Immunotherapy with checkpoint inhibitors has shown to be one of the most effective new strategies to treat some types of cancer. However, stopping the suppression induced by the checkpoints, mainly CTLA-4 and PD-1/PD-L1, makes patients prone to develop different immune reactions that can range from mild organ involvement to life-threatening compromises. This highlights the importance that patients should be carefully evaluated during treatment in order to detect and understand these types of manifestations, and find tools for their management.
ObjectivesTo determine the autoantibody profile of patients under treatment with checkpoint inhibitors (ICI), with or without symptoms suggestive of rheumatological disease associated with the therapy. The clinical and demographical characteristics of such patients were also analyzed.
MethodsPatients diagnosed with cancer and under treatment with ICI were recruited. Sera were evaluated with commercial kits to determine the presence of autoantibodies. The presence of immune-related adverse events (irAEs) was evaluated according to the definitions proposed in the guidelines of the American Society of Clinical Oncology.
ResultsOf the twenty-four patients evaluated, fifteen presented with at least one adverse immunological event, where dermatological and musculoskeletal were the most prevalent. Autoantibodies were obtained from the serum of 22 patients. Nineteen of them had at least one autoantibody, with antinuclear and anti-smooth muscle antibodies being the most prevalent.
ConclusionThe seroprevalence found in our study suggests that autoantibodies may be part of irAEs in patients treated with ICI. However, more studies are required to define the usefulness of autoantibody detection in these patients in order to predict clinical manifestations.
La inmunoterapia con inhibidores de puntos de control (ICC) ha demostrado ser una de las nuevas estrategias más efectivas para tratar varios tipos de cáncer. Sin embargo, al detener la supresión ejercida por los puntos de chequeo, principalmente CTLA-4 y PD-1/PD-L1, algunos pacientes presentan diferentes reacciones inmunológicas (irAE) que pueden ir desde compromiso orgánico leve hasta grave, con riesgo de muerte. Esto hace importante que durante el tratamiento los pacientes sean evaluados cuidadosamente, para lograr detectar y entender este tipo de manifestaciones y encontrar herramientas para su manejo.
ObjetivosDeterminar la presencia de autoanticuerpos en un grupo de pacientes en tratamiento con ICC, con o sin clínica sugestiva de enfermedad reumatológica asociada a la terapia. Además, evaluar las características clínicas y demográficas de dichos pacientes.
MétodosEn este estudio transversal se reclutaron pacientes diagnosticados con cáncer y bajo tratamiento con ICC. En el suero se determinaron autoanticuerpos mediante kits comerciales. La presencia de irAE fue evaluada de acuerdo con las definiciones propuestas en las guías de la Sociedad Americana de Oncología Clínica.
ResultadosDe los 24 pacientes evaluados, 15 presentaron al menos un evento adverso inmunológico, siendo los dermatológicos y los musculoesqueléticos los más prevalentes. Respecto a la presencia de autoanticuerpos, de los 22 sueros analizados 19 presentaban al menos un autoanticuerpo, siendo los anticuerpos antinucleares y antimúsculo liso los más comunes.
ConclusiónLa seroprevalencia encontrada en nuestros pacientes nos sugiere que los autoanticuerpos podrían hacer parte de las irAE en pacientes tratados con ICC. Sin embargo, se requieren más estudios para lograr definir la utilidad de la determinación de autoanticuerpos en estos pacientes como predictores de manifestaciones clínicas.
The presence of tumor antigens makes it easier for the adaptive immune system to eliminate malignant cells due to an antigen-specific response. However, the tumor cells have developed different methods of evasion of the immune response, such as the reduction in the expression of antigens —a phenomenon also called tumor immunoedition— and the inhibition of the response of the immune system by the tumor, especially of the T-lymphocyte-mediated response.
T cells have multiple checkpoints to regulate their activation and prevent the development of autoimmune responses. Within the range of molecules associated with this process, the cytotoxic T lymphocyte antigen 4 (CTLA-4) and the molecule called programmed death 1 (PD-1), stand out for their importance, respectively. Both have an inhibitory function of the immune response, and in fact their expression is altered in different rheumatic diseases.1
Cancer immunotherapy using checkpoint blockade is based on the inhibition of the suppression of immune responses against cancer. This type of treatment has been consolidated as a promising treatment option for patients with different neoplasms.
