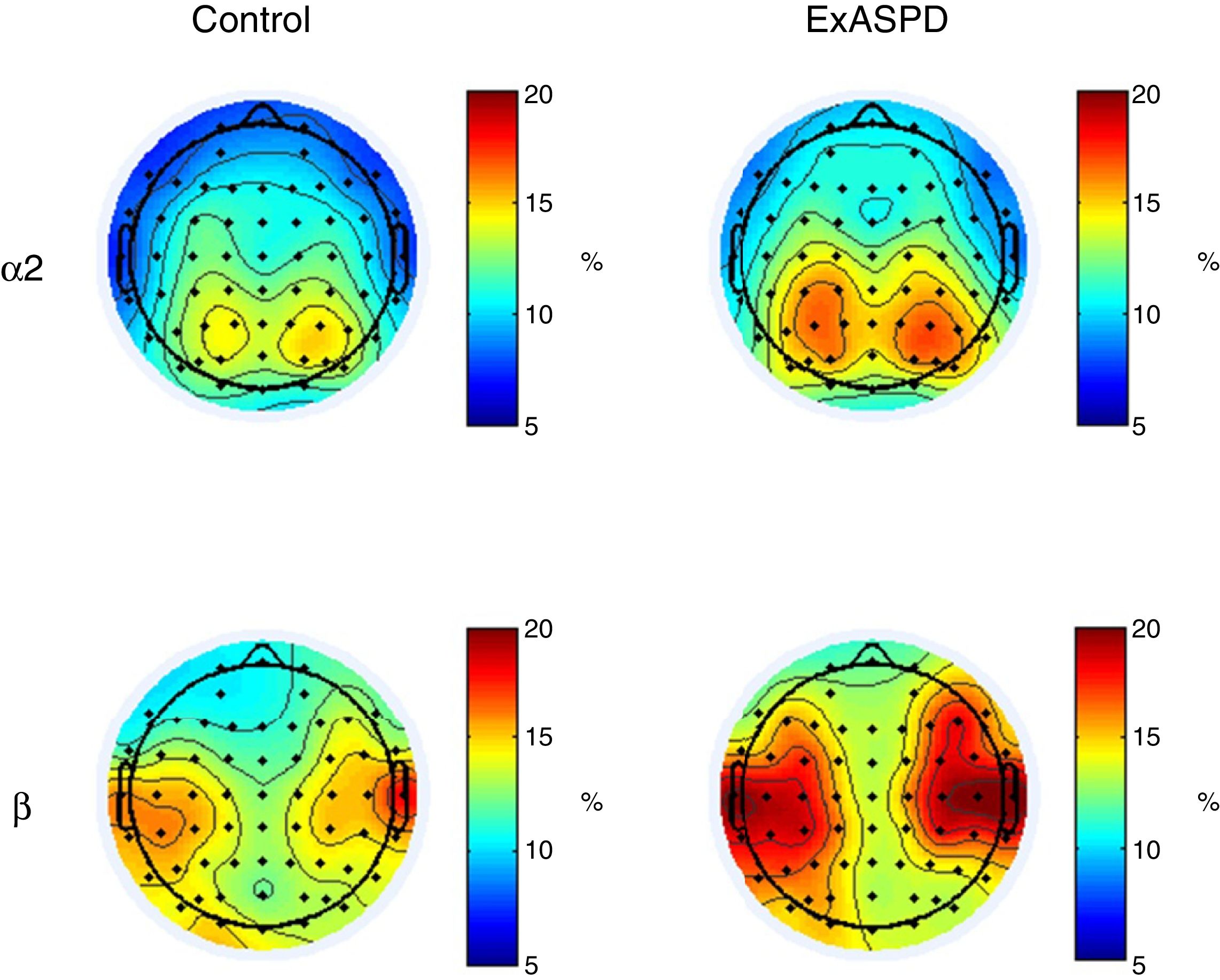
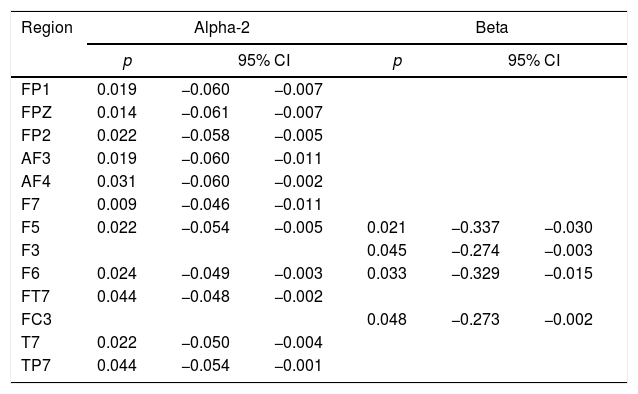
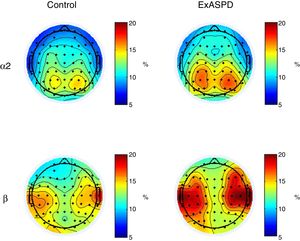
qEEGWhen comparing the spectral power between ExASPD and control, differences were found mainly in alpha-2 (frontal and left temporal region) and beta (left central-temporal region) (Table 2). In all cases, the spectral power was greater in the ExASPD group. Fig. 1 shows the regions with differences in the magnitude of the spectral power between the two groups.
