Patients with systemic lupus erythematosus (SLE) have an increased risk of developing community-acquired infections, as well as those associated with health care. Bacterial infections are the most common and serious while these patients are in hospital.
ObjectiveTo develop, and internally validate, a clinical prediction model for the prognosis of the risk of hospital-acquired bacterial infection in SLE patients, using clinical and laboratory data obtained during the first hours of hospital admission.
MethodsAn analysis was performed on retrospective cohort of patients with SLE older than 16 years and admitted for reasons other than bacterial infection in two highly complex hospitals in Medellín between 2011 and 2016. The characteristics of the patients who developed a bacterial infection were compared between day 3 and day 15 of hospital admission with those who did not develop one. The significant variables in the bivariate analysis were used for the construction of the model using multivariate logistic regression.
ResultsA total of 765 episodes were included, of which 98 (12.8%) presented the outcome of interest. Thirty candidate predictors were considered. The variables incorporated in the final model were: age, neutrophil count, SLEDAI lupus activity score, use of a bladder catheter, use of a central venous catheter in the first 72h, glucocorticoid doses in the previous month, and use of an antimalarial drug in the 3 previous months. The discrimination capacity of the model was acceptable to good (AUC-ROC=0.74; 95% CI, 0.69–0.80). The Hosmer–Lemeshow goodness of fit test (p=.637) suggested adequate calibration.
ConclusionA clinical prediction model of prognostic risk of nosocomial bacterial infection in patients with SLE has been developed. This model is made up of simple clinical and laboratory variables available at the time of hospital admission. External validation and clinical impact studies are required before routine implementation.
Los pacientes con lupus eritematoso sistémico (LES) tienen un riesgo aumentado de padecer infecciones tanto adquiridas en la comunidad como asociadas con el cuidado de la salud. Las infecciones bacterianas son las más frecuentes y graves durante la hospitalización de estos pacientes.
ObjetivoDesarrollar y validar internamente un modelo de predicción clínica de pronóstico del riesgo de infección bacteriana adquirida en el hospital en pacientes con LES usando datos clínicos y de laboratorio obtenidos durante las primeras horas de hospitalización.
MétodosSe analizó una cohorte retrospectiva de pacientes con LES mayores de 16 años hospitalizados por motivos diferentes a infección bacteriana en dos hospitales de alta complejidad de xxx entre 2011 y 2016. Se compararon las características de los pacientes que desarrollaron el desenlace infección bacteriana entre el día 3 y el día 15 de hospitalización con aquellos que no lo presentaron. Las variables significativas en el análisis bivariado fueron consideradas para la construcción del modelo por medio de regresión logística multivariada.
ResultadosSe incluyeron 765 episodios, de los cuales 98 (12,8%) presentaron el desenlace de interés. Se consideraron 35 predictores candidatos. Las variables incorporadas en el modelo final fueron: edad, recuento de neutrófilos, puntaje de actividad lúpica SLEDAI, uso de sonda vesical, uso de catéter venoso central en las primeras 72 horas, dosis de glucocorticoides en el mes previo y el uso de antimalárico en los 3 meses previos. La capacidad de discriminación del modelo fue aceptable a buena (AUC-ROC=0,74; IC 95%: 0,69−0,80). La prueba de bondad de ajuste de Hosmer–Lemeshow (p=0,637) sugirió una adecuada calibración.
ConclusiónDesarrollamos un modelo de predicción clínica de pronóstico del riesgo de infección bacteriana nosocomial en pacientes con LES. El modelo desarrollado está compuesto por variables clínicas y de laboratorio simples disponibles al momento del ingreso al hospital. Se requieren estudios de validación externa y de impacto clínico antes de su implementación rutinaria.
