Hypothyroidism results from inadequate production of thyroid hormone. It is known that there is a relationship between the major psychiatric disorders and hypothyroidism.
ObjectiveTo determine the prevalence of hypothyroidism in patients admitted due to major psychiatric disorders in Montserrat Hospital during the period from March to October 2010.
Material and methodsA descriptive cross-sectional study was conducted on 105 patients admitted to Montserrat Hospital with a primary diagnosis of major psychiatric disorder (major depression, bipolar affective disorder, generalised panic disorder, panic disorder, mixed anxiety-depressive disorder, and schizophrenia) in the aforementioned period. Thyroid stimulating hormone (TSH) was performed to assess the evidence of hypothyroidism.
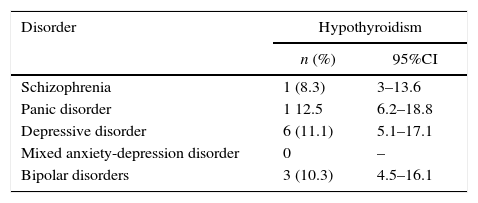
ResultsThe overall prevalence of hypothyroidism was found to be 10.5% (95%CI, 5–16%). It was 12.5% in anxiety disorder, 11.1% in depressive disorder, with a lower prevalence of 10.3% for bipolar disorder, and 9.9% for schizophrenia.
ConclusionsThe overall prevalence of hypothyroidism was found to be less than in the general population, which is between 4.64% and 18.5%, and hypothyroidism was found in disorders other than depression.
El hipotiroidismo resulta de una inadecuada producción de hormonas tiroideas. Es conocido que existe una relación entre los trastornos psiquiátricos mayores y el hipotiroidismo.
ObjetivoDeterminar la prevalencia de hipotiroidismo en los pacientes hospitalizados por trastorno psiquiátrico mayor en la Clínica Montserrat en el periodo de marzo a octubre de 2010.
Material y métodosSe realizó un estudio descriptivo transversal, para el que se seleccionó una muestra de 105 pacientes que ingresaron a la Clínica Montserrat con diagnóstico de trastorno psiquiátrico mayor (depresión mayor, trastorno afectivo bipolar, trastorno de ansiedad generalizada, trastornos de ansiedad, trastorno mixto ansioso-depresivo y esquizofrenia) en el periodo mencionado. Para evaluar el hipotiroidismo se realizó una prueba de Hormona Estimulante del Tiroides (TSH).
ResultadosLa prevalencia general del hipotiroidismo fue del 10,5% (intervalo de confianza del 95%, 5%-16%). Al determinar el hipotiroidismo por diagnóstico, se encontró que había mayor prevalencia en los trastorno de pánico (12,5%) y depresivo (11,1%) y menor en el trastorno bipolar (10,3%) y la esquizofrenia (9,9%).
ConclusionesLa prevalencia general del hipotiroidismo fue menor que en la población general (18,5–4,6%) y se encontró hipotiroidismo en otros trastornos diferentes de la depresión.
