Systemic lupus erythematosus is a chronic, autoimmune, multisystem disease of unknown aetiology, and in which the frequency of alterations in bone mineral density varies between 25% and 74%, although its diagnosis is not standardized.
ObjectiveTo describe the densitometric changes in systemic lupus erythematosus patients, as well as the clinical and demographic characteristics from two reference centres north-western Colombia.
Materials and methodsA cross-sectional study was conducted between January 2013 and December 2014. The data collected included the demographic variables, menopausal status, use of tobacco and alcohol, autoantibodies, organ involvement, medications, as well as the activity and chronicity indices (SLEDAI, SLICC). Densitometric changes were defined according to World Health Organization criteria.
Statistical analysesAbsolute and relative frequencies were calculated for qualitative variables, and medians with interquartile range (IQR) for quantitative variables.
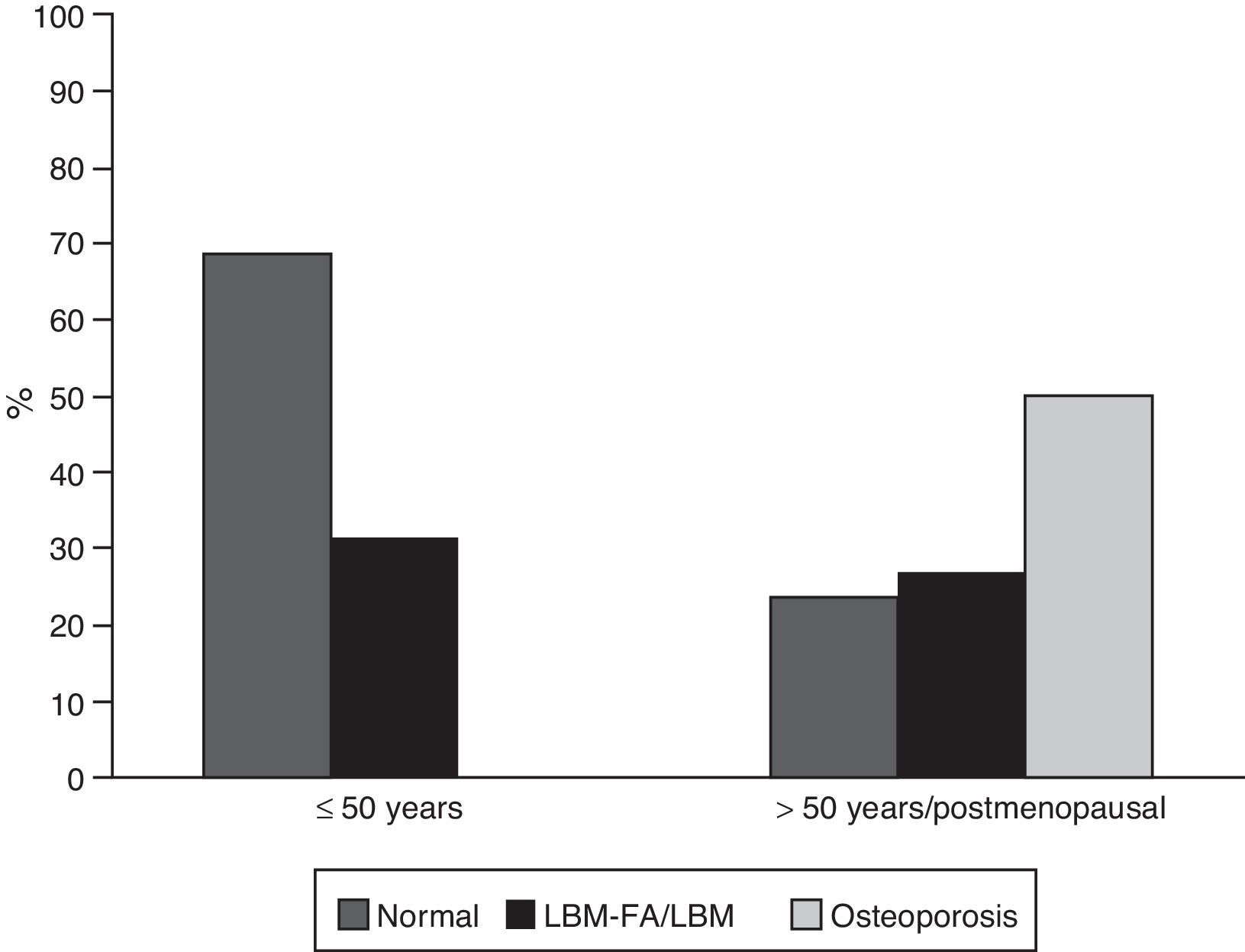
ResultsOf the total 302 patients evaluated, 65 met eligibility criteria. Thirty-one percent of premenopausal patients had low bone mass for age was observed in 31% of pre-menopausal women, with 50% of post-menopausal women showing osteoporosis, and 27% low bone mass. The number of patients with densitometric alterations according to associated factors was, fractures 4, alcohol consumption 2, active smokers 3, anti-Ro antibodies 8, neurological involvement 7, and chronic renal failure 4. Prednisone was used in 53.8%, with a median daily dose of 10mg (IQR 5–52). The median SLEDAI and SLICC was 0 (IQR=0–4) and 0.5 (IQR=0–1.75), respectively.
ConclusionsFew bone densitometry results were found in patients with systemic lupus erythematosus. The frequency of mineral bone disorders was independent of menopausal status. The median dose of prednisone was high in subjects in remission, and without organ damage.