During the course of the disease up to one half of the patients with systemic lupus erythematous (SLE) developed at least one infection.1 Infections in patients with SLE account for between 11 to 23% of the causes for hospitalization2 and between 3.6 and 67% of all deaths of patients with this disease.3 The infections may be of several classes and present in any clinical context. Among the hospital acquired infections, the bacterial infections are the most relevant due to their frequency and severity. Such infections are associated with longer hospital stay, delays in the initiation or continuation of immunosuppressive therapy, ICU admission, increased healthcare costs and higher mortality.4,5
Different risk factors have been reported associated with infection in SLE patients. Some of them include the activity of the disease6; organ involvement or compromised individual systems, particularly lupus nephritis, lung involvement, and central nervous system activity7; leucopenia, lymphopenia and neutropenia8; elevated anti-DNA titers and low levels of complement C3 and C4 fractions9; chronic use of steroids10; the use of methylprednisolone pulses for the treatment of flares11; the use of cyclophosphamide for the treatment of severe manifestations such as the proliferative types of lupus nephritis, and central nervous system involvement,12 inter alia.
Notwithstanding the frequency and the severity of infection in SLE, and the identification of different associated risk factors, the preventive recommendations continue to be very limited.13 The study and implementation of preventive measures ideally requires adequate stratification of the individual risk of infection in clearly identified settings. Such evaluation of the individual risk of infection should be based on predictive models, through a multivariate approach of the problem, rather than the mere identification of isolated risk factors.14 Two predictive models to forecast infection in patients with SLE were found.
Yuhara et al.15 developed a model to estimate the risk of major infection, defined as an infection requiring the use IV antimicrobial agents, 6 months after initiating glucocorticoid therapy. There were 17 infection events, including fungi and mycobacteria. Tejera Segura et al. published a predictive model for major future infection in patients with SLE. The external validation was done using a case control design. The time horizon of the prediction is unclear.16 No model was found to estimate the risk of nosocomial bacterial infection and hence be able to assess and implement individualized and specific preventive or therapeutic strategies.
The purpose of our work was to develop and internally validate a clinical predictive model for the risk of intrahospital bacterial infection in SLE patients over 16 years old, who had been admitted to the hospital for reasons other than an infection, based on the clinical and laboratory data available during the first few hours after hospital admission.
Patients and methodsDesignRetrospective cohort trial for the development and internal validation of a predictive model to forecast the risk of nosocomial bacterial infection in patients with SLE.
ParticipantsPatients of any gender with a diagnosis of SLE, aged 16 years or older, who were admitted to the Hospital Universitario de San Vicente Fundación and the Hospital Pablo Tobón Uribe in Medellín, Colombia between January 2006 and December 2016, and who met the following inclusion criteria: hospitalization for reasons other than infection, no diagnosis of infection during the 48h following admission, no antibiotic therapy in the first 48h of admission, length of stay of more than 72h. The classification criteria of the American College of Rheumatology 1982 updated in 199717,18 were used for the diagnosis of SLE. There were no exclusion criteria.
OutcomesThe outcome variable was the diagnosis of an acquired bacterial infection between day 3 and day 15 of hospitalization, defined as a confirmed infection based on any bacterial microbiological isolate or, in the absence of an isolate, an infection diagnosed by the treating physicians based on symptoms, signs, laboratory tests or imaging studies and which required the use of one or more IV antibiotics and had been diagnosed 48h after hospital admission, in the absence of suspicion of the infection being incubated during such period of time.
PredictorsThe independent variables were the potential predictors for nosocomial infection and were selected after an in-depth analysis of the scientific literature. Each patient’s data were collected from the medical records entered in the first 72h of hospitalization.
The sample size estimate was based on the published reports about the frequency of bacterial infections of the 2 hospitals included. 6,19 The estimates indicated approximately 1200 hospital admissions of SLE patients during the 10 years of observation in the participating institutions, 50% of which received cyclophosphamide or mofetil mycophenolate; of these, 20% developed nosocomial bacterial infection over the course of the next 15 days. 600 eligible hospital episodes were estimated, and 120 bacterial nosocomial infections, allowing for the analysis of at least 12 predictive variables.
Analysis planThe quantitative variables are shown as averages or means, with their respective measures of dispersion; the qualitative variables were expressed as absolute numbers and percentages of the total. The association between the different independent variables and the principal outcome was evaluated using the Student-t test or Mann–Whitney U test, according to the distribution of quantitative variables; the categorical variables were measured with Chi square or Fisher’s exact test.
The quantitative variables were entered into the model continuously, with no dichotomization or mathematical transformation. Data imputation was not considered, and hence only complete cases were analyzed. Bivariate logistic regression analyses were initially conducted with each analysis plan of the independent variables defined previously. The necessary assumptions were the logistic regression were checked. The discrimination ability of each of the potential prediction variables was explored, considering for the final model only the prediction variables with a p value within Wald statistic <0.25, with ≤20% missing data, that exhibited a monotonous behavior and did not present high collinearity among them.
