The safety of electroconvulsive therapy has improved greatly over the last decades, making the potentially adverse effects on memory and other neurocognitive functions the main clinical aspect of concern in the present. In Colombia, the general population and healthcare professionals (even some psychiatrists) seem to have mostly negative opinions towards electroconvulsive therapy treatment, but maybe this could be reconsidered if more information is provided; therefore, the aim of the present study was to evaluate the changes in memory and the severity of the symptoms in a group of patients with severe depression before and after electroconvulsive therapy.
MethodsTwenty-three patients ranging in age from 23 to 70 years from the electroconvulsive therapy service at the San Juan de Dios Clinic (Manizales, Colombia) were recruited in order to assess the effect of electroconvulsive therapy on memory in patients with severe depression. Depressive symptoms and memory were assessed with the Hamilton Depression Scale (HAMD) and Rey Auditory Verbal Learning Test (RAVLT), respectively. The assessment was administered to participants before the initial treatment of electroconvulsive therapy series (0-1 day) and 2 days after their last treatment.
ResultsElectroconvulsive therapy resulted in significant improvement in the rating of depression. There were no significant differences in the five learning trials, delayed recall, learning and forgetting scores from pre-treatment to post-treatment. Significant pre-treatment/post-treatment differences were found in the delayed recognition trial.
ConclusionsPre- and post- electroconvulsive therapy cognitive assessment is a feasible and useful procedure. In general, memory performance does not worsen after electroconvulsive therapy in patients with depression. Only delayed recognition is affected a few days following electroconvulsive therapy, particularly in patients with low educational level and bitemporal (BT) electrode placement.
La seguridad de la terapia electroconvulsiva ha mejorado mucho en las últimas décadas, lo que hace que los efectos potencialmente adversos en la memoria y otras funciones neurocognitivas sean el principal aspecto clínico de preocupación en el presente. En Colombia, la población general y los profesionales de la salud (incluso algunos psiquiatras) parecen tener opiniones mayoritariamente negativas sobre el tratamiento electroconvulsivo, pero quizá esto podría reconsiderarse si se brinda más información; por lo tanto, el objetivo del presente estudio es evaluar los cambios en la memoria y la gravedad de los síntomas en un grupo de pacientes con depresión grave antes y después de la terapia electroconvulsiva.
MétodosSe incluyó a 23 pacientes con edades comprendidas entre los 23 y los 70 años del Servicio de Terapia Electroconvulsiva de la Clínica San Juan de Dios (Manizales, Colombia) para evaluar el efecto de esta terapia en la memoria de pacientes con depresión grave. Los síntomas depresivos y la memoria se evaluaron con la escala de depresión de Hamilton (HAMD) y la prueba de aprendizaje auditivo verbal de Rey (RAVLT) respectivamente. Se evaluó a los participantes antes de la sesión inicial de la serie de terapia electroconvulsiva (0-1 día) y 2 días después de su último tratamiento.
ResultadosLa terapia electroconvulsiva resultó en una mejora significativa en la puntuación de depresión. No hubo diferencias significativas en las puntuaciones de las 5 pruebas de aprendizaje, recuerdo retardado, aprendizaje y olvido desde antes del tratamiento hasta después de este. Se encontraron diferencias significativas antes y después del tratamiento en la prueba de reconocimiento retardado.
ConclusionesLos problemas de memoria pueden evaluarse y caracterizarse de manera práctica tras la terapia electroconvulsiva. La evaluación cognitiva antes y después de la terapia electroconvulsiva es un procedimiento viable y útil. En general, el rendimiento de la memoria no empeora después de la terapia electroconvulsiva en pacientes con depresión. Solo el reconocimiento retardado se ve afectado unos días después, particularmente en pacientes con bajo nivel educativo y colocación de electrodos bitemporales (BT).