Hypothyroidism results from an inadequate production of thyroid hormones1 and is classified into primary, secondary and tertiary forms; in the primary, the alteration is located in the thyroid gland; in the secondary it is caused by a deficit of thyroid stimulating hormone (TSH) production in the pituitary, and the tertiary, which in turn is divided into three grades, is characterised by a deficit in thyrotropin releasing hormone (TRH) production.2 Hypothyroidism and hyperthyroidism have long been associated with neuropsychiatric disorders, either as a cause or as a consequence of the same.3 Thyroid hormone production is governed by the integrity of the hypothalamic-pituitary-thyroid (HPT) axis, in addition to adequate iodine intake. In the thyroid gland, most hormone production involves thyroxine (T4) (80%) and the rest, triiodothyronine (T3). T4 in the periphery is converted into T3 by the action of enzymes that remove iodine molecules (deiodinases). T3 is metabolically more powerful. The measurement of T3 in patients with hypothyroidism is not routinely required but could be useful in patients with thyrotoxicosis and T4 within the “normal” range. The synthesis of thyroid hormones and their secretion is eventually regulated by the HPT axis. Thyroid function control is mediated by TSH and, in turn, TSH is regulated by TRH (synthesised in the paraventricular nucleus of the hypothalamus); TRH binds to its receptors on pituitary thyrotrophic cells (a subpopulation of pituitary cells that secrete TSH).4
A relationship between major psychiatric disorders and hypothyroidism has been described.1,5 It is believed that these psychiatric entities – major depression, bipolar affective disorder (BAD), anxiety and schizophrenia – are disorders that may be related to thyroid dysfunction. Hypothyroidism is a direct and indirect factor in major psychiatric disorders. In depression, the catecholamines in the brain decrease and hypothyroidism reduces cerebral alpha- and beta-adrenergic receptors, thus partially explaining the neuronal hyporeactivity and depressive symptoms.6,7 Comorbidity has been found between schizophrenia and, in particular, schizoaffective disorder and other medical conditions, including acquired hypothyroidism.8 With regard to BAD and hypothyroidism, a study found no significant association between the two, although there was a significant association between a family history of mood disorders in first-degree relatives and patients with hypothyroidism.9 In another study it was reported that patients hospitalised with hypothyroidism had a high risk of being readmitted with depression or BAD compared to a control group.10 Elevated TSH concentrations have been found in rapid-cycling bipolar patients,11 and this has been more common in mixed states than in manic conditions, in addition to low levels of T4.12 Hyperthyroidism and hypothyroidism cause anxiety, which is a common symptom that is frequent in both cases and can occur even before the symptoms of the conditions themselves.13
The studies conducted have aimed to determine the frequency of hypothyroidism in major psychiatric disorders or the frequency of major psychiatric disorders in patients with hypothyroidism. In an American study, 22% of people with treatment-resistant depression had signs of clinical or subclinical hypothyroidism.14 In another study with major depressive patients, elevated TSH levels (4.7–8.2μg/dl) were found in 2.6%.15 In a research study on depressive disorder, a 21% prevalence of subclinical hypothyroidism was found, with said figure reaching 25% in the women-only group.5 The frequency of major depression was higher (56%) in patients who met criteria for subclinical hypothyroidism, compared to 20% of patients without said criteria.16 The prevalence of lifelong depression in patients with subclinical hypothyroidism is approximately double that of the general population.1
There are few studies on the prevalence of hypothyroidism in Colombia, and in particular in the city of Bogotá, and its association with major psychiatric disorders. In Armenia, in a high-risk population over 35 years of age, a prevalence of 18.5% was found in 2009–2010.17 In university students aged 18–30 years, it stood at 4.64%18 in Bogotá. The prevalence of hypothyroidism is known to increase with age and varies with gender and socio-economic status.17 In other studies, its prevalence in the population has been found to fluctuate between 2 and 8%,19,20 or 3 and 18%.21 Among the elderly, it ranges from 0.9 to 5.9%. This figure reaches 14–18.2% when subclinical hypothyroidism is taken into account.17,21 In Latin America, the prevalence of subclinical hypothyroidism in postmenopausal women is 10–40%.17 In another study, we found a prevalence of subclinical hypothyroidism of between 3 and 16% in people over 60 years of age.22
The objective of this study is to determine the prevalence of hypothyroidism in patients hospitalised with a major psychiatric disorder at the Clínica Montserrat from March to October 2010.
Material and methodsThe study is descriptive and cross-sectional and was conducted on a population of patients with a major psychiatric disorder admitted to the Clínica Montserrat in the period between March and October 2010.
The inclusion criteria were male and female patients over 18 years of age (Table 1) who had a major psychiatric disorder (major depression, BAD, generalised anxiety disorder, panic disorder, mixed anxiety-depressive disorder and schizophrenia) as their main diagnosis. The exclusion criteria were: having depression associated with an organic disorder; other psychotic disorders besides schizophrenia; comorbidity with substance dependence or abuse; concomitant use of lithium, potassium iodine, amiodarone, dopamine, prednisone, somatostatin and bexarotene analogues.
A sample of TSH was taken from the selected patients. The type of sampling was non-probability for convenience, with a sample size of 105 patients who met the selection criteria. The study variables were hypothyroidism, gender, age, signs and symptoms and type of psychiatric disorder.
Through information obtained from medical records, hospitalised patients who had a major psychiatric disorder (major depression, BAD, generalised anxiety disorder, panic disorder, mixed anxiety-depressive disorder and schizophrenia) as their main diagnosis were identified and the TSH test performed.