Subsequently, all the optional variables suggested in a multivariate logistic regression model were included. Multiple manual comparisons of the discrimination and calibration properties were done for this selection, using ROC curves and the Hosmer–Lemeshow goodness of fit tests, respectively. Statistical criteria together with clinical judgement and the principle of parsimony were always used as guidelines for the selection of the best model.
Finally, the internal validation of the model was performed using the bootstrapping method, with 200 repeats with no replacement and the stability of the variables finally included was analyzed. All of the analyses used the Stata 12 statistical software.
The study protocol was approved by the Ethics in Research Committee of the Hospital Universitario de San Vicente Fundación and by the Research and Ethics Committee of the Hospital Pablo Tobón Uribe. Due to the retrospective nature of the research, it was rated as minimal risk and hence the obtention of the informed consent was not considered necessary. The current good clinical practices were followed throughout the phases of the project to conduct the clinical research. The anonymity of the data was ensured, and the confidentiality of the information of each patient shall always be protected.
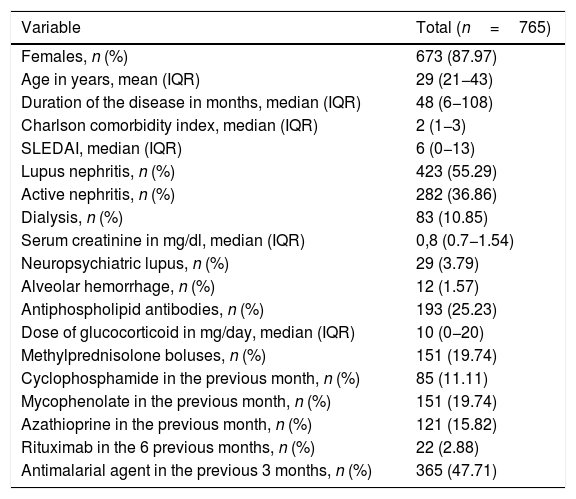
Results2802 episodes of hospital admissions of patients with a discharge diagnosis of SLE who stayed hospitalized for more than 72h were reviewed. Of these, 765 episodes were included, of which 12.8% (n=98) developed an infection. The demographic and clinical characteristics of the patients included are shown in Table 1.
Demographic and clinical characteristics of the study population.
| Variable | Total (n=765) |
|---|---|
| Females, n (%) | 673 (87.97) |
| Age in years, mean (IQR) | 29 (21−43) |
| Duration of the disease in months, median (IQR) | 48 (6−108) |
| Charlson comorbidity index, median (IQR) | 2 (1−3) |
| SLEDAI, median (IQR) | 6 (0−13) |
| Lupus nephritis, n (%) | 423 (55.29) |
| Active nephritis, n (%) | 282 (36.86) |
| Dialysis, n (%) | 83 (10.85) |
| Serum creatinine in mg/dl, median (IQR) | 0,8 (0.7−1.54) |
| Neuropsychiatric lupus, n (%) | 29 (3.79) |
| Alveolar hemorrhage, n (%) | 12 (1.57) |
| Antiphospholipid antibodies, n (%) | 193 (25.23) |
| Dose of glucocorticoid in mg/day, median (IQR) | 10 (0−20) |
| Methylprednisolone boluses, n (%) | 151 (19.74) |
| Cyclophosphamide in the previous month, n (%) | 85 (11.11) |
| Mycophenolate in the previous month, n (%) | 151 (19.74) |
| Azathioprine in the previous month, n (%) | 121 (15.82) |
| Rituximab in the 6 previous months, n (%) | 22 (2.88) |
| Antimalarial agent in the previous 3 months, n (%) | 365 (47.71) |
IQR: interquartile range.
The infections most frequently acquired in the hospital were: bacteriemia (27 cases), urinary tract infection (25 cases), soft tissue infection (19 cases) and pneumonia (15 cases). Microbiological isolates were obtained in 67% of the infections diagnosed. The most frequent microorganisms were: Escherichia coli, Staphylococcus aureus and Klebsiella spp. The most frequent pathogen in the cases of bacteriemia was S. aureus (40.7%).