Electroconvulsive therapy (ECT) administered under anesthesia and muscle relaxation is an effective and rapidly acting treatment for severe depression1,2 leading to significant improvement in health-related quality of life and remission rates above 80%.3,4 The safety of ECT has improved greatly over the last two decades, the potentially adverse effects on memory and neurocognitive functions being the main current area of concern.5–8 While some studies have shown that the cognitive abnormalities associated with ECT are limited to the first days post treatment,7,9 others have reported unfavorable long lasting effects on memory and cognition.10,11
There are several factors that may affect the cognitive performance in patients with depression. The disorder itself may affect memory and cognition;12 the electrode placement may affect anterograde memory (unilateral and bifrontal ECT placement appear to have an advantage over bitemporal placement on specific memory domains, but the evidence is not conclusive);13,14 high educational level seems to protect against verbal memory deficits in individuals with elevated depressive symptoms;15 patients with unipolar depression show smaller impairments in several neurocognitive domains compared to patients with bipolar disorder.16
ECT remains a controversial treatment despite its long history.17 In 2014, the San Juan de Dios Clinic in Manizales, Colombia created a specialized ECT service incorporating all the modern protocols and technology. However, the general public and even health professionals continue to harbor negative attitudes towards the procedure, an attitude that perhaps can be modified if more information is provided.18 The aim of the present study was to evaluate the changes in memory and the severity of symptoms in a group of patients with depression, before and after ECT.
MethodsParticipants and sampleTwenty-three patients ranging in age from 23 to 70 years were recruited from individuals scheduled to begin ECT at the ECT service of the San Juan de Dios Clinic in Manizales, Colombia. Eligibility criteria included a diagnosis of recurrent major depression, hospitalization required, with consecutive admissions in the last year. Patient diagnoses were established by clinical psychiatrist using Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria.19 Study exclusion criteria included comorbidity with neurological disorder, substance abuse/dependence history, cranioencephalic trauma, or intellectual disability.
All patients in this study received ECT as part of an acute course, administered 3 times per week: Monday, Wednesday, and Friday. Informed consent was obtained after the study was fully explained to all the patients and their families. This investigation was approved by the research ethics committee of the University of Caldas and their peers at the San Juan de Dios Clinic.
Clinical and cognitive assessmentDepressive symptoms and memory were assessed with the Hamilton Depression Scale (HAMD)20 and Rey Auditory Verbal Learning Test (RAVLT),21 respectively. The baseline assessment was administered to participants before the initial ECT treatment session (0-1 day) and the second assessment 2 days after their 12th ECT treatment session.
The RAVLT consists of 15 nouns read aloud by the examiner (with a 1-s interval between each word) for 5 consecutive trials (trials 1-5), each trial followed by a free-recall test. Patients were instructed that they would hear a list of 15 words and that they should listen carefully because they would be asked to recall as many words as possible belonging to the list. Instructions were repeated before each trial to minimize forgetting. After a 20-min delay period, each subject was again tested about recalling the words (trial # 6). Then, a list of 30 words was presented to the subjects which included the initial 15 words and patients were asked to recognize the original words (recognition trial).21
The following scores were computed according to a previously described and reported index:22–24
Number of correct responses during in each trial.
Immediate recall score: the sum of all correct responses given in the 5 consecutive trials.
Recognition trial: the number of words recognized correctly from a list of 30 words.
Verbal learning: difference between the number of words correctly recalled after the fifth and the first reading.
Verbal forgetting: difference between the number of words correctly recalled after the fifth reading and at the delayed recall test (trial 6).
ECTECT was administered with an ultra-brief pulse device (Thymatron system IV, Somatics LLC), in an acute course, 3 times per week, until the completion of 12 sessions. Medications used during the ECT procedure were a microdose of rocuronium bromate, followed by succinylcholine (1 mg/kg), and appropriate dosages of propofol or etomidate (the etomidate where used only in patients with an elevate seizure threshold) which were all delivered intravenously. Motor and electroencephalographic seizure manifestations were monitored. All participants were treated initially with the low 0.5 protocol and in cases of elevated seizure threshold with the 2xlp protocol. The electrodes were always positioned bi-frontal, and only in resistant cases the bitemporal electrode placement (BT) were used.
