Brucellosis is a zoonosis that causes a multi-organ granulomatous infection. It has diverse and non-specific clinic features that can make diagnosis difficult. Medical personnel often do not recognize it early. Delayed treatment is associated with high morbidity and even mortality. Its timely diagnosis requires a high index of suspicion. The case is presented of a 35-year-old male zootechnologist, previously healthy, with a progressive picture of two months of evolution of irradiated low back pain to the left hip, nocturnal diaphoresis, and unintentional weight loss. Elevation of acute phase reactants was documented and magnetic resonance imaging found signs of iliopsoas tendonitis and inflammatory changes in the left sacroiliac joint. The IgG and IgM antibodies using an immunoassay for brucella were positive. After establishing antibiotic treatment, a marked clinical improvement, with resolution of the inflammatory process was evident.
La brucelosis es una zoonosis que genera una infección granulomatosa multiorgánica. Tiene una clínica diversa e inespecífica que puede hacer difícil el diagnóstico. Con frecuencia, el personal médico no la reconoce de forma temprana. El retraso en el tratamiento se asocia a una gran morbilidad e incluso mortalidad. Su diagnóstico oportuno requiere un alto índice de sospecha. Se presenta el caso de un hombre de 35 años, zootecnista, previamente sano, con un cuadro progresivo de dos meses de evolución de dolor lumbar irradiado a cadera izquierda, diaforesis nocturna y pérdida no intencional de peso. Se documentó elevación de reactantes de fase aguda y en la resonancia magnética se encontraron signos de tendinitis del psoas y cambios inflamatorios en la articulación sacroilíaca izquierda. Los anticuerpos IgG e IgM por inmuno ensayo para brucella fueron positivos, y luego de instaurar tratamiento antibiótico se evidenció marcada mejoría clínica con resolución del proceso inflamatorio.
Brucellosis is a zoonosis caused by nonmotile aerobic sporogenous Gram-negative bacilli, with intracellular reproduction. The lipopolysaccharide layer of the cell wall shows an endotoxic activity. The infection is characterized by producing a chronic granulomatous infection. In the past it was known as undulant fever, due to the ups and downs in the natural course of the disease. The infection is seen more frequently in developing countries and is practically eradicated in developed countries. According to the World Health Organization (WHO), an incidence of 500,000 cases per year in the world is estimated, mainly in endemic areas such as the Arabian Peninsula, the Mediterranean basin, Indochina, Central Asia and South America.1 It has its highest incidence in people who are in occupational contact with cattle, sheep and goats, among other species, or in individuals who consume unpasteurized dairy products. In fact, it seems that milk from sheep and goats contaminated with Brucella melitensis is the main source of human brucellosis worldwide.2
Brucellosis can affect any organ or system in the body, which is why it is known as one of the “great imitators.” The incubation period is usually one to four weeks, although it can extend beyond several months. It has a great diversity of presentations, ranging from an asymptomatic disease to a deadly one. The most frequent symptoms are arthralgia, fever and asthenia, observed in up to 75%–100% of cases. To a lesser extent, diaphoresis, bad-smelling perspiration, anorexia, myalgia, chills and low back pain may occur. The most common clinical findings are fever and hepatosplenomegaly in up to half of the patients, followed by isolated splenomegaly, peripheral arthritis, sacroiliitis, scrotal edema, nuchal rigidity, and lymphadenopathies.2
Osteoarticular involvement, the most frequent complication, is observed in up to half of the cases of brucellosis. Spinal commitment has a variable incidence from 2% to 65%, being more frequent in men.3 In Colombia, the prevalence in the population at risk ranges between 0.14% and 10.4%. Despite being an entity that is present in the environment and being of mandatory notification, often remains as a forgotten disease.4 In low-income countries, the availability of accurate diagnostic methods is often limited, so a detailed clinical history may support the treatment decision.
An illustrative case documented in a third level hospital of the region of the Coffee Axis (Eje Cafetero) is presented. Initially it represented a clinical challenge, due to the wide differential and the lack of timely diagnostic resources in the setting.