Clinical studies have demonstrated a reduction in the growth of the melanoma and improvement in survival with treatments that block the PD-1 and CTLA-4 pathways. Likewise, monoclonal antibodies that block CTLA-4 and PD-1 have been approved for the treatment of melanoma and lung cancer.
Despite its efficacy, immunotherapy in cancer is not free from problems. By unleashing the potential of the cells of the immune system during the treatment, its reactivity is also directed towards healthy tissues. Both the blockade of CTLA-4 and of PD-1 have adverse effects associated to the immune system that can affect any organ.
For example, during the first weeks of treatment, the appearance of erythema and pruritus in the trunk and the extremities has been described in approximately 40% of the patients with anti-CTLA-4 therapy, and in 20% with anti-PD-1 therapy. Gastrointestinal manifestations are also common. Regarding endocrinopathies, inflammation of the pituitary gland occurs in patients treated with immunotherapy directed at CTLA-4, and hypothyroidism in patients treated with immunotherapy directed towards PD-1.2 Vitiligo, arthralgia, dry mucous membranes and pneumonitis can also appear as additional adverse effects. Even so, the effects are in general moderate and require simple treatments in most cases. Usually, stopping the administration of immunotherapy or providing immunosuppression solves the problem. The persistence of these manifestations can induce an autoimmune disease.
Autoimmune diseases have a long preclinical stage. Treatments with immune checkpoint inhibitors (ICI) can be considered a trigger factor in their development.3 On the other hand, patients treated with ICI do not develop adverse immune effects with the same frequency or with similar severity, for this reason, it is recognized that some patients are more susceptible than others. Collecting data on these patients can provide a deeper look towards the etiology of rheumatic diseases.4
Although the majority of the studies corresponding to specific rheumatologic diseases in patients with ICI are usually retrospective with few patients, it should be highlighted that cases have been reported in which none of the patients has a diagnosis of autoimmune disease previously to the start of the immunotherapy (specifically anti-PD-1/PD-L1), and some of them have developed rheumatoid arthritis, with serological markers such as positive rheumatoid factor and anti-citrullinated peptide (anti-CCP) antibodies.5 The presence of inflammatory arthritis and dry syndrome associated with anti-CTLA-4 y anti-PD-1 therapies has also been reported retrospectively, although the serological markers were negative for the patients with arthritis and the positivity of antinuclear and anti-SSB antibodies was not present in all patients with dry syndrome.6
It is important to recognize that patients are an in vivo model that helps to understand the onset of the autoimmune disease secondary to immunotherapy, and its corresponding clinical study can provide valuable tools for its treatment, among them, the search of autoantibodies.1
Patients and methodsSampleIn this cross-sectional study, patients with a diagnosis of cancer who underwent immunotherapy with ICI during 2018–2019 were recruited. The study was approved by a bioethics committee (FR-310-45).
Immune-related adverse events (irAEs)The clinical evaluation was performed by a rheumatologist to establish the presence of irAE, according to the guidelines of the American Society of Clinical Oncology,7 as follows: In the neurological domain, the presence of peripheral neuropathy, encephalitis, uveitis or aseptic meningitis was assessed. In the digestive domain, the presence of dry symptoms, mucositis, colitis, enteritis or hepatitis was evaluated. The skin was assessed for the presence of rash or vitiligo. Regarding the hematological domain, findings of thrombocytopenia or anemia were sought. The endocrine domain was assessed looking for adrenal insufficiency, hypophysitis, thyroiditis, hypo/hyperthyroidism, autoimmune diabetes, or pancreatitis. Regarding the musculoskeletal domain, arthralgia, arthritis or myalgia were assessed. The cardiovascular system was evaluated searching for myocarditis. The renal system was assessed looking for glomerulonephritis. Finally, the respiratory domain was assessed in search for pneumonitis.
The search for these manifestations was based on clinical suspicion, and it was attributed as the presence of irAE by ruling out other causes supported on the medical history, the physical examination and laboratory, imaging and histopathology parameters when necessary.