Statistical analysesThe results are expressed as mean±SD. Initially all cognitive datasets were examined for normality using the Kolmogorov-Smirnov test with Lilliefors significance correction. For memory and depression level, paired sample Student's t-tests were used to assess changes between pre- and post-ECT. Finally, the association between recognition trial change was assessed with linear regression analysis. All statistical tests were 2-tailed with an α level of .05. The statistical analyses were done using IBM SPSS statistics version 20.25
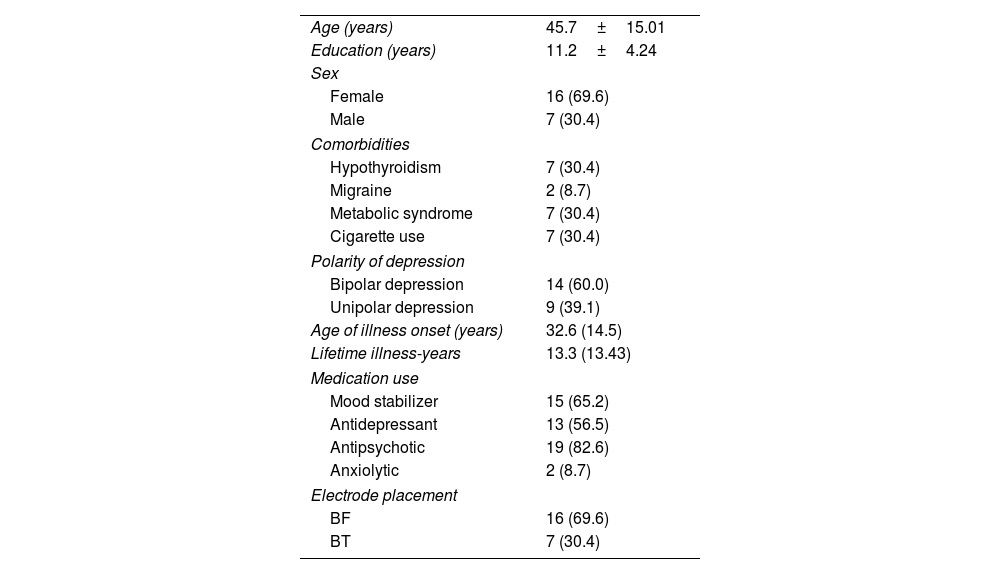
ResultsThe demographic, clinical characteristics, medication use and electrodes placement of patients who were assessed are listed in Table 1.
Demographic and clinical characteristics of patients with depression.
| Age (years) | 45.7±15.01 |
| Education (years) | 11.2±4.24 |
| Sex | |
| Female | 16 (69.6) |
| Male | 7 (30.4) |
| Comorbidities | |
| Hypothyroidism | 7 (30.4) |
| Migraine | 2 (8.7) |
| Metabolic syndrome | 7 (30.4) |
| Cigarette use | 7 (30.4) |
| Polarity of depression | |
| Bipolar depression | 14 (60.0) |
| Unipolar depression | 9 (39.1) |
| Age of illness onset (years) | 32.6 (14.5) |
| Lifetime illness-years | 13.3 (13.43) |
| Medication use | |
| Mood stabilizer | 15 (65.2) |
| Antidepressant | 13 (56.5) |
| Antipsychotic | 19 (82.6) |
| Anxiolytic | 2 (8.7) |
| Electrode placement | |
| BF | 16 (69.6) |
| BT | 7 (30.4) |
Data are expressed as n (%) or mean±standard deviation.