Case presentationA 35-year-old male patient, zootechnician, previously healthy, who consulted due to a clinical picture of two months of evolution consisting in progressive low back pain radiating to the left hip and the left lower limb. The symptom worsened when he leaned the extremity, with an intensity of 10/10 on the subjective pain scale. Concomitantly, he presented unintentional weight loss of approximately 7 kg in two months, asthenia, adynamia, and profuse nocturnal diaphoresis without characteristic odor. The fever was not quantified and there was no history of trauma or any other associated symptomatology. On physical examination, it was found a hemodinamically stable patient, with pain in the left gluteal region that limited the gait. There were no signs of radiculopathy or neurological deficit. Percussion in the sacral region was positive.
The patient had consulted general medicine on multiple occasions and even other specialties such as orthopedics, without obtaining a clear diagnosis. An X-ray of the lumbosacral spine and coxofemoral joint was performed, which did not show significant alterations. He was admitted to the emergency department due to severe low back pain and inability to walk. The initial paraclinical tests showed leukocytosis, neutrophilia, and elevated acute phase reactants, as shown in Tables 1 and 2.
Description of the laboratory studies performed to the patient.
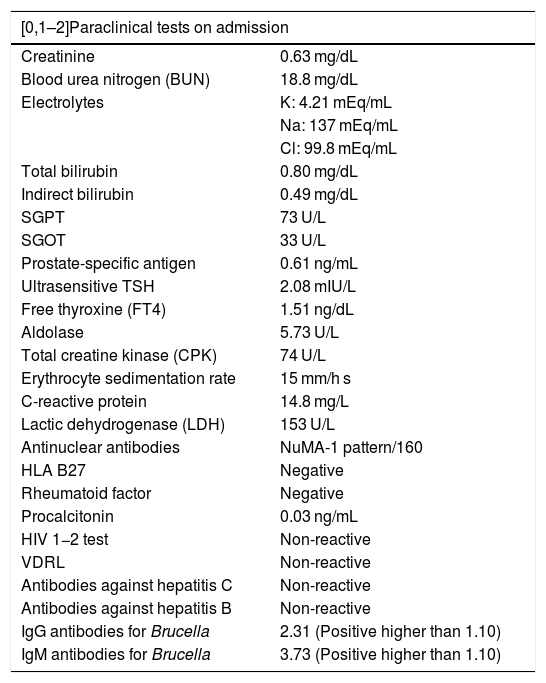
| [0,1–2]Paraclinical tests on admission | |
|---|---|
| Creatinine | 0.63 mg/dL |
| Blood urea nitrogen (BUN) | 18.8 mg/dL |
| Electrolytes | K: 4.21 mEq/mL |
| Na: 137 mEq/mL | |
| Cl: 99.8 mEq/mL | |
| Total bilirubin | 0.80 mg/dL |
| Indirect bilirubin | 0.49 mg/dL |
| SGPT | 73 U/L |
| SGOT | 33 U/L |
| Prostate-specific antigen | 0.61 ng/mL |
| Ultrasensitive TSH | 2.08 mIU/L |
| Free thyroxine (FT4) | 1.51 ng/dL |
| Aldolase | 5.73 U/L |
| Total creatine kinase (CPK) | 74 U/L |
| Erythrocyte sedimentation rate | 15 mm/h s |
| C-reactive protein | 14.8 mg/L |
| Lactic dehydrogenase (LDH) | 153 U/L |
| Antinuclear antibodies | NuMA-1 pattern/160 |
| HLA B27 | Negative |
| Rheumatoid factor | Negative |
| Procalcitonin | 0.03 ng/mL |
| HIV 1−2 test | Non-reactive |
| VDRL | Non-reactive |
| Antibodies against hepatitis C | Non-reactive |
| Antibodies against hepatitis B | Non-reactive |
| IgG antibodies for Brucella | 2.31 (Positive higher than 1.10) |
| IgM antibodies for Brucella | 3.73 (Positive higher than 1.10) |
Description of the hemogram during hospitalization.