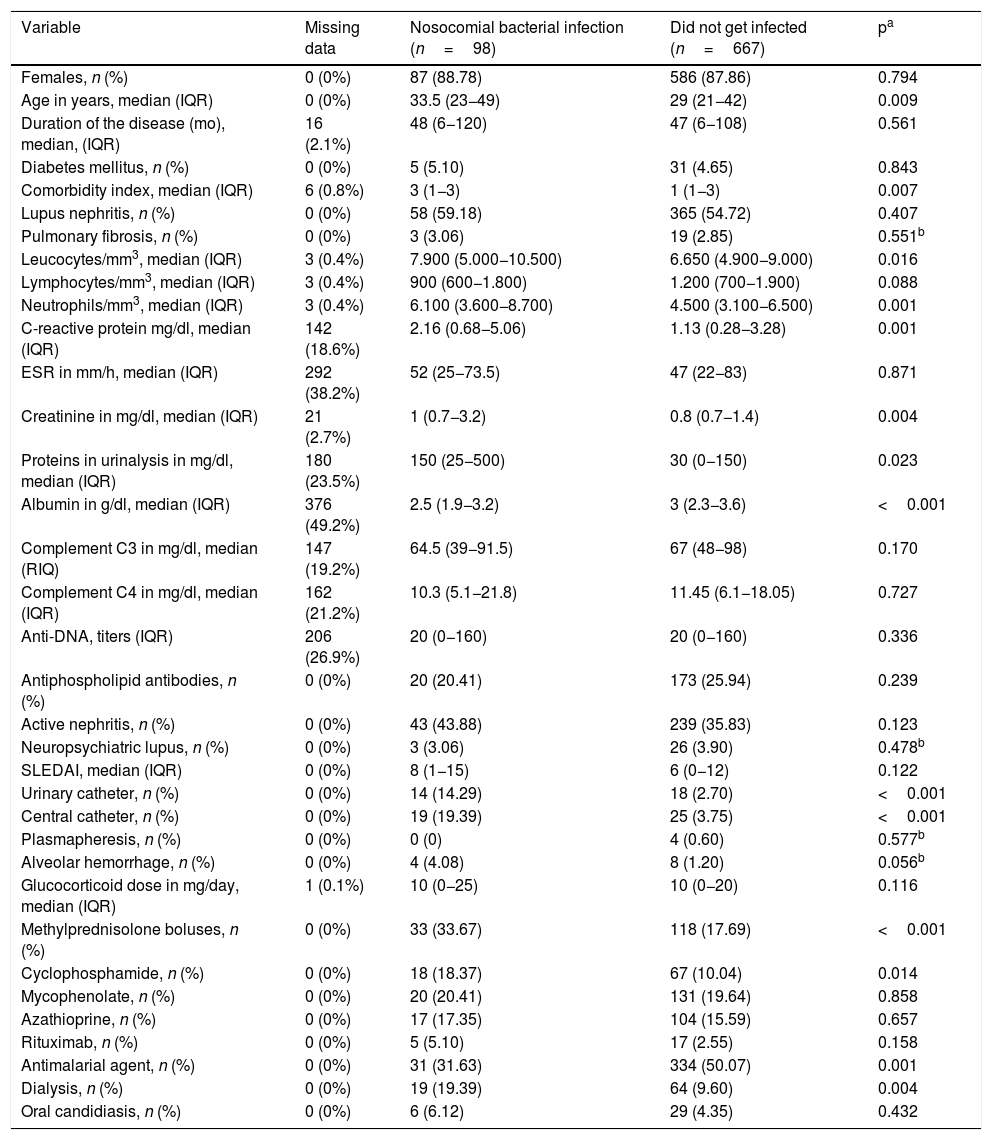
Table 2 illustrates the initial bivariate analysis comparing the patients who developed the infection and those who did not. The patients that became infected during their hospitalization showed significant differences in the following variables as measured at discharge: age, Charlson comorbidity index, leukocytes, neutrophiles, C-reactive protein (PCR), creatinine, proteinuria in isolated sample, need for dialysis, albumin, use of central venous catheter, use of urinary catheter, use of antimalarial agent, methylprednisolone boluses and use of cyclophosphamide.
