Hospitalized patients with systemic autoimmune rheumatic diseases (SARDs) generate high impact in clinical terms.
ObjectivesTo characterize the study population and estimate risk factors associated with the presence of adverse outcomes in hospitalized patients consulting rheumatology at Clínica Imbanaco between January 2013 and December 2019.
MethodsWe analyzed a historical cohort of hospitalized patients who were evaluated by rheumatology. The population was classified as follows: group 1, patients with new onset diagnosed SARDs; group 2, patients with known diagnosed SARDs; group 3, patients without diagnosed SARDs; and group 4, patients with unconfirmed suspicion of SARDs. A composite adverse outcome was defined if at least one of the following occurred: (1) hospital mortality, (2) admission to the intensive care unit, (3) hospital infection, or (4) readmission.
ResultsInformation was collected from 327 hospitalization events (307 patients). The median age was 48 (34−63) years and 222 (72.3%) were women. The composite adverse outcome occurred in 136 (41.5%) hospitalization events. Group 2 had the highest number of adverse outcomes (61/128; 47.6%). The variables associated with the worst outcomes were cardiovascular diagnosis at admission (OR = 4.63; CI: 1.60−13.43; p = 0.005), longer hospital stay (OR = 1.04; CI: 1.01−1.07; p = 0.005), and a treating specialty other than internal medicine (OR = 2.79; CI: 1.26−6.17; p = 0.011). Male sex (OR = 0.29; CI: 0.12–0.66; p = 0.004), having special health coverage (OR = 0.39; CI: 0.15–.099; p = 0.047), and hemoglobin > 11.4 g/dL (OR = 0.82; CI: 0.69–0.99; p = 0.039) were the factors associated with lower odds of developing the composite outcome.
ConclusionsIn this historical cohort, the group of patients with known diagnosed SARDs presented a higher number in percentage terms of adverse outcomes. The most frequent adverse outcomes were admission to the ICU and hospital readmission.
Los pacientes hospitalizados con enfermedades reumáticas o autoinmunes sistémicas (ERAS) generan gran impacto en términos clínicos.
ObjetivosCaracterizar a la población y estimar factores de riesgo asociados con la presencia de desenlaces adversos en pacientes evaluados hospitalariamente por reumatología en la Clínica Imbanaco durante los años 2013–2019.
MetodologíaSe analizó una cohorte histórica de pacientes hospitalizados que fueron evaluados por reumatología. La población se clasificó así: grupo 1, pacientes con ERAS diagnosticada de novo; grupo 2, pacientes con ERAS diagnosticada conocida; grupo 3, pacientes sin ERAS diagnosticada; y grupo 4, pacientes con sospecha no confirmada de ERAS. Se definió un desenlace adverso compuesto si se presentó al menos uno de los siguientes casos: (1) mortalidad hospitalaria; (2) ingreso a la unidad de cuidado intensivo; (3) infección intrahospitalaria; (4) reingreso.
ResultadosEn un total de 327 eventos de hospitalización (307 pacientes), la mediana de edad fue 48 (34−63) años y 222 (72,3%) fueron mujeres. El desenlace adverso compuesto se presentó en 136 (41,5%) eventos. El grupo 2 tuvo mayor número de desenlaces adversos (61/128; 47,6%). Las variables asociadas con peores resultados fueron: diagnóstico inicial cardiovascular (OR = 4,63; IC: 1,60−13,43; p = 0,005), mayor estancia hospitalaria (OR = 1,04; IC: 1,01−1,07; p = 0,005) y tener una especialidad tratante diferente a medicina interna (OR = 2,79; IC: 1,26−6,17; p = 0,011). El sexo masculino (OR = 0,29; IC: 0,12−0,66; p = 0,004), pertenecer a un régimen especial de salud (OR = 0,39; IC: 0,15−0,99; p = 0,047) y tener hemoglobina >11,4 g/dL (OR = 0,82; IC: 0,69−0,99; p = 0,039) fueron factores asociados con menor oportunidad de desarrollar el desenlace compuesto.
ConclusionesEn esta cohorte histórica se encontró que porcentualmente el grupo de pacientes con ERAS diagnosticadas conocidas presentó mayor número de desenlaces adversos, entre los que se destacan para el mismo grupo, el ingreso a UCI y el reingreso hospitalario.
Rheumatic diseases can be accompanied by systemic multi-organ compromise due to autoimmunity.1 They are characterized by pain, chronic and persistent inflammation, impaired functional capacity and deterioration in quality of life. Some of these diseases are associated with decreased life expectancy.2 Autoimmune diseases affect around 3–5% of human beings, especially women; 80% of them of reproductive age.3
The World Health Organization and the International League of Associations for Rheumatology have implemented strategies to impact the identification, prevention and control of these diseases, which are considered a public health problem.4–6 In Colombia, the most frequent rheumatic diseases, according to prevalence estimates, are osteoarthritis (10.81%) and mechanical low back pain (7.24%). Of the inflammatory rheumatic diseases, rheumatoid arthritis (RA) is the most prevalent in the adult population (1.49%), while gout (0.56%), Sjögren's syndrome (0.08%) and systemic lupus erythematosus (SLE) (0.05%) are less prevalent.7 Since RA is one of the most prevalent rheumatic diseases in Colombia, it is relevant to highlight that it has been prioritized as a high-cost disease, because there is a greater probability of complications, it requires a higher consumption of resources of the health system and generates an out-of-pocket expense for patients.8
Regarding hospitalized patients with systemic autoimmune rheumatic diseases (SARDs), the impact of hospital stay in clinical and economic terms has been previously demonstrated.9–11 Several studies describe the course and analyze the hospital outcomes of isolated SARDs, such as SLE and systemic sclerosis,12–14 but there are few publications that report the hospital outcomes in patients with SARDs in general outside the intensive care unit (ICU).10,11,15,16 The mortality of patients with SARDs in the ICU ranges between 17 and 55% (data from Colombia, Argentina, the United States, Spain, France and China), being the infections and the activity of the disease itself the main causes.17–25 The foregoing highlights the importance of studies on this type of diseases, as well as the relevance of the strategies implemented to address the public health problem they represent.