Only TSH was requested because in clinical practice it is considered sufficient for screening, which is what was intended.3 The main initial diagnostic test is TSH measurement. TSH is typically the main marker for the diagnosis of thyroid dysfunction.4 In case of altered TSH values, patients were investigated for signs and symptoms of hypothyroidism.
A form with the diagnostic criteria of each disorder was used to verify the diagnosis. At the Clínica Montserrat, the DSM-IV-TR and ICD-10 criteria were in use at the time of the study. In this study, the DSM-IV-TR criteria were considered.
The patients who underwent TSH testing had been admitted to the institution in the midst of a crisis of their disease. The sample for the examination was taken approximately the week after admission, as soon as the patient and the attending physician signed the informed consent form.
A standardised procedure manual was employed for obtaining the informed consent of both the patient and attending physician, and a form was used to verify signs and symptoms of hypothyroidism, which included a recording of TSH values (Table 2). A guide was used as a procedure for patient selection and identification, along with an organisation chart, informed consent form and a checklist for the study and materials necessary for the TSH determination.
Hypothyroidism signs and symptoms form (only for cases).
| Patient initials: | ||
| Age: | ||
| Gender: | ||
| Diagnosis: | ||
| Signs and symptoms | Yes | No |
| Weight gain | ||
| Constant tiredness and fatigue | ||
| Intolerance to cold | ||
| Constipation | ||
| Dry, rough and thickened skin | ||
| Eyelid oedema | ||
| Dry and brittle hair | ||
| Eyebrow hair loss | ||
| Increased lip and tongue size | ||
| Hoarse voice | ||
| Hypoacusis | ||
| Joint and muscle pain | ||
| Diaphoresis | ||
| Lack of interest in daily activities | ||
| Reduced concentration | ||
| Memory loss | ||
| Sleep disorders | ||
| Amenorrhoea | ||
| Arterial hypertension | ||
| Hyporeflexia | ||
| Tachycardia | ||
| Xerostomia | ||
| Slow speech | ||
| Sadness | ||
| TSH≤0.1μU/ml | ||
| TSH 0.2–2.0μU/ml | ||
| TSH 2.0–4.0μU/ml | ||
| TSH 4.0–10.0μU/ml | ||
| TSH>10.0μU/ml | ||
TSH: thyrotropin.
The hypothyroidism signs and symptoms form was applied to patients with hypothyroidism, which included the TSH values obtained. According to the literature review, some symptoms were specific to hypothyroidism and others could not be differentiated from the underlying disorder, such as a disinterest in daily activities, reduced concentration, constant tiredness and fatigue, and memory loss. The other symptoms deemed to be characteristic of hypothyroidism were more somatic (Table 2).
Hypothyroidism was diagnosed by means of TSH measurement requested by medical personnel (normal TSH values were 0.4–4.0Ul/ml).23
The TSH results were reported to the patient and attending physician, who defined the approach to be followed. The study did not include follow-up.
The data were tabulated in Microsoft Excel; the statistical analyses were performed using the STSS Program, version 15.5. For the qualitative variables, absolute frequencies and percentages were used and, for the quantitative variables, averages and standard deviations; the confidence intervals were found to be 95%. To determine association, the Pearson χ2 test was used, with a significance level of 0.05. For the calculation of the sample size, an expected prevalence of 22% was used, with a 95% confidence level (95%CI), an absolute precision of 6% and a relative precision of 27%, using the EPIDAT 3.1 Program. The ethics committees of Universidad El Bosque and Clínica Montserrat approved the project.
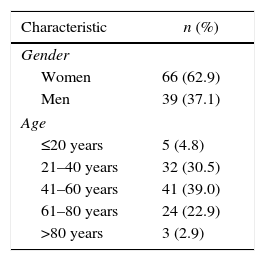
ResultsGeneral characteristicsThe sample was composed of 105 patients. The average age was 47.71±17.2 (18–85) years; the most frequent age group was 41–60 years, followed by 21–40 years. The patients were mostly women (Table 1).