El lupus eritematoso sistémico es una enfermedad autoinmune, multisistémica y crónica, de etiología desconocida, en la cual la frecuencia de alteraciones en la densidad mineral ósea varía entre 25-74%; si bien su diagnóstico no está estandarizado.
ObjetivoDescribir las alteraciones densitométricas en pacientes con lupus eritematoso sistémico, así como las características clínicas y demográficas en 2 centros de referencia del noroccidente colombiano.
Materiales y métodosEstudio transversal realizado entre enero de 2013 y diciembre de 2014. Variables incluidas: demográficas, estado de menopausia, consumo de tabaco y alcohol, autoanticuerpos, compromiso orgánico, medicamentos e índices de actividad y cronicidad (SLEDAI, SLICC). Alteraciones densitométricas definidas según la Organización Mundial de la Salud.
Análisis estadísticoFrecuencias absolutas y relativas para variables cualitativas; mediana con rango intercuartílico (RIQ) para variables cuantitativas.
ResultadosDe 302 pacientes evaluados, 65 cumplieron criterios de elegibilidad. Treinta y uno por ciento de las pacientes premenopáusicas tenían baja masa ósea para la edad; un 50% de las mujeres posmenopáusicas tenían osteoporosis y un 27% baja masa ósea. Número de pacientes con alteraciones densitométricas según factores asociados: fracturas 4, consumo de alcohol 2, fumadores activos 3, anticuerpos anti-Ro 8, afección neurológica 7, falla renal crónica 4. Uso de prednisolona: 53,8%; mediana de dosis diaria: 10mg (RIQ 5-52). Medianas de SLEDAI y SLICC: 0 (RIQ=0-4) y 0,5 (RIQ=0-1,75), respectivamente.
ConclusionesSe encontraron pocas densitometrías óseas en pacientes con lupus; la frecuencia de las alteraciones minerales óseas fue independiente del estado de menopausia. La mediana de dosis de prednisolona fue alta, en sujetos en remisión y sin daño orgánico.