In the reviewed literature, little national and Latin American information was found regarding the characterization of hospital interconsultation of rheumatology, including patients in the ICU and outside of it.15,26 The risk factors associated with adverse outcomes in the Colombian population with SARDs hospitalized outside the ICU are unknown. It is considered relevant to know the course of hospitalization in patients evaluated by rheumatology, in order to take measures that imply improvement in care, in addition to promoting more research in this regard. Taking into account the foregoing, the present study aimed to characterize the patients evaluated in hospital by rheumatology and determine risk factors associated with the presence of adverse outcomes such as hospital-acquired infection, admission to the ICU, mortality or readmission.
MethodologyA historical cohort study was conducted. The available records of hospitalized patients ≥ 18 years who required to be evaluated by rheumatology in the Clínica Imbanaco (CI) of Cali, Colombia, during the years 2013–2019 were included. In the CI, rheumatology is an interconsulting subspecialty. Each episode of hospitalization was defined as an independent event; that is, if during the study period the same patient was hospitalized 3 times, each of the events was analyzed in the database. A readmission (hospitalization for the same cause within the first 30 days after discharge) was not interpreted as a new event, but rather as an adverse outcome.
Classification of the populationThe population was classified as follows: group 1, patients with SARDs diagnosed de novo; group 2, patients with known diagnosed SARDs; group 3, patients without diagnosed SARDs; group 4, patients with unconfirmed suspicion of SARDs. Patients with autoimmune diseases limited to a single organ were not included as SARDs (e.g., inflammatory eye disease, autoimmune hemolytic anemia, subacute cutaneous lupus, pyoderma gangrenosum, psoriasis without joint manifestations, and autoimmune thyroid, liver, pancreatic, inflammatory intestinal or adrenal disease without systemic manifestations). Some of the patients remained in group 4 when they presented findings of systemic involvement to be confirmed or established over time; the majority of them were assigned to group 3.
Adverse outcomesThe presence of the composite adverse outcome was considered when the patients presented at least one of the following related events: (1) in-hospital mortality; (2) admission to the ICU at any time during the hospital stay (in the case of several admissions to the ICU in the same hospitalization, the reason and the initial stay were evaluated); (3) hospital-acquired infection, defined as an infection identified within the first 48 h after admission27; and (4) hospital readmission, defined as hospital admission that occurred within the first 30 days after discharge, for the same cause of initial hospitalization. The choice of these outcomes was based on the literature review. Some of these parameters are widely recognized as indicators of hospital quality.28
Collection of informationAll available electronic medical records, based on the own records of the rheumatology service and the database provided by the computing area of the CI were included. During the procedure of collection of the information, the primary sources were the electronic medical records of the patients who met the eligibility criteria. Not all the records of the rheumatologists of the institution were included due to limitations in the capture of these data. The information was collected with the Magpi + application for data organization (DataDyne Group LLC. Copyright© 2020 Magpi. Version 6.1.12 (6198)), which remained available on the mobile phones and computers of the research team. No interventions were performed on the patients included.
Statistical analysisAll data were analyzed using the Stata 13 statistical package (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP). A descriptive analysis was performed for all the variables. The quantitative variables were described in medians (p25-p75) and the qualitative variables were summarized from absolute and relative frequency measurements. A bivariate analysis was carried out by comparing the variables of the four study groups, using the chi-square or Fisher tests for the qualitative variables. For the quantitative variables, the Mann-Whitney test was used to compare two groups and the Kruskal-Wallis test for more than two groups.
The analysis of the factors associated with the composite adverse outcome was carried out using a conditional logistic regression model, considering that the four study groups presented different risks for developing the adverse event. The magnitude of the association between the variables is described by the odds ratio (OR), adjusted for the study groups. P-values < 0.05 were considered statistically significant.
Ethical considerationsThe research project had the approval of the research and ethics committees of the Universidad Libre de Cali and the CI. This was a retrospective analytical study without any type of intervention on patients. Only the electronic records of the patients evaluated by rheumatology in the hospital setting were reviewed. The confidentiality of the information was maintained, following the principles of the Declaration of Helsinki and the parameters established in Resolution 8430 of 1993 issued by the Ministry of Health of Colombia.