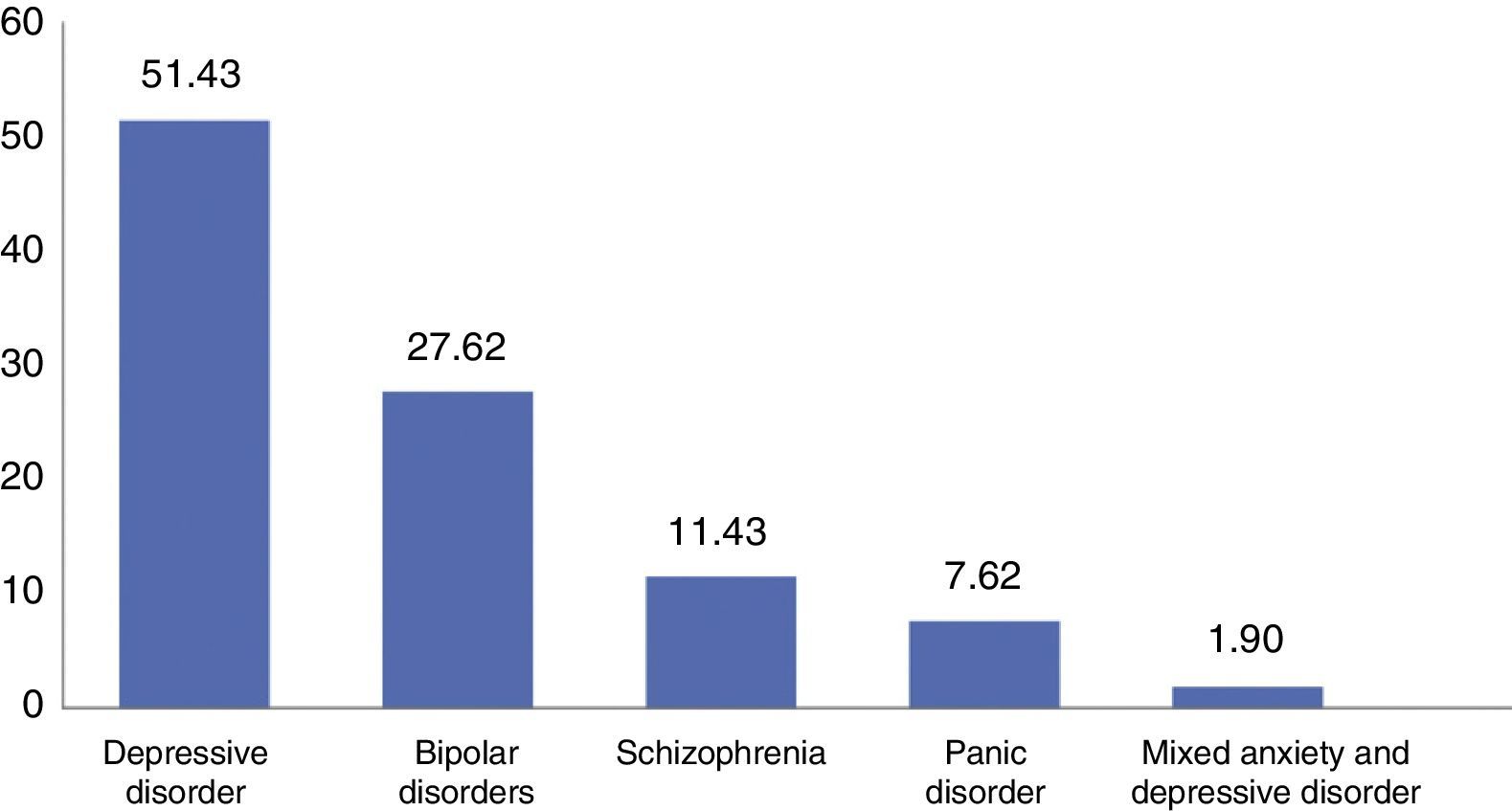
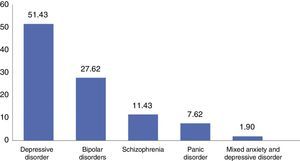
The predominant disorder was depressive disorder (n=51.4), followed by BAD (n=27.6) and schizophrenia (n=11.4), panic disorder (7.6%) and mixed anxiety and depression disorder (1.9%) (Fig. 1).