Systemic lupus erythematosus (SLE) is a chronic autoimmune multisystem disease of unknown aetiology. Its clinical course is characterized by recurrent periods of exacerbation and remission, which generate damage, not only by the disease activity, but also by the cumulative organ damage.1 One of the manifestations of this organ damage is the decrease in bone mineral density (BMD), which manifests itself either as low bone mass (LBM) or osteoporosis in the subgroup of menopausal patients, or as low bone mass for age (LBM-FA) in premenopausal individuals.2 The incidence of these alterations in BMD varies among different studies, depending on the methodology used for their diagnosis, ranging between 25% and 74% for LBM and between 1.4% and 68% for osteoporosis3; between 6% and 42% of these patients suffer fractures, being this the final consequence of the decrease in BMD, causing a significant morbidity and mortality.4
The aetiology of these alterations in BMD is multifactorial and covers from traditional risk factors (such as alcohol and tobacco consumption), to metabolic aspects (vitamin D deficiency, hyperhomocysteinemia), until those derived specifically from the disease, such as the antibodies profile (presence of anti-Ro and absence of anti-Sm), the number of reactivations and the cumulative chronic damage.3 The deleterious effects of the drugs used for the control of the disease, such as cyclophosphamide with its gonadal dysfunction, and especially steroids, by their apoptotic effect on the osteoblasts with increase of osteoclasts, the latter being a recognized risk factor for osteoporosis even in non-lupus population, should also be considered.3,5
At the regional level there is a vacuum of knowledge on this topic, since the studies that address this issue are few6–8 and the methods used for the diagnosis of the alterations in BMD have been variable, not always using bone densitometry (BD), which is the reference standard for the evaluation of such alterations2 using, sometimes, the evidence of fracture by X-rays as a marker of alteration in BMD,9 being difficult to determine the magnitude of this problem in our population.
The purpose of this work was to describe the alterations in the BMD of SLE patients, as well as their clinical and demographic characteristics in two reference centres of the Colombian northwestern region.
MethodologyA cross-sectional study was conducted in patients treated in outpatient and inpatient services of 2 high complexity hospitals between January 2013 and December 2014. Patients older than 18 years with diagnosis of SLE, according to the criteria of the American College of Rheumatology modified in 199710 and with availability of BD were selected. Patients with other systemic diseases such as: rheumatoid arthritis, vasculitis, cancer, chronic kidney disease other than lupus nephropathy; metabolic bone diseases or diagnosis of osteoporosis or LBM prior to the diagnosis of SLE were excluded.
At the time of carrying out the BD the following variables were collected: gender, age, presence of menopause, consumption of alcohol (intake of 3 or more servings per day) or tobacco (any active consumption of cigarettes at the time of inclusion in the study), personal or family history of fracture due to osteoporosis, anti-DNA, serum complement, antibodies against extractable nuclear antigens (Ro, La, Sm, RNP), presence of Raynaud's phenomenon and lupus nephropathy, use of steroids, average dose, type of steroid used, use of methylprednisolone pulses, use of antimalarial agents, disease activity determined by the SLEDAI index,11 as well as a quantification of the organ damage through the SLICC index.12
The information was collected from the clinical records and written down in a previously designed form. Before starting the execution of this study, the researchers conducted a discussion on the protocol, standardized the information gathering process and a pilot test that allowed to adjust this process was conducted with 5 clinical records. The BMD was evaluated by BD and classified in the following way: for patients older than 50 years or postmenopausal the T-score was used as follows: normal between 0 and –0.99; LBM between –1.0 and –2.49, and osteoporosis with values equal to or more negative than –2.5,13 and for subjects younger than 50 years or premenopausal, the Z-score was used as follows: normal between 0 and –1.99 and LBM-FA with values equal to or less than –2.0.14
Based on the information collected it was designed a spreadsheet in Microsoft Excel 2010 (Microsoft Computer Software, Redmond, WA, USA), using validated fields to restrict the entry of information and reduce typing errors. In addition, its handling was carried out by a single researcher to reduce the errors due to multiple manipulations, and before processing the information, its quality and consistency were evaluated.
For the statistical analysis were used absolute and relative frequencies for the qualitative variables and mean with standard deviation or median with interquartile range (IQR) for those quantitative variables, depending on the distribution of the data. The statistical analyses were carried out using the IBM SPSS 20.0 package (IBM Corp. in Armonk, NY, USA).
Ethical considerationsBeing a review of medical records, this research is considered without risk according to resolution 008430 of 1993 of the Ministry of Health of Colombia. The study was approved by the Ethics Committees of the participant institutions and only the information necessary to meet the objectives of this research was obtained from the medical records.