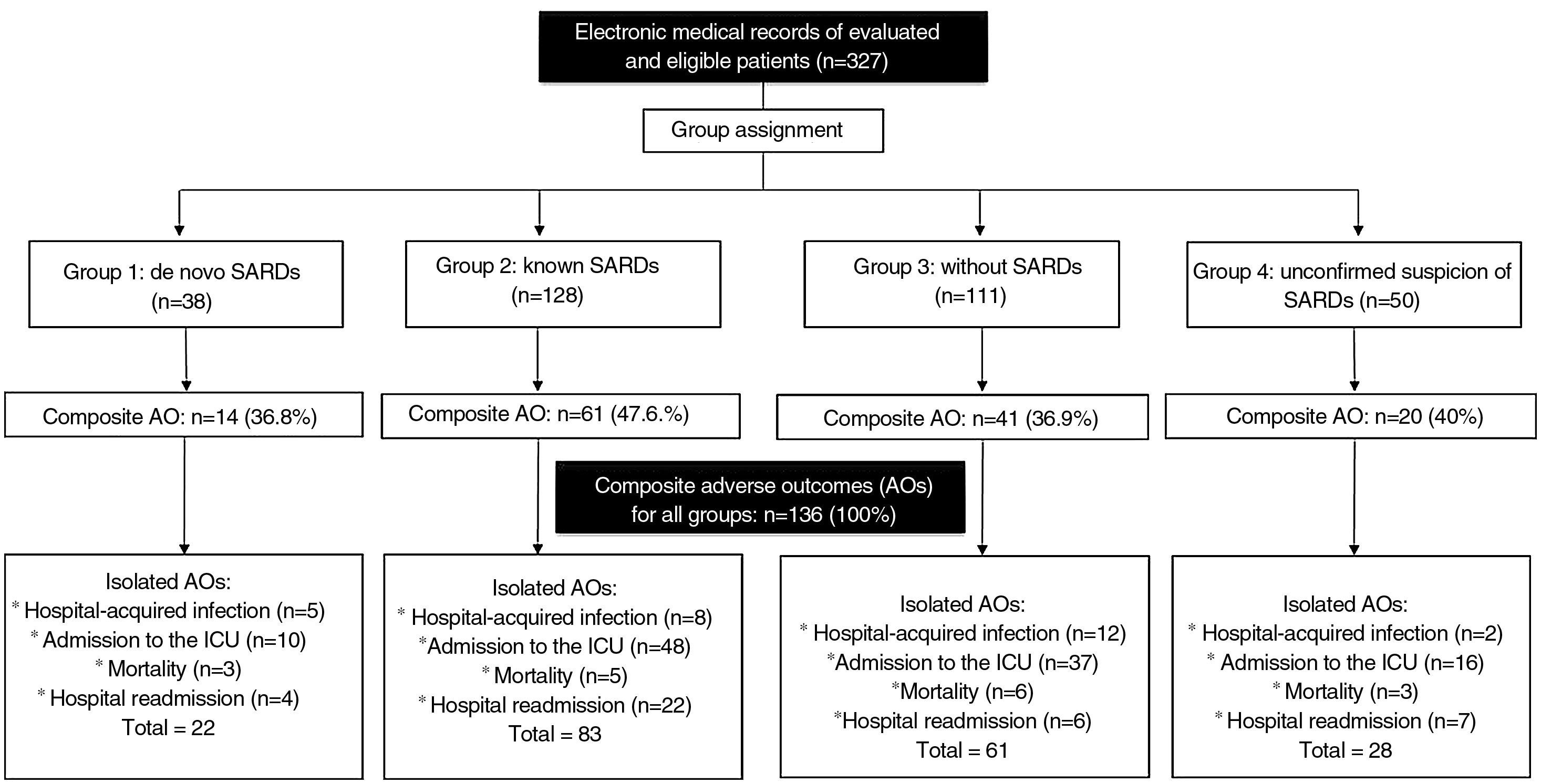
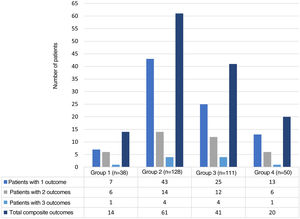
ResultsFor the period between January 2013 and December 2019, it was possible to obtain information on 327 hospital events among 307 patients seen by rheumatology in the CI. Fig. 1 schematically represents the general structure of the study in terms of the conformation of the groups and the presence of the adverse outcomes evaluated: 136 composite outcomes and 194 isolated outcomes. Some patients presented more than one of the isolated adverse outcomes during their hospitalization; therefore, the sum of them is not equal to that of the composite adverse outcome for each study group.
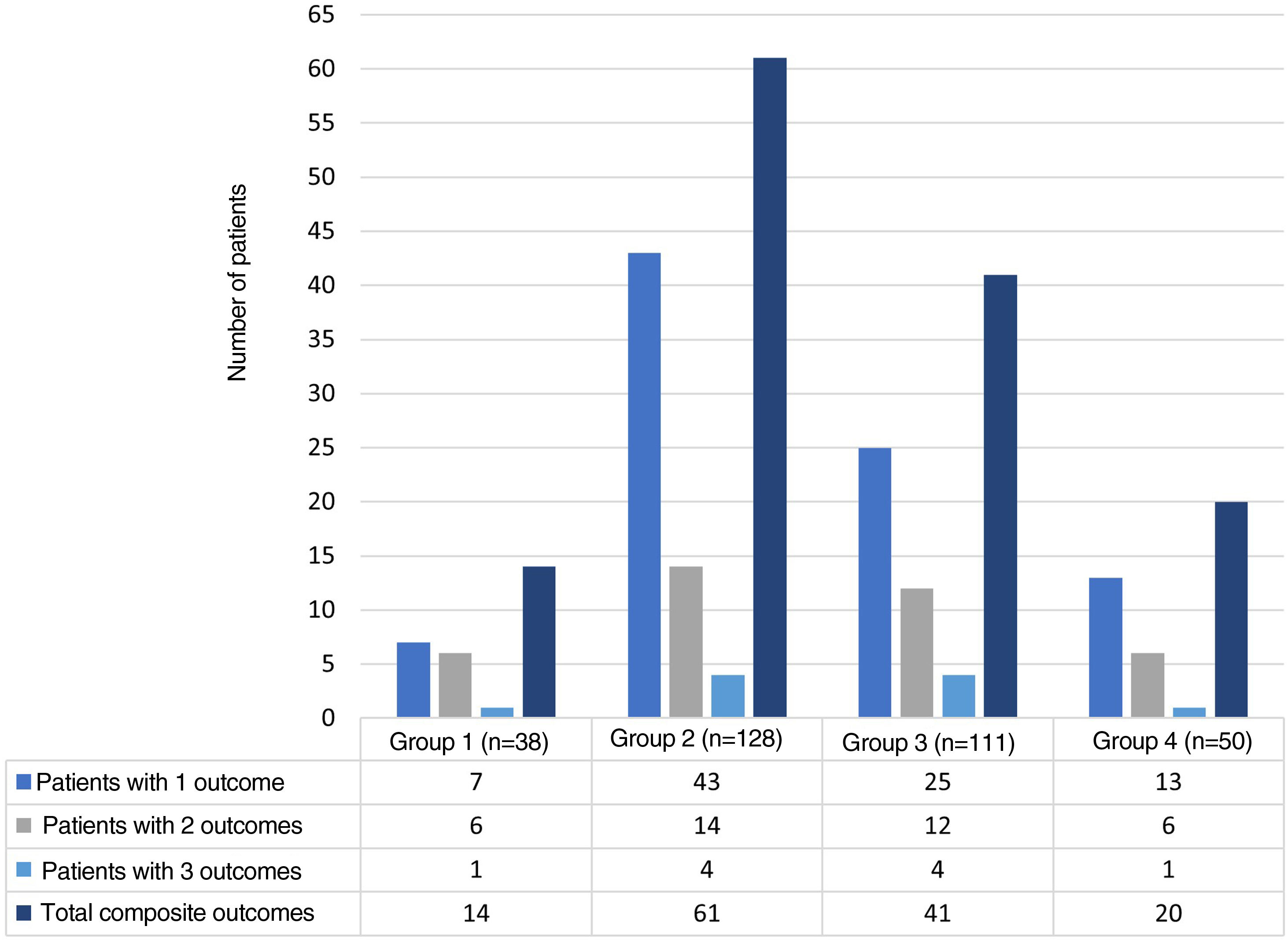
The distribution of the hospitalization events between the study groups was as follows: group 1, 38 (11.6%); group 2, 128 (39.2%); group 3, 111 (33.9%); and group 4, 50 (15.3%) events. The composite adverse outcome occurred in 136 (41.5%) hospital events. Of the total number of events, the frequencies of the outcomes were: admission to the ICU 33.9% (n = 111); readmission 11.9% (n = 39); hospital-acquired infection 8.2% (n = 27) and hospital mortality 5.2% (n = 17). Fig. 2 complements the distribution of the isolated adverse outcomes presented by the patients for each group.
