Obsessive-compulsive disorder is defined by the presence of obsessions and compulsions that cause marked anxiety or distress and has been associated with a disruption in cortico-striato-thalamo-cortical circuitry. After treatment, around 50% of patients continue to experience incapacitating symptoms. Deep-brain stimulation has been shown to be an effective therapeutic alternative to regular treatment.
MethodsCase report.
Case presentationA 54-year-old woman with a diagnosis of treatment-resistant obsessive-compulsive disorder was treated with deep-brain stimulation of the anterior limb of the internal capsule. Molecular imaging before and after the procedure was obtained and correlated with clinical features.
ConclusionsDeep-brain stimulation may be a therapeutic alternative to regular care in treatment-resistant obsessive-compulsive disorder and can be correlated to functional changes in suspected anatomical structures.
El trastorno obsesivo-compulsivo se define por la presencia de obsesiones y compulsiones que ocasionan ansiedad y malestar marcados, y se ha asociado con una alteración en los circuitos cortico-estriado-tálamo-corticales. Tras tratamiento, alrededor de la mitad de los pacientes permanecen con síntomas discapacitantes. La estimulación cerebral profunda ha mostrado ser una alternativa efectiva al tratamiento usual.
MétodosReporte de caso.
Presentación del casoUna mujer de 54 años con diagnóstico de trastorno obsesivo-compulsivo resistente a tratamiento fue tratada con estimulación cerebral profunda del brazo anterior de la cápsula interna. Se obtuvieron imágenes moleculares antes y después de la intervención y fueron correlacionadas con el cuadro clínico.
ConclusionesLa estimulación magnética profunda puede ser una alternativa terapéutica al tratamiento usual en el trastorno obsesivo compulsivo resistente a tratamiento, y puede correlacionarse con cambios funcionales en estructuras anatómicas de sospecha.
Obsessive-compulsive disorder (OCD) is a frequent neuropsychiatric disorder defined by the presence of recurrent and intrusive thoughts, urges, or images that may cause marked anxiety or distress (obsessions), as well as repetitive behaviors or mental acts in response to the obsessions in order to reduce the anxiety produced (compulsions).1 First-line treatment options include the use of selective serotonin reuptake inhibitors (SSRIs), clomipramine, cognitive and exposure-response prevention therapy2. However, even after pharmacological and psychological treatment, around 40-60% of the patients have persisting and incapacitating symptoms.2 The pathophysiology of OCD has been linked to the disruption of cortico-striato-thalamo-cortical (CSTC) loop circuitry, specifically observing hypermetabolism in the orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), and caudate; findings related to the severity and degree of response to treatment.3,4 Deep-brain stimulation (DBS), initially used as treatment for movement disorders,5 represents a therapeutic alternative to patients with treatment-resistant OCD, with a response rate ranging from 10-67% in blind studies.3
We present the case of a woman with treatment-resistant OCD treated with DBS presenting response after the intervention which correlates with changes in the metabolism of CSTC circuitry as demonstrated by 18-fluorodesoxyglucose positron-emission tomography (18FDG-PET) before and after surgery. Written informed consent was obtained prior to submission of this report.
Case presentationA 54-year-old woman was referred to the National Institute of Neurology and Neurosurgery (NINN) in Mexico City with a diagnosis of treatment-resistant OCD. She had a family history of Steinert myotonic dystrophy syndrome in 3 direct family members, and generalized anxiety disorder. She also had a personal history of hypothyroidism which was correctly treated upon arrival.
She was described by her family as an extremely orderly and perfectionist child, with an extensive vocabulary and detailed conversations, presenting with worries regarding her personal hygiene. When she was 10-years-old, she began exhibiting repetitive behaviors such as jumping in the same place 3 times in a row and counting her steps. These behaviors caused mild distress to the patient when not performed correctly or in a repetitive manner, but did not interfere in her studies and daily activities. Later, she manifested repetitive and intrusive symmetry/order thoughts that evoked anxiety if not followed by constant arranging of her bedroom in a specific pattern. When she turned 15, new obsessions related to contamination appeared, provoking cleaning and self-hygiene compulsions which caused secondary dermatitis and interfered with her activities. At this age, she first consulted a psychiatrist, receiving unspecified pharmacological treatments with null response during the next 4 decades. New obsessions appeared progressively, including catastrophic images, aggressive and violent urges, and she began exhibiting hoarding, storing her deceased brothers’ clothing and different types of waste. By her mid-40's, she dedicated her entire time to her obsessions and compulsions and began exhibiting irritability and violent behavior towards her mother and domestic employees when they did not follow her specific routines. Multiple mental-health professionals were consulted, and she received distinct types of treatment, reaching maximum doses and during adequate periods of time (4 selective serotonin reuptake inhibitors, 1 tricyclic antidepressant, 4 augmenting antipsychotics, 2 antiepileptic treatments, and multiple benzodiazepines). Due to non-response, she was referred to our facilities for further evaluation and therapeutic approach.