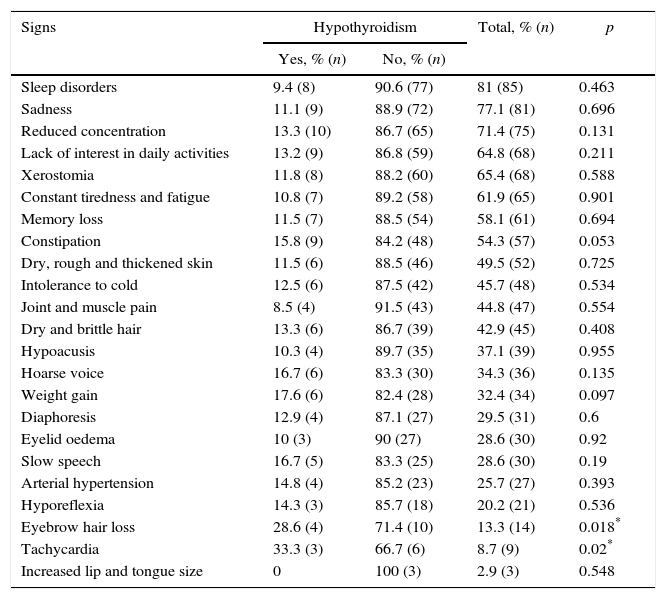
The most frequent signs and symptoms found in patients with hypothyroidism are sleep disorder, sadness, reduced concentration, disinterest in daily activities, xerostomia, constant tiredness and fatigue, memory loss and constipation, which are very common in patients with anxiety disorder, especially those with depression and in hypothyroidism; hypothyroidism with eyebrow hair loss and tachycardia was found to be statistically significant (p<0.05) (Table 3).
Distribution of signs and symptoms.
| Signs | Hypothyroidism | Total, % (n) | p | |
|---|---|---|---|---|
| Yes, % (n) | No, % (n) | |||
| Sleep disorders | 9.4 (8) | 90.6 (77) | 81 (85) | 0.463 |
| Sadness | 11.1 (9) | 88.9 (72) | 77.1 (81) | 0.696 |
| Reduced concentration | 13.3 (10) | 86.7 (65) | 71.4 (75) | 0.131 |
| Lack of interest in daily activities | 13.2 (9) | 86.8 (59) | 64.8 (68) | 0.211 |
| Xerostomia | 11.8 (8) | 88.2 (60) | 65.4 (68) | 0.588 |
| Constant tiredness and fatigue | 10.8 (7) | 89.2 (58) | 61.9 (65) | 0.901 |
| Memory loss | 11.5 (7) | 88.5 (54) | 58.1 (61) | 0.694 |
| Constipation | 15.8 (9) | 84.2 (48) | 54.3 (57) | 0.053 |
| Dry, rough and thickened skin | 11.5 (6) | 88.5 (46) | 49.5 (52) | 0.725 |
| Intolerance to cold | 12.5 (6) | 87.5 (42) | 45.7 (48) | 0.534 |
| Joint and muscle pain | 8.5 (4) | 91.5 (43) | 44.8 (47) | 0.554 |
| Dry and brittle hair | 13.3 (6) | 86.7 (39) | 42.9 (45) | 0.408 |
| Hypoacusis | 10.3 (4) | 89.7 (35) | 37.1 (39) | 0.955 |
| Hoarse voice | 16.7 (6) | 83.3 (30) | 34.3 (36) | 0.135 |
| Weight gain | 17.6 (6) | 82.4 (28) | 32.4 (34) | 0.097 |
| Diaphoresis | 12.9 (4) | 87.1 (27) | 29.5 (31) | 0.6 |
| Eyelid oedema | 10 (3) | 90 (27) | 28.6 (30) | 0.92 |
| Slow speech | 16.7 (5) | 83.3 (25) | 28.6 (30) | 0.19 |
| Arterial hypertension | 14.8 (4) | 85.2 (23) | 25.7 (27) | 0.393 |
| Hyporeflexia | 14.3 (3) | 85.7 (18) | 20.2 (21) | 0.536 |
| Eyebrow hair loss | 28.6 (4) | 71.4 (10) | 13.3 (14) | 0.018* |
| Tachycardia | 33.3 (3) | 66.7 (6) | 8.7 (9) | 0.02* |
| Increased lip and tongue size | 0 | 100 (3) | 2.9 (3) | 0.548 |
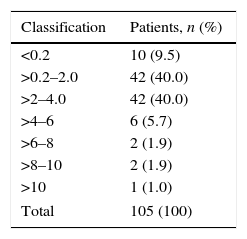
The mean TSH was 2.66±3.76 (0.01–36.4), with heterogeneous variability (coefficient of variation [CV], 141%). The TSH values obtained are shown in Table 4. Most patients, 84 (80%), had TSH levels of between 0.2 and 4.0; 10 patients (9.5%) had it below 0.2, another 10 between 4.0 and 10.0 (9.5%), and one patient was greater than 10 (1%). The overall prevalence was 10.5% (n=11), with a 95%CI of 5–16%; the sampling error was 28.67%. When determining hypothyroidism by diagnosis, a higher prevalence was found in anxiety and depressive disorders (Table 5).