As for the general characteristics, the median age was 48 (34−63) years and 222 (72.3%) participants were women. The majority of patients were mestizos (78.5%). 30.9% of the population had university studies. Regarding the type of health care regime, 59% of the patients belonged to a special regime, which included prepaid medicine, private medicine and medical insurance policies. The general characteristics of the population are shown in Table 1. The origin of the referral was spontaneous demand for 64.5% of the events; the rest came from outpatient clinics or from other hospitals.
General characteristics of the population.
| Variables | Group 1 | Group 2 | Group 3 | Group 4 | General | p-value |
|---|---|---|---|---|---|---|
| n (%) | 38 (12.4) | 112 (36.5) | 110 (35.8) | 47 (15.3) | 307 (100) | |
| Sex | 0.806 | |||||
| Female | 25 (65.8) | 82 (73.2) | 81 (73.6) | 34 (72.4) | 222 (72.3) | |
| Male | 13 (34.2) | 30 (26.8) | 29 (26.4) | 13 (27.6) | 85 (27.7) | |
| Age (years)a | 53 (30−59) | 51 (35−68) | 44 (34−62) | 46 (31−65) | 48 (34−63) | 0.348 |
| Ethnicityb | 0.066 | |||||
| Mestizo | 28 (73.8) | 85 (75.9) | 92 (83.7) | 36 (76.7) | 241 (78.5) | |
| Afro-descendant | 4 (10.5) | 11 (9.8) | 6 (5.4) | 1 (2.1) | 22 (7.2) | |
| Indigenous | 1 (2.6) | 2 (1.8) | – | 2 (4.2) | 5 (1.6) | |
| Other | 1 (2.6) | – | – | – | 1 (0.3) | |
| No data | 4 (10.5) | 14 (12.5) | 12 (10.9) | 8 (17) | 38 (12.4) | |
| Educational level | 0.005 | |||||
| University | 5 (13.1) | 35 (31.2) | 46 (41.8) | 9 (19.2) | 95 (30.9) | |
| High school | 11 (28.9) | 26 (23.3) | 21 (19) | 15 (31.9) | 73 (23.8) | |
| Primary | 4 (10.5) | 11 (9.8) | 6 (5.4) | 1 (2.1) | 22 (7.2) | |
| Technical | – | – | 1 (0.9) | 2 (4.2) | 3 (1) | |
| No data | 18 (47.4) | 40 (35.7) | 36 (32.7) | 20 (42.6) | 114 (37.1) | |
| Health regime | 0.012 | |||||
| Special | 18 (47.4) | 65 (58) | 75 (68.2) | 23 (48.9) | 181 (59) | |
| Contributory | 7 (18.4) | 31 (27.7) | 16 (14.5) | 16 (34.1) | 70 (22.8) | |
| Subsidized | 13 (34.2) | 16 (14.3) | 19 (17.3) | 8 (17) | 56 (18.2) | |
| Provenance | 0.591 | |||||
| Cali | 26 (68.4) | 79 (70.5) | 88 (80) | 35 (74.5) | 228 (74.3) | |
| Valle del Cauca (not Cali) | 6 (15.8) | 17 (15.2) | 14 (12.7) | 5 (10.6) | 42 (13.7) | |
| Colombia (outside the Valle del Cauca) | 5 (13.1) | 14 (12.5) | 8 (7.3) | 6 (12.8) | 33 (10.7) | |
| Other country | 1 (2.6) | 2 (1.8) | – | 1 (2.1) | 4 (1.3) | |
| Comorbiditiesc | ||||||
| AHT | 11 (28.9) | 63 (49.2) | 26 (23.4) | 13 (26) | 113 (34.5) | < 0.001 |
| Hypothyroidism | 7 (18.4) | 36 (28.1) | 19 (17.1) | 9 (18) | 71 (21.7) | 0.183 |
| Heart diseased | 5 (13.1) | 23 (17.9) | 8 (7.2) | 5 (10) | 41 (12.5) | 0.086 |
| CKDe | 4 (10.5) | 26 (20.3) | 6 (5.4) | 2 (4) | 38 (11.6) | 1.000 |
| Diabetes mellitus | 3 (7.8) | 14 (10.9) | 11 (9.9) | 3 (6) | 31 (9.5) | 0.830 |
| Dyslipidemia | 4 (10.5) | 7 (5.4) | 8 (7.2) | 5 (10) | 24 (7.3) | 0.539 |
| Cancerf | 1 (2.6) | 9 (7.0) | 8 (7.2) | 1 (2) | 19 (5.8) | 0.499 |
| Depressive disorder | 3 (7.8) | 5 (3.91) | 7 (6.3) | 4 (8) | 19 (5.8) | 0.534 |
| Obesity | 1 (2.6) | 5 (3.9) | 5 (4.5) | 2 (4) | 13 (3.9) | 1.000 |
| COPD | 1 (2.6) | 4 (3.1) | 6 (5.4) | 2 (4) | 13 (3.9) | 0.812 |
| Venous insufficiency of the lower limbs | – | 5 (3.9) | 2 (1.8) | 1 (2) | 8 (2.4) | 0.683 |
| Osteoporosis | 1 (2.6) | 1 (0.7) | 2 (1.8) | 2 (4) | 6 (1.8) | 0.356 |
| Tuberculosis | – | 2 (1.5) | 3 (2.7) | – | 5 (1.5) | 0.724 |
| CVD | 1 (2.6) | 2 (1.5) | – | 1 (2) | 4 (1.2) | 0.304 |
| HIV infection | – | – | 2 (1.8) | – | 2 (0.6) | 0.461 |
| Otherg | 4 (10.5) | 5 (3.9) | 15 (13.5) | 10 (20) | 34 (10.4) | 0.005 |
CVD: cerebrovascular disease; COPD: chronic obstructive pulmonary disease; CKD: chronic kidney disease; AHT: arterial hypertension; HIV: human immunodeficiency virus.