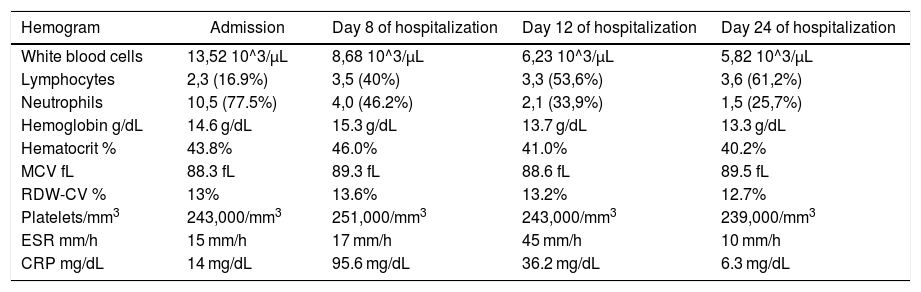
| Hemogram | Admission | Day 8 of hospitalization | Day 12 of hospitalization | Day 24 of hospitalization |
|---|---|---|---|---|
| White blood cells | 13,52 10^3/μL | 8,68 10^3/μL | 6,23 10^3/μL | 5,82 10^3/μL |
| Lymphocytes | 2,3 (16.9%) | 3,5 (40%) | 3,3 (53,6%) | 3,6 (61,2%) |
| Neutrophils | 10,5 (77.5%) | 4,0 (46.2%) | 2,1 (33,9%) | 1,5 (25,7%) |
| Hemoglobin g/dL | 14.6 g/dL | 15.3 g/dL | 13.7 g/dL | 13.3 g/dL |
| Hematocrit % | 43.8% | 46.0% | 41.0% | 40.2% |
| MCV fL | 88.3 fL | 89.3 fL | 88.6 fL | 89.5 fL |
| RDW-CV % | 13% | 13.6% | 13.2% | 12.7% |
| Platelets/mm3 | 243,000/mm3 | 251,000/mm3 | 243,000/mm3 | 239,000/mm3 |
| ESR mm/h | 15 mm/h | 17 mm/h | 45 mm/h | 10 mm/h |
| CRP mg/dL | 14 mg/dL | 95.6 mg/dL | 36.2 mg/dL | 6.3 mg/dL |
Five days after admission to the emergency service, the patient presented a fever peak and during follow-up the specific polymerase chain (PCR) and erythrocyte sedimentation rate (ESR) values continued to rise. Upon directed questioning, it was found that in addition to having regular contact with cattle, sheep and goats due to his profession, he had consumed unpasteurized goat milk, which raised the suspicion of a possible brucellosis. A series of blood cultures of the patient did not document growth of Brucella. In addition, a serology for Brucella was performed on one of the goats in the place where the patient worked for the Colombian Agricultural Institute (ICA). The final report was negative. Other paraclinical tests such as the bone marrow culture or antibodies against Brucella are not routinely available in the environment, but they were requested and sent to an external laboratory.
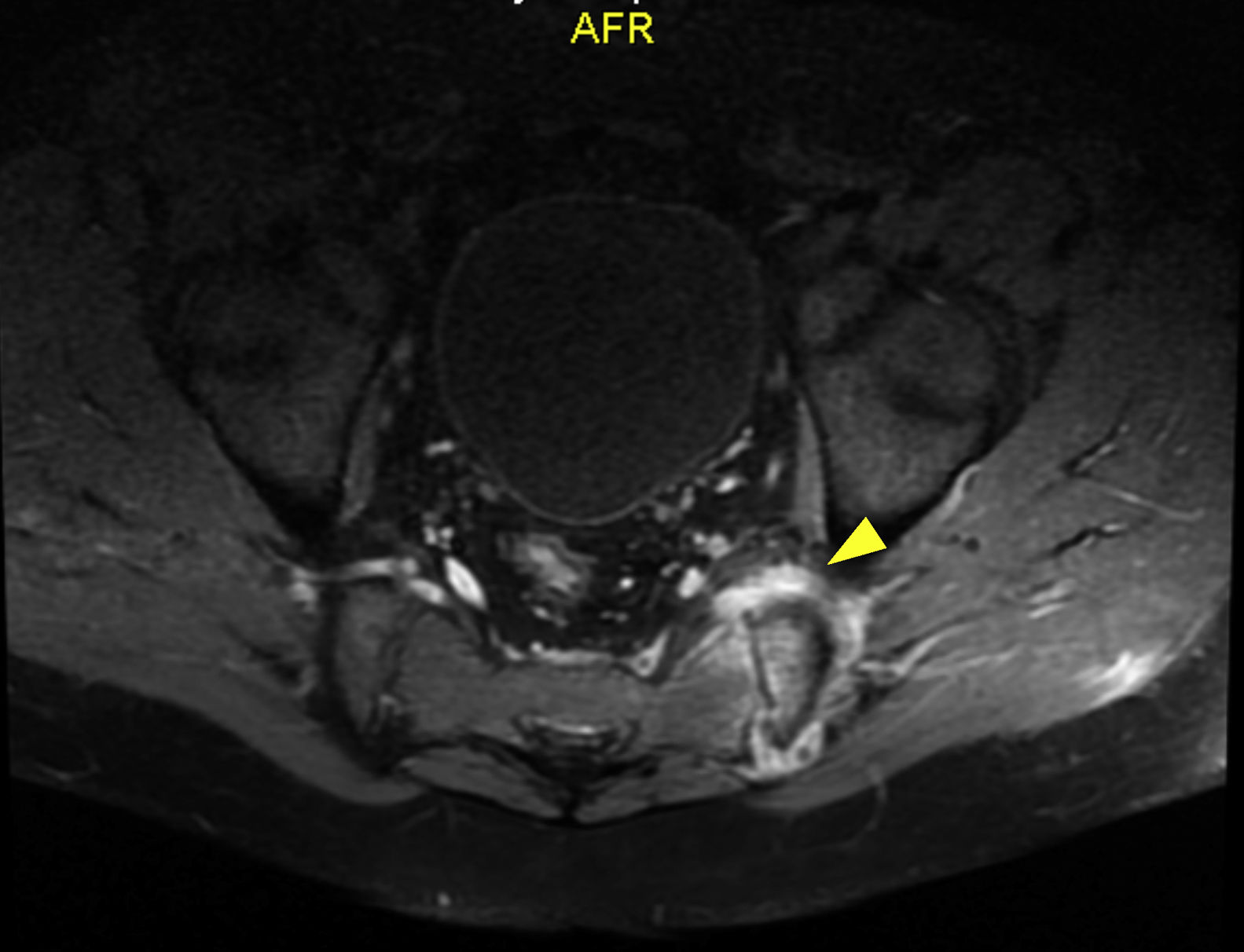
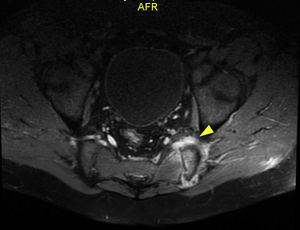