The 21–56% prevalence of hypothyroidism in depressed patients, or twice that of the general population found in the literature,1,5,14–16 is higher than the prevalence found in this study on thyroid disorders in patients hospitalised for a depressive disorder (11.1%). As for the population studied, major psychiatric disorders are seen to appear more frequently in the middle and older adult population, predominantly in women.
The highest hypothyroidism frequency was not expected to appear in anxiety disorders, but in depression, which ranked second.
DiscussionIn this cross-sectional study, which included a sample of 105 patients with an average age of 47.71±17.2 years (62.9% women), it was found that the overall prevalence of hypothyroidism of 10.5% is lower than in the general population (4.64–18.5%),17,18 considering that the figure of 18.5% corresponds to people aged over 35 with a high risk, mostly women, with 45.7% from strata 1 and 2 and 58.6% from strata 3 and 4. The 4.64% group concerns a population aged between 18 and 30 years. The hypothesis raised (“the prevalence of hypothyroidism in patients hospitalised for a major psychiatric disorder is higher than that found in the general population”) was not confirmed.
The lower prevalence found in our study may be related to the fact that the study population with a prevalence of 18% included a larger number of women (62.9–99.0%) and the majority of the sample came from socio-economic strata 1 and 2 (45.7%) and 3 and 4 (58.6%).17 Our study did not determine the stratum, but the sample corresponds to people from a clinic whose patients probably belonged to socio-economic strata above 4, i.e. people with a better socio-economic status.
In light of the above findings, it can be stated that a person experiencing hypothyroidism may also have an anxiety disorder or a depressive disorder; however, this study is not conclusive with regard to a person with a major psychiatric disorder having a greater risk of concomitant hypothyroidism in relation to the general population.
It has already been mentioned that hypothyroidism can be associated with BAD, depression and cognitive impairment (particularly in older adults). Depressed patients show several alterations in the hypothalamic-pituitary-thyroid axis, such as a flattening in the circadian rhythm of thyroid hormone secretion, with an absence of the normal nocturnal peak of TSH secretion, elevation of TRH concentrations in some cases of depression and a flattened response in the TRH stimulation test, which is somewhat unspecific, since it is also seen in maniacs and alcoholics. A reversal of this situation occurs once mood is restored.24
Among the results obtained on the frequency of hypothyroidism symptoms, xerostomia was found to be common (68%), which may be a result of both hypothyroidism and a medication side effect, especially in patients who use anti-depressants or anti-psychotics.
Moreover, it is important to note that the main symptoms of hypothyroidism, such as dry skin, intolerance to cold and sparse and brittle hair, were not the most frequent in the study. Despite these results, it is important to note that symptoms of hypothyroidism may be underestimated in patients with a major psychiatric disorder, because in some cases the symptoms may be similar. As such, in addition to taking the patient's symptoms into account, it is necessary to evaluate them with the TSH test, since patients with these psychiatric disorders may be related to hormonal dysfunction.
Certain clinical features are more relevant in the diagnosis of hypothyroidism, such as insomnia and xerostomia. These need to be taken into account in relation to the exploration of signs and symptoms.
To our knowledge, this would be the first study published in Bogotá to investigate the presence of thyroid disorder in the indicated population.
Other measures such as stimulation with TRH and anti-thyroid antibodies, among others, or ultrasound studies can undoubtedly contribute to a greater diagnostic approach.