If all hospitalization events are considered, among patients in group 2, the most common underlying diagnosis was SLE (42.1%), followed by RA (26.5%), Sjögren’s syndrome (11.7%), systemic sclerosis (10.1%), vasculitis (6.2%), crystalline arthropathy (gout and calcium pyrophosphate crystal arthropathy) (4.6%) and other diagnoses such as inflammatory muscle disease, sarcoidosis and antiphospholipid syndrome (10.1%). In the same group, 75% had been previously followed-up by rheumatology and 82.8% received immunomodulatory treatment: steroids (66.4%), antimalarials (30.4%), azathioprine (15.6%) and biologic agents (12.5%).
The median hospital stay for the four groups was 11 (6−22) days. The general characteristics of hospitalization by groups are shown in Table 2. The median time between the onset of symptoms and admission was 6 (2–14) days (opportunity for consultation) and the median time between the request for interconsultation with rheumatology and the response to it was 9.5 (4–31) hours. Regarding the ward in which the patients remained during most of their stay, 295 (90.2%) of all the events occurred in general hospitalization, 23 (7%) in the ICU, and the rest in other wards such as the coronary care unit or obstetrics/gynecology.
Characteristics of the population during hospitalization.
| Variables | Group 1 | Group 2 | Group 3 | Group 4 | General | P-value |
|---|---|---|---|---|---|---|
| n (%) | 38 (11.6) | 128 (39.1) | 111 (33.9) | 50 (15.2) | 327 (100) | 0.862 |
| Main initial diagnosisa | ||||||
| Cardiovascular | 8 (21) | 27 (21) | 18 (16.2) | 6 (12) | 59 (18) | |
| Infectious | 4 (10.5) | 28 (21.8) | 5 (4.5) | 12 (24) | 49 (14.9) | |
| Neuromuscular or ocular | 4 (10.5) | 12 (9.3) | 28 (25.2) | 4 (8) | 48 (14.7) | |
| Respiratory | 5 (13.1) | 11 (8.5) | 14 (12.6) | 4 (8) | 34 (10.5) | |
| Musculoskeletal | 5 (13.1) | 10 (7.8) | 7 (6.3) | 11 (22) | 33 (10) | |
| Hematooncological | 6 (15.7) | 5 (3.9) | 12 (10.8) | 5 (10) | 28 (8.5) | |
| Fever as a sign not associated with a system | 1 (2.6) | 10 (7.8) | 12 (10.8) | 1 (2) | 24 (7.4) | |
| Gastrointestinal or hepatic | 1 (2.6) | 7 (5.4) | 7 (6.3) | 2 (4) | 17 (5.3) | |
| Renal | 3 (7.8) | 6 (4.6) | 5 (4.5) | 1 (2) | 15 (4.6) | |
| Activity/flare of the rheumatic disease | 1 (2.6) | 10 (7.8) | – | – | 11 (3.4) | |
| Other | – | 2 (1.5) | 3 (2.7) | 4 (8) | 9 (2.7) | |
| Received immunosuppressive scheme | 26 (68.4) | 65 (50.7) | 33 (29.7) | 21 (42) | 145 (44.3) | <0.001 |
| Admission to ICU | 10 (26.3) | 48 (37.5) | 37 (33.3) | 16 (32) | 111 (33.9) | 0.637 |
| De novo requirement of RRT | 7 (18.4) | 6 (4.6) | 3 (2.7) | 21 (42) | 21 (18.9) | 0.352 |
| Days of hospital stayb | 17 (7−32) | 11 (7−21) | 12 (7−19) | 9 (5−22) | 11 (6−22) | 0.172 |
| Hospital readmission | 4 (10.5) | 22 (17.1) | 6 (5.4) | 7 (14) | 39 (11.9) | 0.032 |
| Laboratory testsb,c | ||||||
| White blood cell count (cells/μL) | 9,170 (6,820–13,170) | 8,920 (5,830–12,859) | 10,130 (6,930–13,230) | 9.515 (6,790–13,670) | 9,130 (6,695–13,085) | 0.704 |
| Lymphocyte count (cells/μL) | 1,100 (740–1,560) | 1,200 (700–1,770) | 1,840 (1,080–2,700) | 1,580 (970–2,050) | 1,390 (850–2,180) | 0.001 |
| Hemoglobin (g/dL) | 9.4 (8–10.4) | 10.1 (8.4–11.9) | 11.4 (9.5–13.1) | 10.9 (8.4–12.8) | 10.4 (8.6–12.4) | 0.001 |
| Platelets (cells in thousands/μL) | 217 (152–306) | 244 (145–347) | 271 (177–348) | 264 (201–339) | 259,5 (170,5–340,5) | 0.313 |
| Creatinine (mg/dL) | 1 (0.65–2) | 0.9 (0.7–1.28) | 0.77 (0.62–1.01) | 0.75 (0.68–1.07) | 0.81 (0.67–1.15) | 0.006 |
| CRP (mg/L) | 65.8 (20.9–122) | 59.6 (23.6–133) | 58.7 (7.4–126.8) | 41 (13.6–121) | 58.6 (14.3–130.5) | 0.539 |
CRP: C-reactive protein; RRT: renal replacement therapy; ICU: intensive care unit.