Regarding other paraclinical tests performed, positive ANAs titers (1/160 NuMA pattern) and a negative rheumatoid factor were found, and for this reason the diagnostic suspicion of an infectious condition was dismissed and a rheumatologic disorder such as spondiloarthritis was considered. Management with non-steroidal anti-inflammatory drugs (NSAIDs) and sulfasalazine was started. The report of the magnetic resonance imaging (MRI) of the lumbar spine showed sacroiliitis (Fig. 1). After two weeks of empirical treatment with non-steroidal anti-inflammatory drugs and immunomodulators, the patient presented a partial improvement in pain, but continued losing weight and with functional limitation. The measurement of HLA B27 was reported as negative.
Given the persistence of the symptoms, an infectious process such as tuberculosis (also frequent in the environment and with the possibility of lumbar involvement), mycosis, brucellosis or other bacterial agents was reconsidered. A MRI with STIR sequence was performed at the level of sacroiliac joints, which documented persistence of sacroiliitis due to articular and periarticular inflammatory changes, tendonitis of the left psoas and sacroiliac arthritis of the left side, which suggested a possible collection at this level. This collection was studied by computerized axial tomography (CAT)-guided puncture. The cultures and PCR for bacteria, mycobacteria, fungi and tuberculosis were negative; PCR for Brucella was not performed due to the non-availability of the test in this setting.
After 22 days of hospitalization, the report of the IgG and IgM antibodies for Brucella, which have been requested at the beginning of the clinical picture and referred to an external laboratory, finally arrived. Since the result was positive, antibiotic treatment with gentamicin, rifampicin and doxycycline was started, after which complete resolution of the symptoms was observed. At six months of follow-up, the patient was asymptomatic and without evident lesions in the control MRI of the sacroiliac joints.