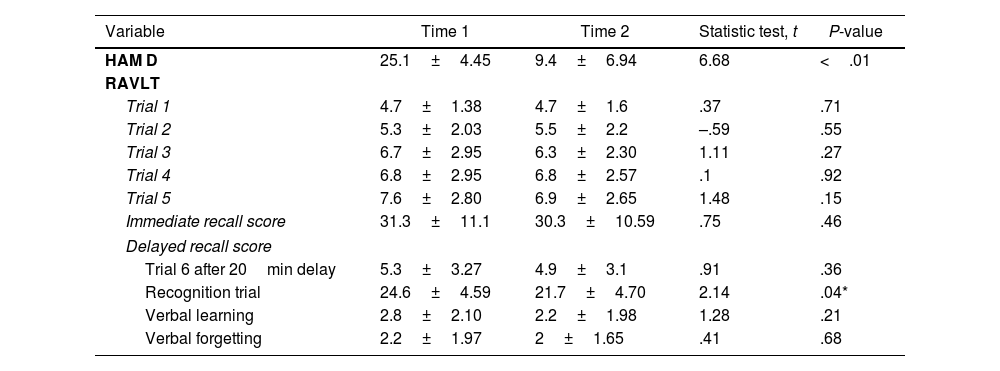
Table 2 summarizes mean pre- and post-ECT ratings of HAMD scale and RAVLT performance. The ECT led to a significant improvement in the rating of depression. There were no significant differences in ratings between time 1 and time 2 (Table 2) for the 5 learning trials, delayed recall, learning and forgetting scores. A significant time 1/time 2 difference was found in the recognition trial.
Paired t-test comparison of Hamilton Depression Scale and RAVLT before and after a course of ECT in depression.
| Variable | Time 1 | Time 2 | Statistic test, t | P-value |
|---|---|---|---|---|
| HAM D | 25.1±4.45 | 9.4±6.94 | 6.68 | <.01 |
| RAVLT | ||||
| Trial 1 | 4.7±1.38 | 4.7±1.6 | .37 | .71 |
| Trial 2 | 5.3±2.03 | 5.5±2.2 | –.59 | .55 |
| Trial 3 | 6.7±2.95 | 6.3±2.30 | 1.11 | .27 |
| Trial 4 | 6.8±2.95 | 6.8±2.57 | .1 | .92 |
| Trial 5 | 7.6±2.80 | 6.9±2.65 | 1.48 | .15 |
| Immediate recall score | 31.3±11.1 | 30.3±10.59 | .75 | .46 |
| Delayed recall score | ||||
| Trial 6 after 20min delay | 5.3±3.27 | 4.9±3.1 | .91 | .36 |
| Recognition trial | 24.6±4.59 | 21.7±4.70 | 2.14 | .04* |
| Verbal learning | 2.8±2.10 | 2.2±1.98 | 1.28 | .21 |
| Verbal forgetting | 2.2±1.97 | 2±1.65 | .41 | .68 |
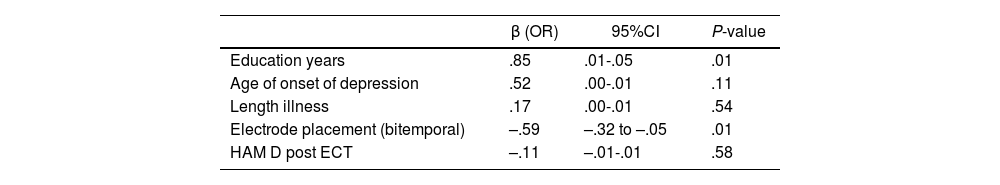
The simple linear regression analysis revealed that there was a significant association between change in recognition trial and years of education and electrode placement. After ECT, scores in the recognition trial got worse in patients with a lower educational level and bitemporal electrode placement (adjusted R square=.31; F test=2.97; P-value=.04) (Table 3).
Single linear regression of factors associated with recognition trial post ECT.
| β (OR) | 95%CI | P-value | |
|---|---|---|---|
| Education years | .85 | .01-.05 | .01 |
| Age of onset of depression | .52 | .00-.01 | .11 |
| Length illness | .17 | .00-.01 | .54 |
| Electrode placement (bitemporal) | –.59 | –.32 to –.05 | .01 |
| HAM D post ECT | –.11 | –.01-.01 | .58 |
Adjusted R2=.31; F-test=2.97; P-value=.04.