Autoantibody testA serum sample was analyzed for: anti-nuclear (ANA), anti-gastric parietal cells (anti-GPC), antimitochondrial (AMA), anti-smooth muscle (anti-SMA) antibodies, while anti-double stranded DNA antibodies (anti-dsDNA) were assessed by indirect immunofluorescence. Anti-thyroid peroxidase antibodies (anti-TPO) were evaluated by chemiluminiscence. Anti-cyclic citrullinated peptides and extractable nuclear antigen antibodies were evaluated by enzyme-linked immunosorbent assay (ELISA). The titers of rheumatoid factor were measured by immunoturbidimetry.
This autoantibody panel was performed in a third party diagnostic laboratory, with standardized commercial techniques. The personnel of the laboratory were blinded to the clinical and therapeutic information.
Statistical analysesDescriptive statistics were calculated. The different variables are presented as mean and standard deviation (SD), or as median and interquartile range, depending on the nature of the distribution according to a Kolmogorov-Smirnov test. The qualitative variables are presented as percentages. The software used was the Prism 5 (GraphPad Prism, California, USA).
ResultsDemographic characteristics of the patientsThe demographic characteristics of the patients are shown in Table 1. 24 patients with diagnosis of cancer in treatment with con ICI were recruited. The mean age was 63 years (SD ± 13.5). Fourteen patients (58.3%) were diagnosed with non-small cells lung cancer (NSCLC), 7 (29.2%) with melanoma and 3 (12.5%) with renal cell carcinoma (RCC). The data of 22 patients showed a median since the diagnosis of cancer of 26 months (IQR 4−108). Sixteen patients (66.7%) were in treatment with nivolumab and 8 (33.3%) with pembrolizumab. The mean time of treatment with ICI of 20 patients was 12.4 months (SD ± 9.6).