Half of the entire population was treated by the specialty of internal medicine. The other treating clinical specialties included neurology and other subspecialties of internal medicine. The main reason for the interconsultation with rheumatology was the activity of the underlying rheumatic disease (the next most frequent reasons were joint syndrome, neuromuscular or ocular disorders, and hematooncological disorders) (the percentages of the causes of interconsultation with rheumatology are shown in Appendix B, Table S1 of the additional material). Among the hospitalization events in groups 1 and 2 of the study, the most frequent final rheumatological diagnoses were SLE, 70/166 (42.2%); RA, 29/166 (17.5%); vasculitis, 14/166 (8.4%); Sjögren’s syndrome, 14/166 (8.4%); systemic sclerosis, 13/166 (7.8%); crystal arthropathy, 10/166 (6%) and others 16/166 (9.6%). The main diagnoses that led to hospitalization were cardiological, the most frequent being: heart failure, coronary syndrome, atrial or ventricular tachyarrhythmia, and cardiomyopathy.
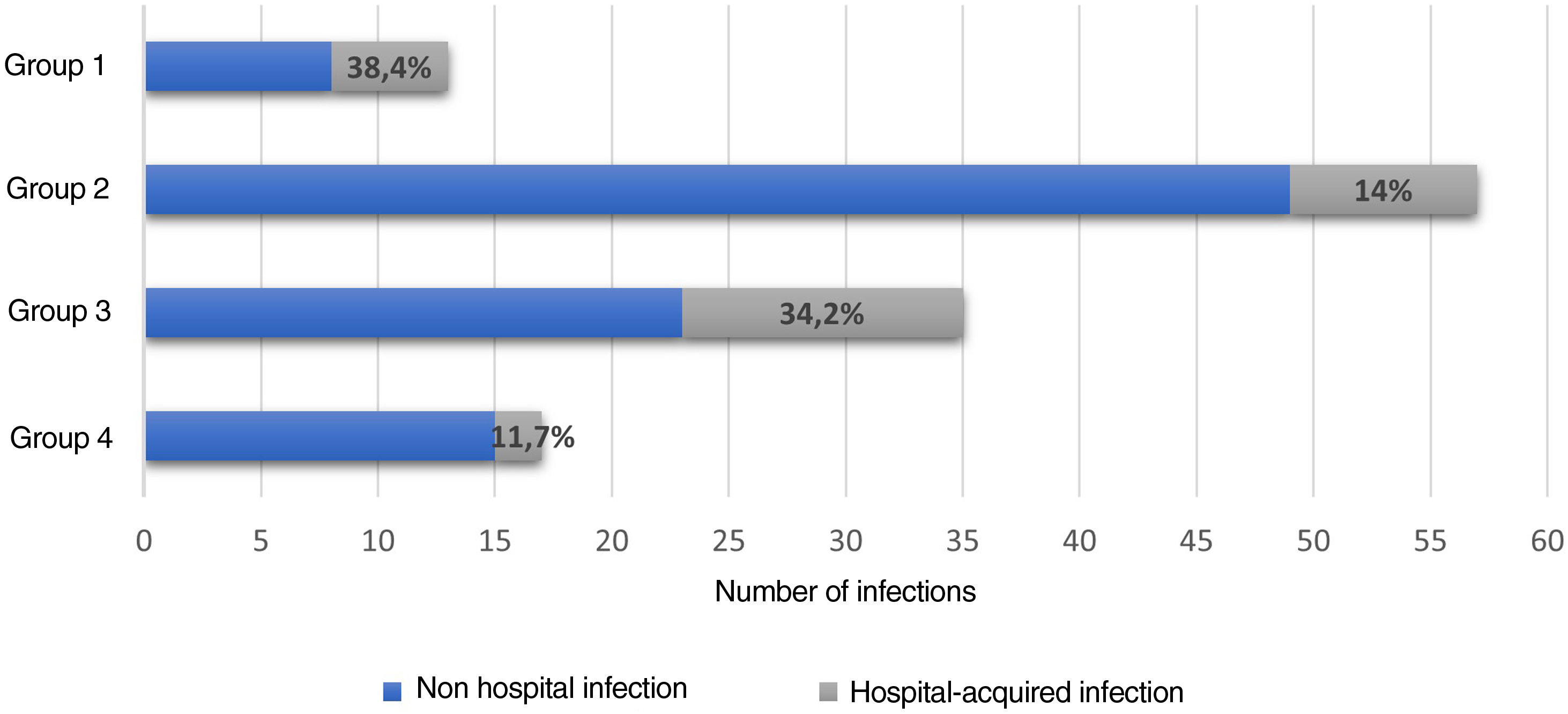
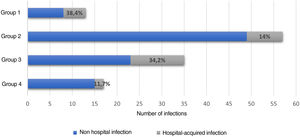
Hospital-associated infections occurred in 122 events (37.3%), among these, 22.1% (27/122) corresponded to nosocomial infections. Fig. 3 shows the distribution by groups of the patients who presented infections. Antibiotic treatment was used in 41.2% of all hospital events, with a distribution by groups as follows: group 1, 14/38 (36.8%); group 2, 60/128 (46.8%); group 3, 41/111 (36.9%); and group 4, 20/50 (40%).
With reference to repeated events for the same patient, 18 patients had 2 admissions recorded as different events in the study, while one patient had 3 admissions recorded as independent events. Among them, the majority (14/19 patients) belonged to group 2 during the 2 or 3 hospital events. As for mortality, among the 327 records, 17 patients died, distributed as follows: group 1, 3 (7.8%); group 2, 5 (3.9%), group 3, 6 (5.4%); and group 4, 3 (6%) (The patients who died are characterized in Appendix B, Table S2 of the additional material).