This study compared the performance on the RAVLT in a sample of patients with depression before and after ECT. As expected, a significant clinical improvement in depression was observed following ECT. We found no significant group differences in the learning curves, but there was a significant difference in delayed recognition memory. Patients with low educational levels and bitemporal electrode placement showed lower post-treatment scores in delayed recognition memory.
There were no differences in the learning curves, this could be explained with the novel developments in ECT on stimulus characteristics (pulse width, frequency), since recent studies suggest lower risk of cognitive impairment.26 Another way to possibly explain this is that memory remains impaired in patients with recurrent and severe depression for months after remission.27,28 Evidence found in research suggest that learning curve improves in the long term with ECT in depressive patients.29,30 Hence why, it is necessary to follow up the changes in learning curves in order to evaluate them.
Our findings are in agreement with previous clinical trials showing efficacy of ECT on depression and its impact on cognitive functions.7,31–34 The scientific literature also suggests that recognition memory worsens in patients exposed to bilateral ECT31 and in other memory domains.34 The impairment in delayed recognition memory can be explained by the Signal Detection Theory (SDT) that describes how perceivers separate meaningful information from “noise”.35,36 This may be clinically relevant when patients need to discriminate between two options that are perceptually similar, and a misclassification or decision could bring negative consequences. While in our study the impairment in delayed recognition memory was found 2 days after the last ECT session, there is evidence that cognitive abnormalities are generally limited to the first 3 days posttreatment,9 therefore it is necessary to explore in detail how the memory impairment evolves over time.
With regard to electrode placement, the literature reports that bitemporal electroconvulsive therapy (BT ECT) is generally associated with greater cognitive side effects than unilateral and bifrontal electroconvulsive therapy (BF ECT),37,38 and that there are no significant differences between patients given BF ECT and those given unilateral electroconvulsive therapy (UL ECT).32,33,39 In line with these previous studies, we found that BT ECT worsened recognition memory more than BF ECT. However, at least one report concludes that the cognitive profile of BF ECT is not substantially different from that of BT ECT.13 This discrepancy may be also explained by differences in stimulus doses across the protocols used in the various studies, the cognitive batteries utilized and the small sample sizes.
Another variable associated with delayed recognition memory was educational level, which is related with cognitive reserve (CR),40,41 and is a factor that may affect the neurocognitive effects of ECT. In line with our findings, there is evidence which indicates that memory performance following ECT treatment is considerably worse in patients with lower CR operationalized by a combination of educational level and occupational attainment,42 and that higher CR may reduce later retrograde autobiographical memory changes.43 Our findings reinforce previous results from other studies which suggest that baseline cognitive status may predict cognitive side effects after ECT.43,44
LimitationsThe primary study limitations are its quasi-experimental design, small sample size and the use of only one instrument to measure memory. This study used a quasi-experimental, pretest-posttest design; therefore, causal inferences from its findings requires caution. The study lacks a control group, making it difficult to differentiate between ECT effects and the effects of time, medication, and depression. It is necessary a control group with patients without memory impairment to evaluate the changes after ECT, or evaluate the memory in patients without ECT to find another causal or associated variables that play a role in memory.
The impairment in recognition memory might be due to the short interval between the last ECT session and the memory assessment, hence why a study with long term follow up is necessary to evaluate the recognition memory. The small study group limits the statistical power of the analysis and it may be a source of type 2 errors. Therefore, our study requires replication with a large sample size, and it is necessary to assess the long-term effects of ECT on recognition memory and depression level. Longitudinal studies addressing development and change over longer time periods in memory assessment are necessary. Future studies should investigate a neuropsychological and clinical complete status, and use other memory instruments to prevent the learning of the patient through the evaluation with the same instrument in a short interval between assessments.
ConclusionsThis study suggests that delayed recognition memory may be affected a few days after ECT treatment, particularly in patients with low educational level and BT electrode placement. Memory functions should be systematically followed up and evaluated in efforts to minimize their negative impact on the wellbeing of the patients.
Conflicts of interestsAll authors declare no conflict of interests that could influence their work.
The authors would like to thank San Juan de Dios Clinic and the University of Caldas for the help to carry out the project.