In clinical practice, it is common to find TSH values above the normal range with normal T4 and T3 values. The need to carry out a comprehensive assessment, a potential treatment and the urgency of the latter have not been clearly established. An aggressive management of these alterations would be indicated in the case of a pregnant woman, in people over 60 years of age or in case of an increased risk due to thyroid dysfunction.25
Subclinical hypothyroidism (early thyroid dysfunction or compensated primary hypothyroidism) has been defined as elevated TSH with normal concentrations of T4 and T3 in an asymptomatic patient. However, before making a decision on the need for definitive treatment, a diagnosis should be made to distinguish between transient thyroiditis in the recovery phase and non-thyroid disease with changes in the hormonal profile,26 or even laboratory interference (heterophile antibodies).27 In these circumstances, an additional TSH test is recommended after 6–8 weeks in order to assess whether the TSH elevation persists, thereby supporting the diagnosis of primary hypothyroidism. If TSH elevation persists (>6mIU/l) with goitre and high titres of anti-peroxidase (thyroid microsomal) (anti-TPO) antibodies, the probability of progression to a clinical form is 4–5% per year.26,28
In a study that analysed the possible consequences of TSH elevation, it was concluded that: (a) TSH elevation with normal free T4 does not necessarily mean thyroid failure; (b) patients with positive thyroid antibodies and particularly those with TSH concentrations >10 are at a high risk of hypothyroidism; (c) the typical symptoms (specific thyroid, cardiovascular, neurological and psychiatric symptoms and, finally, alterations of risk factors for arteriosclerosis) seem to occur in a large and variable percentage of patients; and (d) some of the symptoms, especially the cardiovascular ones, appear to be treatable with levothyroxine, while others – like most changes in lipid metabolism – may not be influenced by the normalisation of TSH. It is concluded that TSH and free T4 screening appears justified in older women, in whom the prevalence of the disease is approximately 20%. However, “symptoms” of subclinical hypothyroidism, such as high cholesterol or depression, should only be treated in patients with TSH levels >10mIU/l and only with great caution in other cases in order to avoid an unnecessary overdose, with a risk of atrial fibrillation.29
These considerations are reaffirmed with the findings of this work. Taking into account the previous findings and, according to the TSH values obtained, only a patient with TSH values >10 would require treatment.
The study limitations are related to the fact that only one TSH measurement was performed and no other parameters were investigated; neither a history of thyroid function was taken nor a follow-up scheduled, since this was a cross-sectional investigation. In addition, there is a scarcity of research on the prevalence of hypothyroidism in Colombia, which prevents wider comparisons in relation to the results.
ConclusionsThere are no differences in the prevalence of hypothyroidism among the population hospitalised with a major psychiatric disorder and the general population. However, until studies on this association are extended, we must continue to take into account the probability of hypothyroidism among the population hospitalised with these disorders, especially in case of anxiety, major depression and BAD, albeit with the same considerations as for the general population. Anxiety disorders presented the highest prevalence of hypothyroidism.
The question therefore arises as to whether a TSH test should be requested in order to assess the thyroid function of patients with the abovementioned psychiatric disorders. Studies could address TSH values above the 25th percentile of the normal reference range and the implications of the same for severe and recurrent depressive disorders, as noted.7 Alternatively, they could determine what approach to follow when a psychiatric patient has a report with elevated TSH values.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
FundingThis article is based on the thesis “Prevalence of hypothyroidism in major psychiatric disorder in patients hospitalised at the Clínica Montserrat in Bogota from March to October 2010”. The research was funded by the Universidad El Bosque.
Conflicts of interestThe authors have no conflicts of interest to declare.
To Universidad El Bosque for its support and funding of this work and the Colombian Nervous System Institute (“Instituto Colombiano del Sistema Nervioso”) for its support.
Please cite this article as: Vargas Navarro P, Ibañez Pinilla EA, Galeano España A, Noguera Bravo AM, Milena Pantoja S, Suárez Acosta AM. Prevalencia de hipotiroidismo en trastorno psiquiátrico mayor de pacientes hospitalizados en la Clínica Montserrat en el periodo de marzo a octubre de 2010. Rev Colomb Psiquiat. 2017;46:140–146.