Comparison of the spectral power between the ExASPD and control groups.
| Region | Alpha-2 | Beta | ||||
|---|---|---|---|---|---|---|
| p | 95% CI | p | 95% CI | |||
| FP1 | 0.019 | −0.060 | −0.007 | |||
| FPZ | 0.014 | −0.061 | −0.007 | |||
| FP2 | 0.022 | −0.058 | −0.005 | |||
| AF3 | 0.019 | −0.060 | −0.011 | |||
| AF4 | 0.031 | −0.060 | −0.002 | |||
| F7 | 0.009 | −0.046 | −0.011 | |||
| F5 | 0.022 | −0.054 | −0.005 | 0.021 | −0.337 | −0.030 |
| F3 | 0.045 | −0.274 | −0.003 | |||
| F6 | 0.024 | −0.049 | −0.003 | 0.033 | −0.329 | −0.015 |
| FT7 | 0.044 | −0.048 | −0.002 | |||
| FC3 | 0.048 | −0.273 | −0.002 | |||
| T7 | 0.022 | −0.050 | −0.004 | |||
| TP7 | 0.044 | −0.054 | −0.001 | |||
Only results with significant difference are shown.
Topographical maps illustrating differences in spectral power in alpha-2 and beta between ex-combatants with antisocial personality disorder (ExASPD) and controls. The blue regions indicate a relative power of 5%, which increases up to 20% (red areas). In general, greater spectral power is found in the ExASPD than in the controls.
To determine the relationship between the spectral changes found and the PCL-R, a linear correlation analysis was performed using the Pearson test. For the spectral energy in alpha-2, a positive relationship was found between the electrodes T7 and TP7 and the PCL-R. That is, as the spectral energy increases in this band, so did the PCL-R score. A positive relationship was also found for the spectral energy in beta for the electrodes F5, F3 and FC3. Table 1 shows the correlation values in which it was significant.
DiscussionIn this study, differences were found in the resting qEEG of a group of ex-combatants of the Colombian armed conflict with ASPD compared to a control group. Specifically, we found that the spectral power of the EEG in the frequency bands alpha-2 and beta was higher in ExASPD. These differences correlate directly with 16 of the 20 items in the structured PCL-R interview with which the participants with ASPD were classified. The results strengthen the hypothesis that in ASPD there are changes in brain electrical activity that can be detected by EEG. Moreover, these findings constitute an advance in the study of the neurophysiology of ex-combatants of the Colombian armed conflict.
In this study, the prevalence of ASPD was 68.4% (13 of the 19 participating ex-combatants), classified according to the PCL-R scale ≥21 and fulfilling the diagnostic criteria for ASPD according to the ICD-10. Other studies in similar populations also found high prevalences of the disorder. Espriella et al.29 assessed 76 ex-combatants and found that 20 of them had some type of personality disorder: four met diagnostic criteria for borderline personality disorder; 15, for ASPD (11.25%); and one, for schizoid personality disorder. However, the objective of the above study was to assess the prevalence of psychoactive substance use, and the finding of ASPD was secondary and diagnosed according to DSM-IV criteria. Even so, the samples in both studies are too small to talk about a population prevalence.
According to several authors, ASPD may be explained by the presence of post-traumatic stress disorder (PTSD) in ex-combatant populations.12,30–32 However, an investigation conducted by Baldovino Pérez evaluated 192 demobilised Colombian guerrillas and found that PTSD and ASPD were not comorbid.33 In our population, with characteristics similar to those of Baldovino Pérez, only one of the participants met the DIGS interview model criteria for PTSD. We therefore presume that aggressive behaviour and violent acts are not explained by such comorbidity.
Of the qEEG findings, the increase in the spectral power in beta had already been reported by several authors who worked with similar populations. The studies by Calzada-Reyes et al. are closest to our population, as they worked with Cuban prisoners with violent backgrounds.8 They reported an increase in beta in the left parietal-temporal regions, which is consistent with our findings, as well as an increase in this same band for bilateral occipital areas, where we did not find significant differences. Lindberg et al. also reported an increase in spectral power in beta for occipital regions when comparing people with violent histories classified with ASPD to controls.2 However, the results of the above studies are not directly comparable because of differences in methodology. In those studies, they recorded the EEG at rest with the eyes closed, while we performed the EEG with eyes open. That may account for the difference in activation in the occipital area, as it affects the primary visual area.