Among the study groups, it was not identified that any of them had a greater chance of developing the composite adverse outcome. However, the factors associated with the presence of adverse events were adjusted for the four groups. It was found that the variables associated with worse results were: initial cardiovascular diagnosis (OR = 4.63; CI: 1.60−13.43; p = 0.005), hospital stay > 8 days (OR = 1.04; CI: 1.01−1.07; p = 0.005) and having been treated by a specialty other than internal medicine (OR = 2.79; CI: 1.26−6.17; p = 0.011). While the male gender (OR = 0.29; CI: 0.12−0.66; p = 0.004), belonging to a special health regime (OR = 0.39; CI: 0.15−0.99; p = 0.047) and having hemoglobin (Hb) > 11.4 g/dL (OR = 0.82; CI: 0.69−0.99; p = 0.039) were factors associated with a lower odds of developing the composite adverse event, as shown in Table 3. Variables that had a loss of information greater than 20% and those in which there was no clinical or statistical relevance were excluded from the multivariate analysis.
Multivariate analysis of association with the composite adverse outcome.
| Variables | Presence of the composite adverse outcome | OR | p-value | |
|---|---|---|---|---|
| Yes | No | |||
| n (%) | 136 (41.6) | 191 (58.4) | ||
| Sex | ||||
| Male | 25 (18.3) | 64 (33.5) | 0.29 (0.12–0.66) | 0.004 |
| Female | 111(81.6) | 127 (66.4) | Ref. | |
| Age (years)a | 46 (34–63) | 51 (34–65) | 1.02 (0.99–1.04) | 0.172 |
| Health regime | ||||
| Contributory | 30 (22) | 44 (23) | 1.23 (0.43–3.50) | 0.692 |
| Special | 140 (102.9) | 52 (27.2) | 0.39 (0.15–0.99) | 0.047 |
| Subsidized | 21 (15.4) | 40 (20.9) | Ref. | |
| Comorbidities | ||||
| Arterial hypertension | 61 (44.8) | 52 (27.2) | 1.07 (0.46–2.4) | 0.877 |
| Heart disease | 25 (18.3) | 16 (8.3) | 1.73 (0.56–5.32) | 0.339 |
| T2DM | 25 (13.3) | 13 (6.8) | 0.67 (0.49–2.11) | 0.491 |
| CKD | 9 (6.6) | 10 (5.2) | 1.39 (0.39–4.95) | 0.608 |
| Previous follow–up by rheumatology | 50 (36.7) | 66 (34.5) | 0.58 (0.19–1.72) | 0.327 |
| Opportunity to consult (days)a,b | 4 (1–14) | 7 (3–15) | 0.98 (0.96–1.00) | 0.223 |
| Treating specialty | ||||
| Other clinical specialty | 55 (40.4) | 48 (25.1) | 2.79 (1.26–6.17) | 0.011 |
| Surgical specialty | 29 (21.3) | 30 (15.7) | 1.76 (0.71–4.40) | 0.223 |
| Internal medicine | 52 (38.2) | 113 (59.1) | Ref. | |
| Main initial diagnosisc | ||||
| Cardiovascular | 38 (27.9) | 21 (10.9) | 4.63 (1.60–13.43) | 0.005 |
| Infectious | 23 (16.9) | 26 (13,6) | 1.39 (0.54–3.54) | 0.493 |
| Previous rheumatological treatment | 59 (43.3) | 65 (34) | 1.22 (0.39–3.87) | 0.734 |
| De novo requirement of RRT | 19 (13.9) | 2 (1) | 3.46 (0.64–18.61) | 0.148 |
| Days of hospital staya | 18 (10–31) | 8 (5–15) | 1.04 (1.01–1.07) | 0.005 |
| Hemoglobin (g/dL)a | 9.1 (8–11) | 11.4 (9.9–12.9) | 0.82 (0.69–0.99) | 0.039 |
| Creatinine (mg/dL)a | 0.91 (0.6–1.74) | 0.78 (0.68–1.06) | 0.94 (0.74–1.19) | 0.605 |
| CRP (mg/L)a | 83.6 (40.3–156.7) | 35.1 (8.9–108.3) | 1.00 (1.00–1.01) | 0.102 |
Ref: group that was used as comparator to establish the OR; T2DM: type 2 diabetes mellitus; CKD: chronic kidney disease; OR: odds ratio; CRP: C-reactive protein; RRT: renal replacement therapy.
The p-values of the variables with statistical significance are highlighted in bold.
In our cohort, we found risk factors and protective factors associated with the presentation of a composite adverse outcome in the hospitalized patients evaluated by rheumatology. The composite adverse outcome had an equitable distribution among the four groups. However, in terms of percentages, the group of patients with known diagnosed SARDs (group 2) had more adverse outcomes and new hospitalizations. The foregoing could be related to the accumulated damage in this group, which was not measured in this study. For patients with de novo SARDs, the findings suggest that if their care is conducted appropriately, the results could be encouraging, despite having a longer hospital stay.
The older age, the greater number of days in the ICU, and cardiovascular involvement on admission have been previously identified as factors associated with mortality.17,18 In our analysis, the age did not turn out to be a factor associated with adverse outcomes, which could be related to the inclusion of a population younger than those reported in other cohorts, in which the average age ranged between 52 and 62 years,15,29 while in this research the median was 48 (34−63) years. In addition, we found a predominance of the female sex, a finding that coincides with that described for SARDs. The male sex was associated with lower odds of developing the composite adverse outcome, which should be corroborated in studies that include a larger and more diverse male population in terms of main diagnoses.
In studies of patients with SLE, it has been demonstrated that the high burden of comorbidity, mainly of cardiovascular or neoplastic origin, has a negative impact on the course of the disease.30 Despite the limitations for the comparison with what was found in our cohort, this being a population with multiple pathologies and with a majority of patients outside the ICU, what was found correlates with the available evidence in patients with SARDs. However, the presence of neoplasia was not found as a variable associated with the adverse outcome.