Bivariate analysis in accordance with the development of the bacterial nosocomial infection.
| Variable | Missing data | Nosocomial bacterial infection (n=98) | Did not get infected (n=667) | pa |
|---|---|---|---|---|
| Females, n (%) | 0 (0%) | 87 (88.78) | 586 (87.86) | 0.794 |
| Age in years, median (IQR) | 0 (0%) | 33.5 (23−49) | 29 (21−42) | 0.009 |
| Duration of the disease (mo), median, (IQR) | 16 (2.1%) | 48 (6−120) | 47 (6−108) | 0.561 |
| Diabetes mellitus, n (%) | 0 (0%) | 5 (5.10) | 31 (4.65) | 0.843 |
| Comorbidity index, median (IQR) | 6 (0.8%) | 3 (1−3) | 1 (1−3) | 0.007 |
| Lupus nephritis, n (%) | 0 (0%) | 58 (59.18) | 365 (54.72) | 0.407 |
| Pulmonary fibrosis, n (%) | 0 (0%) | 3 (3.06) | 19 (2.85) | 0.551b |
| Leucocytes/mm3, median (IQR) | 3 (0.4%) | 7.900 (5.000−10.500) | 6.650 (4.900−9.000) | 0.016 |
| Lymphocytes/mm3, median (IQR) | 3 (0.4%) | 900 (600−1.800) | 1.200 (700−1.900) | 0.088 |
| Neutrophils/mm3, median (IQR) | 3 (0.4%) | 6.100 (3.600−8.700) | 4.500 (3.100−6.500) | 0.001 |
| C-reactive protein mg/dl, median (IQR) | 142 (18.6%) | 2.16 (0.68−5.06) | 1.13 (0.28−3.28) | 0.001 |
| ESR in mm/h, median (IQR) | 292 (38.2%) | 52 (25−73.5) | 47 (22−83) | 0.871 |
| Creatinine in mg/dl, median (IQR) | 21 (2.7%) | 1 (0.7−3.2) | 0.8 (0.7−1.4) | 0.004 |
| Proteins in urinalysis in mg/dl, median (IQR) | 180 (23.5%) | 150 (25−500) | 30 (0−150) | 0.023 |
| Albumin in g/dl, median (IQR) | 376 (49.2%) | 2.5 (1.9−3.2) | 3 (2.3−3.6) | <0.001 |
| Complement C3 in mg/dl, median (RIQ) | 147 (19.2%) | 64.5 (39−91.5) | 67 (48−98) | 0.170 |
| Complement C4 in mg/dl, median (IQR) | 162 (21.2%) | 10.3 (5.1−21.8) | 11.45 (6.1−18.05) | 0.727 |
| Anti-DNA, titers (IQR) | 206 (26.9%) | 20 (0−160) | 20 (0−160) | 0.336 |
| Antiphospholipid antibodies, n (%) | 0 (0%) | 20 (20.41) | 173 (25.94) | 0.239 |
| Active nephritis, n (%) | 0 (0%) | 43 (43.88) | 239 (35.83) | 0.123 |
| Neuropsychiatric lupus, n (%) | 0 (0%) | 3 (3.06) | 26 (3.90) | 0.478b |
| SLEDAI, median (IQR) | 0 (0%) | 8 (1−15) | 6 (0−12) | 0.122 |
| Urinary catheter, n (%) | 0 (0%) | 14 (14.29) | 18 (2.70) | <0.001 |
| Central catheter, n (%) | 0 (0%) | 19 (19.39) | 25 (3.75) | <0.001 |
| Plasmapheresis, n (%) | 0 (0%) | 0 (0) | 4 (0.60) | 0.577b |
| Alveolar hemorrhage, n (%) | 0 (0%) | 4 (4.08) | 8 (1.20) | 0.056b |
| Glucocorticoid dose in mg/day, median (IQR) | 1 (0.1%) | 10 (0−25) | 10 (0−20) | 0.116 |
| Methylprednisolone boluses, n (%) | 0 (0%) | 33 (33.67) | 118 (17.69) | <0.001 |
| Cyclophosphamide, n (%) | 0 (0%) | 18 (18.37) | 67 (10.04) | 0.014 |
| Mycophenolate, n (%) | 0 (0%) | 20 (20.41) | 131 (19.64) | 0.858 |
| Azathioprine, n (%) | 0 (0%) | 17 (17.35) | 104 (15.59) | 0.657 |
| Rituximab, n (%) | 0 (0%) | 5 (5.10) | 17 (2.55) | 0.158 |
| Antimalarial agent, n (%) | 0 (0%) | 31 (31.63) | 334 (50.07) | 0.001 |
| Dialysis, n (%) | 0 (0%) | 19 (19.39) | 64 (9.60) | 0.004 |
| Oral candidiasis, n (%) | 0 (0%) | 6 (6.12) | 29 (4.35) | 0.432 |
IQR: interquartile range.