Demographic characteristics.
| Total number of patients | n = 24 |
| Mean age, years (± SD) | 63 (13.5) |
| Male, n (%) | 11 (45.8) |
| Female, n (%) | 13 (54.1) |
| Race, n (%) | |
| Caucasian | 1 (4.1) |
| Black | 3 (12.5) |
| Mestizo | 16 (67) |
| Indeterminate | 4 (16.6) |
| Type of cancer, n (%) | |
| Melanoma | 7 (29.2) |
| NSCLC | 14 (58.3) |
| RCC | 3 (12.5) |
| Conventional chemotherapy, n (%)* | 17 (85) |
| Time since diagnosis, median in months (IQR)** | 26 (4−108) |
| Type of molecule, n (%) | |
| Nivolumab | 16 (66.7) |
| Pembrolizumab | 8 (33.3) |
| Time under treatment with ICI, mean in months (±SD)** | 12.4 (± 9.6) |
RCC: renal cell carcinoma; NSCLC: non-small cell lung cancer.
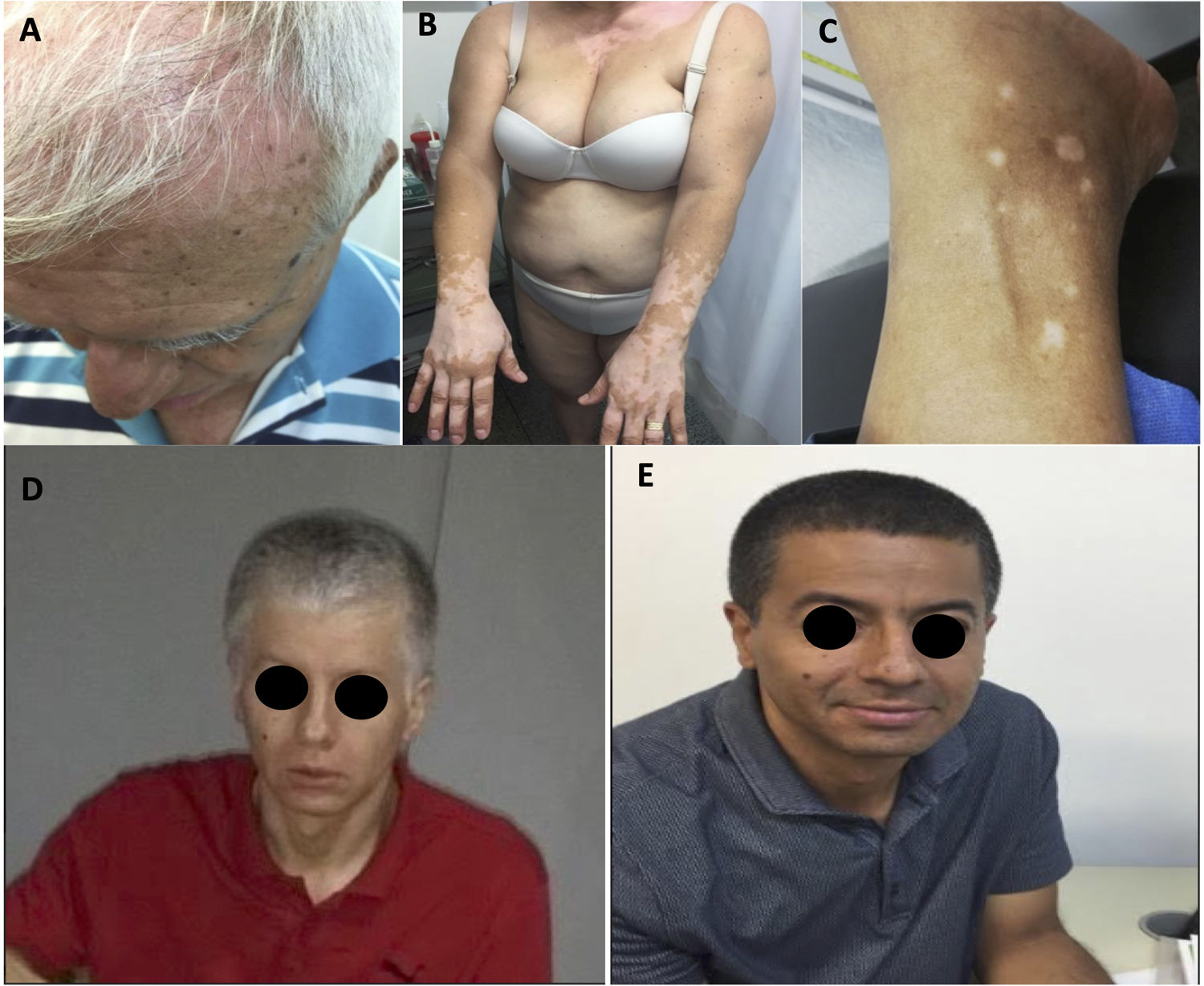
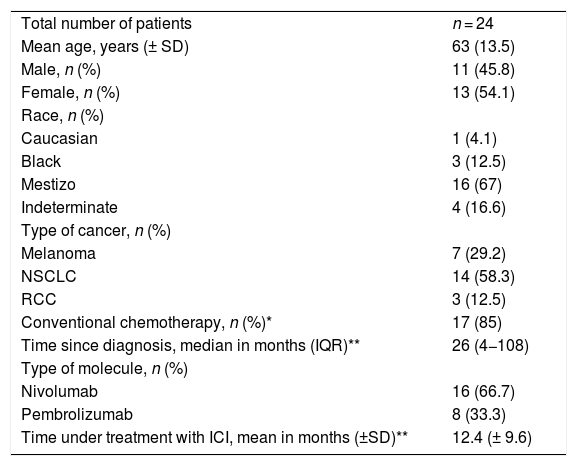
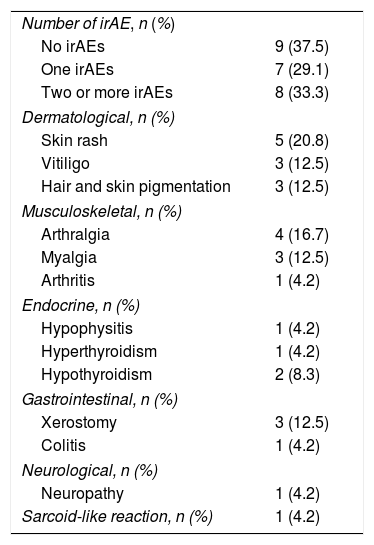
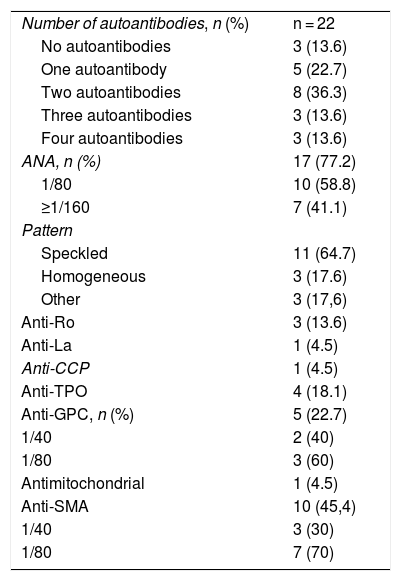
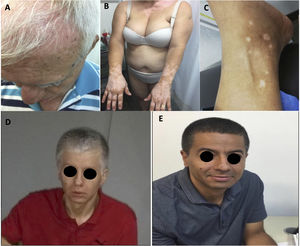
A total of 15 patients had one or more adverse events related to the immune system (irAE) (Table 2). The most common irAEs were dermatological and musculoskeletal. Five patients had skin rash (20.8%), 3, vitiligo (12.5%), and other 3, skin and hair repigmentation (12.5%) (Fig. 1A-E). Five patients had arthralgia (20.8%), 3 had myalgia (12.5%) and one had arthritis (4.2%). One patient had a cutaneous and pulmonary sarcoid-like reaction.8 Other less frequent irAEs are listed in Table 2.
Immune-related adverse events (irAE).
| Number of irAE, n (%) | |
| No irAEs | 9 (37.5) |
| One irAEs | 7 (29.1) |
| Two or more irAEs | 8 (33.3) |
| Dermatological, n (%) | |
| Skin rash | 5 (20.8) |
| Vitiligo | 3 (12.5) |
| Hair and skin pigmentation | 3 (12.5) |
| Musculoskeletal, n (%) | |