It has previously been demonstrated that the most frequent reason for consulting the emergency department in patients with SARDs is cardiovascular (25%), followed by an infectious cause (15%),31 which is consistent with what was reported in our study, where cardiac pathology was the most common cause of admission. In patients with SARDs, early arteriosclerosis and cardiovascular disease are important causes of morbidity and mortality and are dependent on chronic inflammation, immunosuppressive therapy, as well as on the duration and activity of the SARD. The foregoing is independent of the prevalence of classic cardiovascular risk factors in this type of patients.32,33 However, the most common comorbidities in patients of all groups were: arterial hypertension, heart disease, chronic kidney disease, type 2 diabetes mellitus, and dyslipidemia.
Regarding the distribution of the patients seen by rheumatology, it could be said that half of the population evaluated actually had SARDs, while the other half did not, or did not meet sufficient criteria to be classified as such. This finding was similar to that found in a cohort of Chilean patients hospitalized and evaluated by rheumatology (57%),15 while in a Colombian study with patients older than 13 years, hospitalized and outpatient, it was found that 43% had systemic and 57% organ-specific diseases.26
In 20.2% of the events, the reason for rheumatology interconsultation was due to known SARD activity. In patients without SARDs, the most common reasons for consultation were neuromuscular or ocular disorders and joint syndrome. The foregoing is similar to that was reported by Pacheco et al., where 24.3% of the interconsultations were from patients with active SARDs, while in those without known SARDs the cause was musculoskeletal pain or arthritis.15 This sets out the relevance that the approach of patients by a basic clinical specialty such as internal medicine would have. In this cohort, it was identified that not having a treating specialty such as internal medicine was associated with a greater odds of presenting the composite adverse outcome.
The median hospital stay in our population was 11 (6−22) days, lower than that reported in a similar population, whose average was 18 (2–58) days.15 Among the patients with SARDs, it has been demonstrated that a hospital stay longer than 14 days is related to the severity of the disease and serious complications.16 However, when the care of patients with rheumatic pathologies is carried out by a multidisciplinary team, the hospital length of stay is reduced.10
Infections and exacerbations or complications of SARDs are the most common and potentially reversible causes of admission to the ICU.17–24 In this research, hospital-acquired infections occurred in 8.26% of the population and were more common in groups 1 and 3. This percentage was lower than that recorded in a study of patients with SLE (12.5%), which also showed that there was a greater probability of hospital-acquired infection when the hospital stay was longer than 7 days.34
The patients with SARDs often have comorbidities and coexisting drug therapies that impact the course of the disease. It has been reported that mortality is not different or increased compared to patients in the ICU without SARDs.35 In our research, one third of the patients were admitted to the ICU, this being the most common adverse outcome. Approximately half of the admissions to the ICU occurred in patients of groups 1 and 2, and SLE was the most common diagnosis, which is similar to that reported in cohorts of patients with SARDs in the ICU. In these patients, the mortality was 10.3%, a lower percentage compared to what was found in other series, which ranged between 17 and 55%.22–24
The 30-day readmission rate for different pathologies varies between 5 and 19.6%.36 Readmission, which was the second most frequent adverse outcome in this cohort, was present in 11.9% of the hospital events. The foregoing is similar to what was found in a study in which diseases of the hematopoietic organs and the immune system demonstrated to be those with the highest probability of readmission at 30 days, which was associated with greater economic impact.37. Hospital readmissions, in any pathology, are costly events, related to high morbidity and mortality and potentially preventable.38
In this study, the overall mortality was 5.2%. Bernal-Macias et al.21 reported a value of Hb < 8 g/dL as a relevant marker for mortality of the patients with SARDs in the ICU (OR = 16.1; p = 0.001). In our research, Hb > 11.4 g/dL was associated with a lower odds of developing the composite adverse outcome. 47% of the deceased patients belonged to groups 1 and 2. It was found that, although there were no statistically significant differences between groups in relation to the presence of the composite outcome, group 2 presented more adverse outcomes in percentage terms. In addition, it was associated with a higher number of new hospitalizations and more hospital readmissions. The foregoing could be related to the presence of accumulated damage in SARDs, a concept that has been applied mainly in SLE and vasculitis.39,40
In our cohort, the patients with de novo SARDs had a median stay of 17 (7−32) days, longer than the other groups, which could be related to the severity of the disease. However, this same group presented fewer adverse outcomes than patients in group 2, which may suggest that if the care of a patient with de novo SARDs is done properly, the results could be encouraging and that the accumulated damage generates a significant impact on patients with known SARDs.
The research had some limitations: first, the records were incomplete in some of the electronic medical records, mainly in terms of sociodemographic characteristics, especially ethnicity, in which there was a loss of information greater than 20%; second, being able to group patients with unconfirmed suspicion of SARDs implied certain complexity in some cases, because during hospitalization there was not enough information to define the existence or not of a systemic compromise; third, some hospitalization events could not be included in the sample because the system did not allow to export the 100% of the interconsultations conducted by the rheumatologists of the clinic; fourth, the external validity of this research should be interpreted according to each population, considering that it was carried out in a single center and that the conditions of the population could be different, as well as the models of medical care in each hospital.
ConclusionsCardiovascular comorbidities represented a key condition for the course of the hospitalization and the presence of adverse outcomes in patients with or without SARDs. This cohort showed the importance of the presence of a clinical specialty such as internal medicine in the prevention of adverse hospital outcomes. Teaching on the diagnostic and therapeutic approach to SARDs should be part of the training of human talent in health.
The characterization that this study presents of the patients evaluated in the hospital by rheumatology can serve as the basis for a new line of research that seeks to resolve the knowledge gaps in the continuum of care in rheumatology. To our knowledge, this Colombian cohort is the first to show hospital interconsultation in rheumatology without excluding patients outside the ICU and those without SARDs. The external validity of these findings needs to be corroborated in other studies.
FundingThis research has not received any specific grant from agencies in the public, commercial or non-profit sectors.
Conflict of interestThe authors declare that they have no conflict of interest in the present work.
Please cite this article as: Muñoz-Urbano M, Morales MA, Arbeláez-Cortés Á. Desenlaces hospitalarios en pacientes evaluados por reumatología en una cohorte histórica colombiana. Rev Colomb Reumatol. 2022;29:160–170.