DiscussionThe etiologic agent of brucellosis is an aerobic intracellular gram-negative coccobacillus that is shed in large amounts in the urine, milk, placental fluid, and other fluids of the infected animal. The species identified have been named primarily for the animal origin or the characteristics of the infection. Among these, four have significant human pathogenicity: Brucella melitensis (sheep), Brucella suis (pigs), Brucella abortus (cattle) and Brucella canis (dogs). The most invasive and pathogenic type of human brucellosis is due to B. melitensisfollowed by B. abortus and B. suis.2–4
The incidence of this disease exceeds 200 cases per 100,000 inhabitants in developing regions such as the Middle East, Africa and Latin America, with a geographical distribution closely related to the distribution of animal brucellosis. The prevalence of infection by Brucella in the population at risk in Colombia ranges between 0.14% and 10.4%.5 There are additional data on the seroprevalence in veterinary students, which amounts to 18.4%. The main clinical manifestations reported in these cases were headache, fever and osteoarticular involvement.6
The initial clinical manifestations are nonspecific, such as diaphoresis, weight loss, fatigue, or fever, which may be recurrent, mild, or prolonged. These symptoms were compatible with those presented in the patient. The literature also describes chills, myalgia, asthenia, adynamia, anorexia, joint pain, low back pain and headache.7 The preponderant symptom of brucellosis is acute onset fever, being one of the causes of febrile neutropenia in endemic areas.8
The physical exam may also be nonspecific. Hepatomegaly or splenomegaly is present in 33% of cases, lymphadenopathy in 10%; and findings such as peripheral arthritis, nuchal rigidity, scrotal edema and sacroiliitis can be present, the latter evidenced in the patient.9
As previously described, brucellosis is considered one of the great imitators along with diseases such as tuberculosis, malaria and syphilis. Therefore, its diagnosis must be based on a thorough clinical history and physical examination. The common factors are usually: constitutional symptoms, nonspecific febrile illness and an epidemiological nexus of exposure to the pathogen. In brucellosis, osteoarticular involvement is the most common complication, whether in the form of peripheral arthritis, sacroiliitis or spondylitis. The latter occurs mainly in people of productive age, with involvement of the lumbar spine in more than half of the cases, mimicking acute low back pain or lumbar disc herniation, unilaterally in the vast majority of cases.
In addition, it has been seen simultaneously with olecranon bursitis, humeral osteomyelitis and abscess of the iliacus muscle.9 Spondylitis caused by Brucella usually begins in the upper plate due to its rich vascularization and can cause bone destruction, even in the initial stages. It can be diagnosed in imaging studies by means of the Pons' sign, in which an erosion of the anterosuperior vertebral margin is observed.10
Among the suggestive findings on the MRI, which is the method of choice both for diagnosis and for the follow-up of the response to treatment, the presence of intraarticular fluid, subchondral bone marrow edema, joint enhancement after contrast, and soft tissue edema can be observed. Some of these findings can also be present in the acute phase of the disease. Manifestations such as bone erosions, joint space alterations, subchondral sclerosis, and ankylosis are evidence of chronic disease.11–15
As for the paraclinical tests in brucellosis infection, the findings are usually nonspecific. Slightly elevated erythrocyte sedimentation rates, C-reactive protein, and liver enzyme levels are usually seen, in addition to anemia, thrombocytopenia and leukocytosis. The isolation of the bacteria in blood or tissue samples is required to establish the diagnosis of this disease, with a percentage of positive cultures ranging between 15 and 70%. Bone marrow cultures are the gold standard, due to the tropism of the bacteria for the reticuloendothelial system, in such a way that greater sensitivity is demonstrated in patients with a chronic form of brucellosis. The use of automated cultures has accelerated the isolation of this pathogen, thus reducing the time to establish the diagnosis.
Another very useful diagnostic method is the measurement of class M, G and A immunoglobulins by ELISA, with positive antibody levels to consider infection. On the other hand, a standard test of bacterial antigen agglutination in serum, with positive titers for infection of 1:160, can be used for patients with a history of contact with animals.16 These last two, both the enzyme-linked immunoassay and the conventional serological tests are comparable in the diagnosis of the disease. However, a negative serology does not exclude the diagnosis, so it is strongly recommended to use more than one test in probable cases of brucellosis. An important advance is the PCR.17
In the reported case, we tried to isolate the bacteria by different available media, without success. The PCR for Brucella and other advanced diagnostic methods are not routinely available. It should be clarified that serological tests and PCR are processed in reference laboratories such as the National Institute of Health (INS) and the Colombian Institute of Tropical Medicine (ICMT), and their processing must be paid for in a private way, since they are not covered by the health benefit plan. The Colombian Agricultural Institute (ICA) performs measurement of class M, G and A immunoglobulins by ELISA.