| Arthralgia | 4 (16.7) |
| Myalgia | 3 (12.5) |
| Arthritis | 1 (4.2) |
| Endocrine, n (%) | |
| Hypophysitis | 1 (4.2) |
| Hyperthyroidism | 1 (4.2) |
| Hypothyroidism | 2 (8.3) |
| Gastrointestinal, n (%) | |
| Xerostomy | 3 (12.5) |
| Colitis | 1 (4.2) |
| Neurological, n (%) | |
| Neuropathy | 1 (4.2) |
| Sarcoid-like reaction, n (%) | 1 (4.2) |
The results of the antibody panel are shown in Table 3. Nineteen of 22 patients (whose autoantibody panel was carried out successfully) had at least one autoantibody (86.4%), 5 had one antibody, 8 had two antibodies and 6 had three of more. The most commonly found autoantibodies were ANA: 17 patients tested positive (77.2%), 10 with titers of 1/80 (58.8%) or higher, and 11 of the 17 had a speckled pattern (64.7%). One patient had positive anti-CCP at low titers (23.4 IU/mL). None of the patients tested positive for anti-dsDNA, anti-Sm, anti-RNP, or RF.
Autoantibodies.
| Number of autoantibodies, n (%) | n = 22 |
| No autoantibodies | 3 (13.6) |
| One autoantibody | 5 (22.7) |
| Two autoantibodies | 8 (36.3) |
| Three autoantibodies | 3 (13.6) |
| Four autoantibodies | 3 (13.6) |
| ANA, n (%) | 17 (77.2) |
| 1/80 | 10 (58.8) |
| ≥1/160 | 7 (41.1) |
| Pattern | |
| Speckled | 11 (64.7) |
| Homogeneous | 3 (17.6) |
| Other | 3 (17,6) |
| Anti-Ro | 3 (13.6) |
| Anti-La | 1 (4.5) |
| Anti-CCP | 1 (4.5) |
| Anti-TPO | 4 (18.1) |
| Anti-GPC, n (%) | 5 (22.7) |
| 1/40 | 2 (40) |
| 1/80 | 3 (60) |
| Antimitochondrial | 1 (4.5) |
| Anti-SMA | 10 (45,4) |
| 1/40 | 3 (30) |
| 1/80 | 7 (70) |
ANA: antinuclear antibodies; Anti-CCP: anti-citrullinated cyclic peptide antibodies; Anti-GPC: anti-gastric parietal cells antibodies; Anti-SMA: anti-smooth muscle antibodies; Anti-TPO: anti-thyroid peroxidase antibodies.