The following variables were excluded from the logistic regression analysis, due to the percentage of missing data >20%: erythrocyte sedimentation rate, proteinuria in isolate sample, serum albumin, complement C4 and anti-DNA. Of these, isolated proteinuria and albumin showed a significant difference. Comorbidity as a variable did not display monotony and hence was excluded.
A correlations matrix suspected high collinearity between the leukocyte and neutrophil variables (0.89) and between C3 and SLEDAI (−0.55). The leukocytes variable was excluded (on the grounds that the neutrophils variable could contribute with more information, independent from the lymphocytes variable neutrophils) and complement C3 (due to more missing data). The initial complete model finally included the following variables: age, lymphocytes, neutrophils, PCR, creatinine, active nephritis, SLEDAI, use of urinary catheter, use of central venous catheter, alveolar hemorrhage, daily dose of methylprednisolone, cyclophosphamide, use of antimalarial agent and dialysis. The variables creatinine, active nephritis and dialysis may be strongly correlated, so only dialysis was included because of a smaller number of missing data.
The analysis of complete cases using PCR would only allow for the inclusion of 604 observations out of 765 in total; PCR was therefore excluded, to make better use of the available information. Looking for the most parsimonious model, the variables alveolar hemorrhage, cyclophosphamide, lymphocytes, rituximab and glucocorticoid pulses were ruled out step by step, based on the statistical significance and performance observed. The final analysis of complete cases included 762 of 765 episodes.
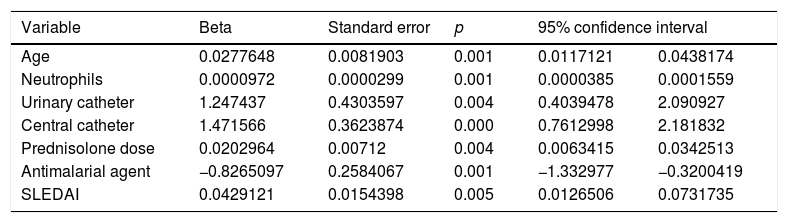
The final model included the following predictors: age, neutrophils, SLEDAI, use of urinary catheter, use of central venous catheter, dose of glucocorticoids in the month prior to hospitalization, and the use of one antimalarial agent during the 3 months prior to hospital admission. Table 3 shows the coefficients for each predictor with their corresponding standard error. The ROC area under the curve of the final model was 0.74 (95% CI 0.69−0.80). The Hosmer–Lemeshow goodness of fit test evidenced adequate calibration (p=0.637). A cut-off point of 0.16 provides the best balance between sensitivity (55.1) and specificity (82.5). Through re-sampling, an internal validation of the model was conducted, resulting in stability of all the estimated coefficients.
Variables included in the final predictive model.
| Variable | Beta | Standard error | p | 95% confidence interval | |
|---|---|---|---|---|---|
| Age | 0.0277648 | 0.0081903 | 0.001 | 0.0117121 | 0.0438174 |
| Neutrophils | 0.0000972 | 0.0000299 | 0.001 | 0.0000385 | 0.0001559 |
| Urinary catheter | 1.247437 | 0.4303597 | 0.004 | 0.4039478 | 2.090927 |
| Central catheter | 1.471566 | 0.3623874 | 0.000 | 0.7612998 | 2.181832 |
| Prednisolone dose | 0.0202964 | 0.00712 | 0.004 | 0.0063415 | 0.0342513 |
| Antimalarial agent | −0.8265097 | 0.2584067 | 0.001 | −1.332977 | −0.3200419 |
| SLEDAI | 0.0429121 | 0.0154398 | 0.005 | 0.0126506 | 0.0731735 |
A prognostic model was developed to predict the risk of acquiring a bacterial nosocomial infection for patients with lupus who are admitted to the hospital for reasons other than infection. The variables included were: age, neutrophils, SLEDAI, use of urinary catheter, use of central venous catheter dose of glucocorticoids in the month previous to hospital admission, and use of one antimalarial agent in the three months prior to admission.