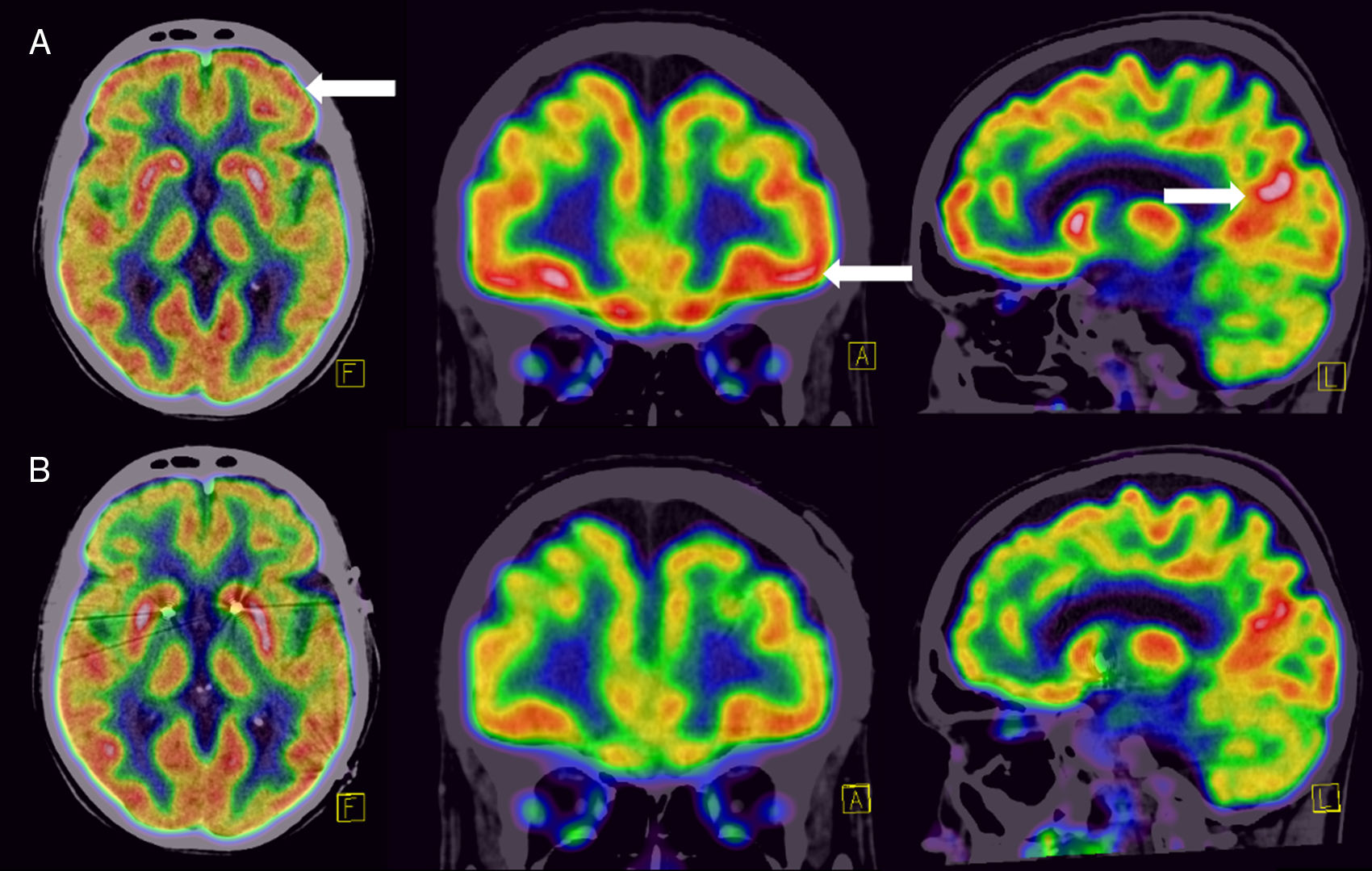
Upon arrival, neuropsychiatric evaluation was limited due to the presence of constant ordering compulsions and productive speech regarding her order and contamination obsessions, but remained insightful. At the time, she was receiving 200 mg of sertraline, 2 mg of risperidone, 15 mg of mirtazapine, 7 mg of clonazepam, and 0.05 mg of clonidine. An initial Yale-Brown obsessive-compulsive scale (YBOCS) score of 40 was calculated. After reviewing her medical history and response to earlier treatments, her case was deliberated by the Psychosurgery Committee of the NINN, and she was offered DBS as a therapeutic alternative for treatment-resistant OCD, which she accepted. As part of her diagnostic and therapeutic-oriented work-up, an 18FDG-PET was obtained, showing marked hypermetabolism in the OFC, prefrontal dorsolateral cortex, and posterior cingulate cortex (figure A). Bilateral DBS (Medtronic® 3391) was installed with an inhibitory frequency in the nucleus accumbens (NA) bilaterally. During transurgical stimulation, she presented laughter and an inappropriate affect, which remitted after adjusting the stimulator parameters.