The evidence of autoantibodies in patients treated with ICI is limited, mainly due to the low frequency of their presentation after treatment with such molecules.9 Previous studies of patients treated with ICI report a low prevalence of autoantibodies, such as that of Calabrese et al.,10 who evaluated 13 patients without pre-existing autoimmune disease and only one patient who had arthritis as irAE tested positive for anti-dsDNA y ANA. Cappelli et al.6 previously reported 13 patients treated with ICI who expressed rheumatic irEAs. Only 3 of them developed autoantibodies under treatment with ICI. These reports are in contrast with our study: 86.4% of our serum samples (19 out of 22) tested positive for at least one autoantibody and 14 had at least one irAE.
63% of the total of patients (15 out of 24) had at least one irAE, which is similar to the pooled incidence obtained in a meta-analysis that included 36 clinical trials and showed a range of 56–76%.11 We observed that one of the most common were dermatological, in particular skin rash and vitiligo. These results are similar to those of a study with 296 patients with melanoma in treatment with nivolumab.12 When it comes to vitiligo, ICIR-BIOGEAS reported that 353 of 368 patients (96%) with vitiligo had melanoma.13 This phenomenon also occurred in our patients, where 2 of the 3 who developed vitiligo also had melanoma, which supports the hypothesis of cross-reactivity between melanoma antigens and melanocytes.14 Another dermatological irAE we observed was hair and skin repigmentation, which was previously reported.15
There are some limitations in our study. First, our number of samples is small and not randomized, which could led to a selection bias. Second, we had a very heterogeneous sample, without an equal number in each subgroup. Third, the cross-sectional nature of the study prevented us to observe any progression of the irAEs or of the levels of autoantibodies over time. Fourth, the study did not have a control group. Fifth, patients undergoing therapy with nivolumab and pembrolizumab were recruited; but patients with any other checkpoint inhibitor such as atezolizumab, ipilimumab, avelumab, durvalumab or tremelimumab, among others, were not included. Finally, we cannot be sure if our patients already had autoantibodies before treatment or if they appeared as a result thereof.
The higher seroprevalence of AA found in our study suggests that it could be useful to explore the presence of baseline AA before starting treatment; positive AA titers and clinical manifestations could be followed-up, providing clues to any association between both. In addition, it is important to look for AA when an irAE develops, because such AA could explain the presence of the adverse event. Although we found an association between some AA and irAE, the usefulness of the autoantibodies needs further research. It is worth noting that the AAs were not widely evaluated in different studies with irAE; therefore, they could be useful as biomarkers in decision-making for treatment and follow-up of these patients.
Conflict of interestThe authors declare that they have no conflict of interest.
Authors participationTomás Urrego Callejas and Juan Felipe Soto Restrepo contributed equally to this manuscript.
FundingThe funding entity was the Colombian Association of Rheumatology (Asociación Colombiana de Reumatología) (ASOREUMA), Bogotá, Colombia. The ID of the grant was: ASOREUMA - 2018-23516.
We are grateful to ASOREUMA for financing the project. To the Young Researcher and Sustainability Programs of the University of Antioquia, to the Research Unit of the Instituto de Cancerología Clínica las Américas-Auna, and to the patients for their support to the research.
Please cite this article as: Urrego-Callejas T, Soto-Restrepo JF, Sandoval-Álvarez S, Chvatal-Medina M, Gómez R, Vásquez G. Autoanticuerpos: una manifestación de los eventos adversos inmunológicos de la inmunoterapia en cáncer. Rev Colomb Reumatol. 2022;29:3–8.