This predictive model comprises clinical and laboratory variables that are simple and easy to measure during the first few hours after hospital admission. The area under the curve ROC of 0.74 indicates an acceptable to good discriminative ability. The dependent variable was restricted to bacterial infections because these are the most common and severe. These infections are associated with a longer hospital stay, higher rates of ICU admission and lower survival rates.4,5
Additionally, nosocomial infections due to other microorganisms probably have different risk/predictive factors that may cause confusion and limit the clinical applicability if a mixed outcome is used. Infections diagnosed after 14 days of hospitalization were not included, because these may not probably be influenced by some of the variables measured close to the admission date, which is considered to be the suitable time to establish the risk of developing an infection.
A systematic search of the literature failed to identify specific prediction models for bacterial nosocomial infection in patients with SLE, but other authors have built predictive models of infection for these patients, using different definitions, populations and methodologies: Yuhara et al. designed a model to predict major infection of any etiology, within six months of initiating glucocorticoid therapy for a lupus activity episode requiring hospitalization. The final predictors were: level of serum albumin, level of serum creatinine and use of prednisone at a dose ≥ 60mg/day without methylprednisolone pulses.15
Tejera Segura et al. developed an infection prediction model in SLE, based on the Spanish cohort RELESSER-T, which they called SLESIS (SLE severe infection score) and included patients over 46-years old, Latin American ethnicity, dose of prednisolone >10mg/day, male sex, previous SLE-associated hospital admission, Katz severity index and a history of infection anytime. The model was subject to external validation in an independent cohort and the ROC area was 0.66 (95% CI 0.56−0.71).16 The long-term prediction horizons and the compound outcomes used in these 2 models limit their potential clinical application.
The most frequent nosocomial bacterial infection in our study was bacteriemia. Marcos et al. described 48 episodes of bacteriemia in 38 patients with SLE at the Hospital Clínic in Barcelona; 41% of the events were nosocomial, with S. aureus as the most frequent isolate, and 55% had a central venous catheter in place.20 Bacteremia was the fourth most frequent severe infection and the principal cause of death from infection in the Spanish RELESSER-T5 registry. Rua-Figueroa et al. conducted a case control trial based on that cohort to describe the characteristics of the bacteremia; the most frequent pathogens were E. coli (29.8%), S. aureus (16.7%) and Salmonella spp. (10.5%); 24.6% had a central venous catheter in place, 35.1% of the events were hospital-acquired and in the bivariate analysis, the antimalarial agents offered protection against the development of hospital-acquired bacterial infections.21
Similar to our study, Rúa-Figueroa et al. identified the age of diagnosis as an independent risk factor associated with infection, evidencing that lupus probably has an increased impact on the immune system as age progresses.5 Likewise, Mok et al. indicated that age is an important factor affecting the clinical manifestations and the prognosis of SLE; infection is a major cause of mortality in late onset SLE.22,23
Although in our study, inserting a central venous line and a urinary catheter during the first 72h of hospitalization was a predictor for nosocomial bacterial infection, other studies have not reported these variables as significant in their multivariate analyses6,20,21,24–29; however, it is widely acknowledged that they do represent a risk factor for blood-borne infections.30
Glucocorticoids are a risk factor for SLE infection, consistently reported in multiple trials; this association is dose and usage-time-dependent.9,27,31–38 Few studies have characterized the specific relationship between hospital-acquired infection and the use of glucocorticoids prior to hospitalization; some reported that it behaved as a risk factor in the multivariate analysis,15,26,27,39 but not in others.6,25,29
In our study, a higher neutrophil count during the first hours of hospitalization was associated with nosocomial bacterial infection. A finding of neutrophilia in SLE patients usually points to an already existing infection or to a transient glucocorticoid effect.40,41 A likely explanation for our finding could be that the group with severe hospital-acquired bacterial infection required higher doses of glucocorticoids, including methylprednisolone pulses and were receiving a higher previous outpatient dose, because of increased activity of the disease, as reflected in higher SLEDAI scores and the need for hospital admission because of lupus activity in half of the patients.