We also found an increase in alpha-2 power (10.0–13.0Hz) in the frontal and left temporal regions. For that same band of frequencies, Lindberg et al. reported a decrease in power in occipital and temporal areas.2 Once again, these differences may be the result of variations in the recording methods. Authors have reported that in closed-eye recordings it is difficult to control for the participant entering an early phase of sleep, which would modulate the frequency bands. In particular, it has been demonstrated that the alpha band increases in environments with little light or when the eyes are closed, and this is accompanied by a decrease in the beta band.34
When relating the PCL-R with the qEEG, from 25% to 60% of the items in the interview showed significant correlation with the spectral power in the alpha-2 and beta frequency bands (Table 1). In the alpha-2 band, six of the eight items of the variable F1 of the scale (corresponding to the facets “interpersonal relationships” and “emotionality”) and six of the ten items of the variable F2 (“lifestyle” and “antisocial”) had a direct correlation in frontal and temporal regions of the left hemisphere. Another smaller group of items had correlation with the beta band (4/8 items of F1 and 1/5 items of F2) in the same regions, in line with the findings of Calzada-Reyes et al.8 It is striking that the strongest correlation is with variable F1, which has better evidence of association with psychopathy and ASPD when compared to the relationship between psychopathic personality disorder and F2.
These findings show that the group of ex-combatants with ASPD have differences in spontaneous electrical activity in several brain regions which are also related to some behavioural characteristics of the disorder. The modulation found in alpha could be a correlate of altered cortical regulation and the manifestation of responses to maladaptive behavioural impulses.35 The EEG activity in the beta band is considered an index of the level of brain activation, and the differences found in this band could therefore be interpreted as evidence of lack of inhibition of the central nervous system (hyperexcitability) in the group of people with ASPD.19,36 It should be noted that the areas where there were correlations between the qEEG and the behavioural measurements (frontal and temporal areas) have been related to impairment in control of impulses, the selection and control of social behaviour, the production of moral judgements, sexual behaviour, affective regulation and an increase in aggressive and violent behaviour.37,38 These changes are characteristic of ASPD, suggesting that the alpha-2 and beta frequency bands may be related to the dysfunctional behaviour associated with the disorder. Similarly, the correlations may be linked to superficial emotional reactions and the development of socially inappropriate behaviour.
In the EEG signal processing stage, we included techniques aimed at filtering interference in brain electrical activity. This adds reliability to our results by guaranteeing that the differences reported are due to changes in the physiological measurement between ExASPD and controls, rather than possible recorded artefact. A step for correction of eye movements was included using the analysis of independent components, a technique widely used in recent years for its sensitivity in extracting and removing the interference of this and other types of brain signal artefacts.27 Moreover, the recording was made in a Faraday chamber, which removes interference from any external source such as the electrical system or other electronic devices.39
We feel it is necessary to reiterate that this is the first study of resting qEEG in ExASPD to be reported in the literature, and that it therefore constitutes an initial guide to understanding the neurophysiology underlying the personality disorders of participants in a warlike conflict. Specifically, the description of possible modifications in the processing of information, which would be one of the generating characteristics of antisocial behaviour, becomes a useful tool in the development of intervention strategies for the ex-combatant population, and even for people with the disorder outside a war environment. This is more relevant in a context of peace negotiations in Colombia, where there is an interest in the demobilisation and reintegration of ex-combatants into society. Studies of this type can support strategies for reintegration programmes.
The main limitation of this study is the number of participants involved. A larger sample is needed to control variables such as a history of alcohol consumption, smoking and use of psychoactive substances such as cocaine and tetrahydrocannabinol. These substances have been related to electrophysiological changes in the brain.40,41 A larger sample would also enable conclusions to be reached on the neurophysiological characteristics of this population with greater certainty.
ConclusionsThis study shows an increase in the spectral power in the resting EEG of ex-combatants of the Colombian armed conflict in the left frontal and temporal regions of the alpha-2 and beta bands which are related to dysfunctional behaviour characteristic of ASPD. These findings strengthen the hypothesis of modulation in basic brain electrical activity in ASPD and constitute a first step towards a better understanding of the disorder in this population.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
CODI Project “Emotional inhibitory control in the aggressive and violent behaviour of ex-combatants of illegal armed groups in Colombia”, with Universidad de Antioquia code 2566.
Please cite this article as: Ramos C, Duque-Grajales J, Rendón J, Montoya-Betancur A, Baena A, Pineda D, et al. Cambios en el EEG en reposo de exparticipantes en el conflicto armado colombiano con trastorno de personalidad antisocial. Rev Colomb Psiquiat. 2018;47:90–97.