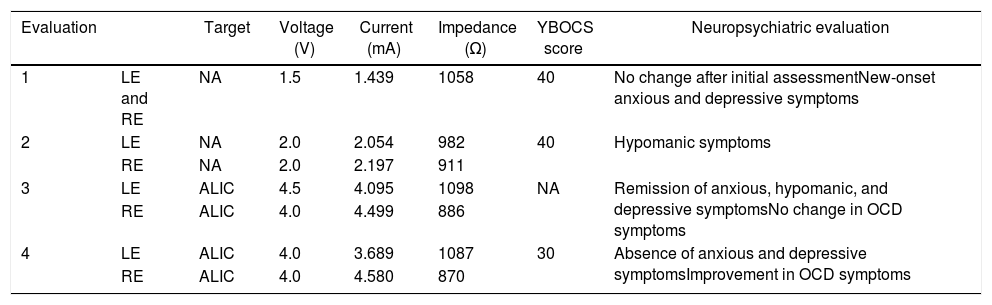
A month after DBS was installed, she was re-evaluated by our team. She presented no response in OCD symptoms, and instead, had developed distressing anxiety and depressive symptoms that elicited violent behavior towards her family members. DBS parameters were adjusted monthly with little response and presenting with a hyper-productive speech (table). Due to non-response and new-onset anxious and depressive symptoms, she was admitted to the Neuropsychiatry Department for further evaluation. After new deliberation by the Psychosurgery Committee, the DBS site was changed to the anterior limb of the internal capsule, bilaterally, and parameters were modified. During the first week, this change was followed by a normalization of her speech, disappearance of depressive symptoms, and improvement in anxiety, with no improvement in OCD symptoms. After a month, response was observed in OCD symptomatology, with a YBOCS score of 30 (25% change). She no longer presented violent behavior nor depression, and her obsessions and compulsions diminished drastically, being able to perform some of her daily chores. A new 18FDG-PET was obtained, showing a normalization of the metabolism in the anatomical sites that showed disruption in the first study (figure B).
Deep-brain Stimulation Parameters and Clinical Correlation.
| Evaluation | Target | Voltage (V) | Current (mA) | Impedance (Ω) | YBOCS score | Neuropsychiatric evaluation | |
|---|---|---|---|---|---|---|---|
| 1 | LE and RE | NA | 1.5 | 1.439 | 1058 | 40 | No change after initial assessmentNew-onset anxious and depressive symptoms |
| 2 | LE | NA | 2.0 | 2.054 | 982 | 40 | Hypomanic symptoms |
| RE | NA | 2.0 | 2.197 | 911 | |||
| 3 | LE | ALIC | 4.5 | 4.095 | 1098 | NA | Remission of anxious, hypomanic, and depressive symptomsNo change in OCD symptoms |
| RE | ALIC | 4.0 | 4.499 | 886 | |||
| 4 | LE | ALIC | 4.0 | 3.689 | 1087 | 30 | Absence of anxious and depressive symptomsImprovement in OCD symptoms |
| RE | ALIC | 4.0 | 4.580 | 870 |
ALIC: anterior limb of the internal capsule; LE: left electrode; NA: nucleus accumbens; OCD: obsessive-compulsive disorder; RE: right electrode; YBOCS: Yale-Brown obsessive compulsive scale.
Response-rates for DBS in OCD vary according to the therapeutic target (NA: 10-56%; internal capsule: 50%); with a change in YBOCS scores of 6.8-17.5 after 12 to 21-month follow-up.3 Due to initial non-response and appearance of neuropsychiatric side-effects after stimulation in the NA, the target was changed to the anterior limb of the internal capsule as proven beneficial in earlier studies.6 Our patient presented initial improvement in depressive and anxious symptoms (during the first days of the last DBS adjustment), as reported previously.7 However, OCD symptoms may have a later response.7 Hypermetabolism in CSTC circuitry (specifically OFC, ACC, and caudate) have been associated with clinical changes and response to DBS, finding that was replicated in the present case.3 Interestingly, a euphoric response to site stimulation may predict a better response in OCD symptoms as rated by the YBOCS,8 which could be illustrated by this report. Side effects include transient hypomanic states and disinhibition as reported previously,9 which we found as an increase in speech production and goal-oriented activity in our patient. Although a 25% reduction in OCD was observed in our patient after 1-month follow-up, increasing response rates may be found after longer observation.7,9
ConclusionsThe use of DBS represents potential advantages over ablative procedures such as capsulotomy and cingulotomy (such as less complications9), which could be related to neurogenesis, modification of astrocyte activity, increase in cerebral regional flow, and electrotaxis, resulting in neuromodulation.7 DBS may be a therapeutic alternative to regular care in treatment-resistant OCD and can be correlated to functional changes in suspected disrupted anatomical structures, although continuous pharmacological treatment is recommended.
Conflict of interestsRodrigo Pérez Esparza has served as a speaker for Johnson & Johnson. The rest of the authors declare no conflicts of interest, including any financial, personal, or other relationship with people or organizations that could influence inappropriately the current report.
We would like to acknowledge the PET/CT Molecular Imaging Unit of the NINN for their support in acquiring the images presented and their interpretation.
This case report was presented in the medical reunion “X Jornadas de Médicos Residentes de Institutos Nacionales de Salud y Hospitales de Alta Especialidad”, Instituto Nacional de Cancerología, Ciudad de México, November 15, 16 and 17.