Due to the initial nonspecific clinical manifestations in the patient, it was necessary to make a meticulous differential diagnosis. Other diagnostic possibilities such as tuberculous sacroiliitis, syphilis, rheumatic diseases such as axial spondyloarthritis and oncological diseases such as Hodgkin lymphoma, metastasis, among others, were also taken into account. The possibility of involvement by tuberculosis and mycoses was contemplated, but the cultures obtained for fungi, tuberculosis and other bacteria were negative; extension images of the thorax and abdomen did not show alterations.
Other agents such as S. aureus, E. coli and Salmonella can also cause sacroiliac infection, with musculoskeletal symptoms indistinguishable from those presented by the patient, with findings documented in images of thinning of the periarticular fatty tissue layer, increased size of the adjacent muscles, appearance of abscesses and presence of destructive bone changes. The infection by these agents is extremely rare and is related to well-recognized risk factors such as: being an intravenous drug user or having previous trauma, endocarditis, immunocompromise, and cutaneous, respiratory, or genitourinary infections, clinical conditions that were not present in the patient.
Spondyloarthropathies are another of the most important differential diagnoses of sacroiliac involvement. Sacroiliitis is a major criterion for its diagnosis, associated with minor criteria such as arthritis, dactylitis and enthesitis. In the peripheral case and in ankylosing spondylitis, bilateral sacroiliitis and the presence of syndesmophytes are common, while in psoriatic arthritis and reactive arthritis, manifestations of asymmetric and paravertebral sacroiliitis are more frequent. In these pathologies, it is necessary to look for extraarticular, mucocutaneous, ocular, and gastrointestinal and genitourinary tract manifestations, among others, to support their diagnosis.14
Ankylosing spondylitis was initially suspected in the patient, given that he had pain with inflammatory characteristics and an MRI image of the sacroiliac joints with STIR sequence that showed bone marrow edema, associated with elevated CRP and ESR. The probability of this entity decreased due to the poor response of pain to treatment with NSAIDs and negative HLA B27, in addition to the asymmetric involvement seen on MRI with a very acute time of evolution of the disease.
The follow-up of patients with brucellosis is of utmost importance due to the possibility of relapses in up to 10% of the cases in the first year after infection, whose presentation is usually milder than the initial clinical picture. The correlation of the risk factors in the clinical history was key to guide the diagnosis of sacroiliitis secondary to infection with Brucella, but it was not easy to confirm it due to the unavailability of tests for this bacterium in the environment and the plausibility of other diagnoses. Starting the treatment scheme in a timely manner can prevent morbidity and complications. Follow-up is essential due to the probability of relapses. Sacroiliitis due to brucellosis is a differential diagnosis to be taken into account.
ConclusionBrucellosis is a zoonosis that is still a public health problem in some areas of Colombia and its impact can be underestimated. The studies conducted in the country for human brucellosis have been limited to the determination of prevalence in high-risk personnel such as slaughterhouse workers. The non-specific manner in which the disease occurs, the capture problems at the local level and the low percentage of patients who go to health centers or clinics lead to underreporting and underregistration of the cases that actually occur in the country. Thus, the existing studies on Brucella are sporadic.18
It is important that professionals who are particularly exposed, such as livestock farmers, veterinarians and slaughterhouse workers, avoid the risk that exists from contact with infected animals, by using adequate protective clothing such as gloves that cover the entire forearm, high rubber boots, aprons and masks, elements that allow easy cleaning and disinfection after use, or disposable. Care must also be taken with the intake of contaminated milk and milk derivatives, which must undergo a process that guarantees the elimination of brucella by pasteurization at an industrial level and minimize the risk of biological accidents in vaccinators.
FundingThis work has not received any type of funding.
Conflict of interestThe authors declare that they have no conflict of interest.
Please cite this article as: Bolaños Toro OF, Saldarriaga Rivera LM, Murcia Rojas EJ, Hoyos Pulgarin JA. Sacroiliitis por brucelosis: Un diagnóstico diferencial para tener presente. Rev Colomb Reumatol. 2022;29:145–150.