Another possibility is that the higher neutrophil count reflects activity of the disease.42 Alternatively, this higher neutrophil count could be an indication that the infection was being incubated during the first few hours of admission but not yet diagnosed; however, the suspicion of this latter scenario was one of the exclusion criteria in the trial.
Moreover, a higher SLEDAI score was found to result in higher risk of developing bacterial nosocomial infection probably as a reflection of more severe SLE, and the subsequent need for a stronger immunosuppressive therapy. This association is consistent with the reports from other research studies, which identified risk factors for hospital-acquired infections in SLE patients.43 Ramírez Gómez et al. found that a higher mean SLEDAI score at admission was associated with a higher risk of nosocomial infection (OR 4.43; 95% CI 1.16–16.8; p=0.03).6 Navarro-Zarza et al. found that a Mex-SLEDAI score ≥6 was a risk factor for infection in outpatients and inpatients.29 In the study by Duffy et al. a score of >8 was the most sensitive and specific variable to predict infection in hospitalized SLE patients.25
The apparent protective effect of antimalarial agents in the development of infections has been reported by several authors. Ruiz-Irastorza et al., for instance, found in their case control study based on the Lupus-Cruces cohort, that treatment with antimalarial agents was associated with a 16-fold lower risk of developing severe infection (OR 0.06; 95% CI 0.02−0.18).7 Sisó et al. analyzed the influence of using chloroquine or hydroxychloroquine prior to the diagnosis of lupus nephritis in a cohort with 204 patients during an average 148-month follow-up. The subjects exposed to these medications had less infections (11 vs. 29%; p=0.006).44 Feldman et al., in their robust cohort of Medicaid users, reported that the basal use of hydroxychloroquine was associated to a lower risk of a primary severe infection (HR 0.73; 95% CI 0.68−0.77).31
Our study has several limitations. Due to its retrospective nature, the data of some independent variables were missing in over 20% of the episodes, including some significant variables in the bivariate analysis, such as albumin, reported as a predictor of infection in some trials. It would be interesting to analyze the predictive power of albumin in nosocomial infection in a prospective trial. The number of outcomes obtained was small and the ideal ratio of an independent variable per at least every 10 outcomes was not achieved.45 The episodes in which the viral, fungal or parasitic infections were the reason for hospitalization (4.83%) were not excluded, since the goal was to predict nosocomial infection of bacterial etiology; however, these infections may have affected some independent variables, such as the total and differential leukocyte count and the ESR and PCR values
The clinical prediction model developed and internally validated, requires at least an external validation study prior to assessing its potential clinical impact in controlled trials. The probable clinical applications of this model include the implementation and assessment of interventions aimed at reducing the risk of infection in these patients by means of a closer follow-up, immunosuppression adjustments, preventive isolation and the use of prophylactic antimicrobials, inter alia.
ConclusionsA clinical prediction model was built to forecast the risk of nosocomial bacterial infection in inpatients with SLE. The model developed comprises simple clinical and laboratory variables, available at the time of hospital admission. The suggested model estimates the risk of a clearly defined severe outcome, which develops in a short-term horizon. This makes the model highly valuable for clinical use. External validation and clinical impact studies are needed prior to the routine implementation of the model.
FinancingThis article received financial support from the Colombian Association of Rheumatology.
Conflict of interestsThe authors have no conflict of interests to disclose.
The authors express their deepest felt gratitude to the Ethics in Research Committee of Hospital Universitario San Vicente Fundación and to the Research and Ethics Committee of Hospital Pablo Tobón Uribe, in Medellín.
Please cite this article as: Restrepo-Escobar M, Castaño-González P, Galvis-García M, Morales-Maya L, Urrego T, Sandoval-Álvarez S, et al. Desarrollo y validación interna de un modelo de predicción clínica del riesgo de infección bacteriana nosocomial en pacientes con lupus eritematoso sistémico. Rev Colomb Reumatol. 2021;28:95–103.