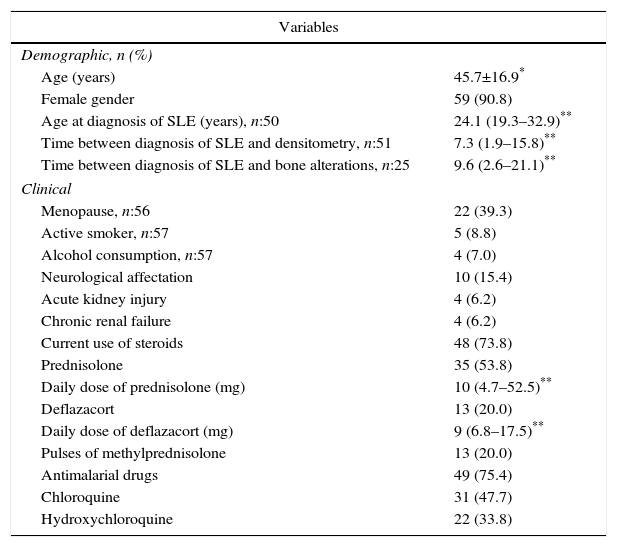
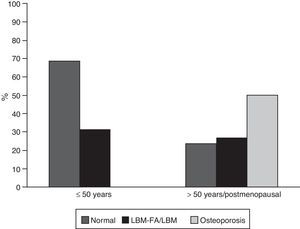
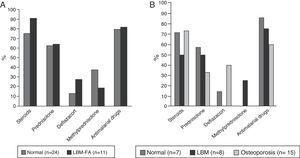
ResultsGeneral characteristicsOf the 302 patients evaluated during the study period, 65 (21.52%) met the eligibility criteria; of them, 59 (90.8%) were women, with a mean age of 45.7 years (standard deviation: 16.9). Of the 6 men in this cohort, 4 were younger than 50 years and only one of them was classified with LBM-FA, the remaining 2 were older than 50 years and they had osteoporosis. Thirty-five subjects (53.8%) were patients younger than 50 years of age or premenopausal; in this group 11 (31%) were classified with LBM-FA; of the patients older than 50 years or menopausal, 15 individuals (50%) were diagnosed with osteoporosis and 8 (27%) with LBM (Fig. 1). Six out of 59 (10.2%) individuals had personal antecedents of fractures, of whom 4 had osteoporosis and 2 had LBM-FA.
Regarding the traditional risk factors, it was found that 4 out of 57 patients (7%) consumed alcohol, 2 of them were older than 50 years of postmenopausal and presented osteoporosis; 5 (8.8%) were active smokers, of whom 2 were men and presented LBM-FA and osteoporosis; according to their corresponding age groups, the totality of the characteristics of the patients can be seen in Table 1.
Demographic and clinical characteristics of patients with SLE.
| Variables | |
|---|---|
| Demographic, n (%) | |
| Age (years) | 45.7±16.9* |
| Female gender | 59 (90.8) |
| Age at diagnosis of SLE (years), n:50 | 24.1 (19.3–32.9)** |
| Time between diagnosis of SLE and densitometry, n:51 | 7.3 (1.9–15.8)** |
| Time between diagnosis of SLE and bone alterations, n:25 | 9.6 (2.6–21.1)** |
| Clinical | |
| Menopause, n:56 | 22 (39.3) |
| Active smoker, n:57 | 5 (8.8) |
| Alcohol consumption, n:57 | 4 (7.0) |
| Neurological affectation | 10 (15.4) |
| Acute kidney injury | 4 (6.2) |
| Chronic renal failure | 4 (6.2) |
| Current use of steroids | 48 (73.8) |
| Prednisolone | 35 (53.8) |
| Daily dose of prednisolone (mg) | 10 (4.7–52.5)** |
| Deflazacort | 13 (20.0) |
| Daily dose of deflazacort (mg) | 9 (6.8–17.5)** |
| Pulses of methylprednisolone | 13 (20.0) |
| Antimalarial drugs | 49 (75.4) |
| Chloroquine | 31 (47.7) |
| Hydroxychloroquine | 22 (33.8) |
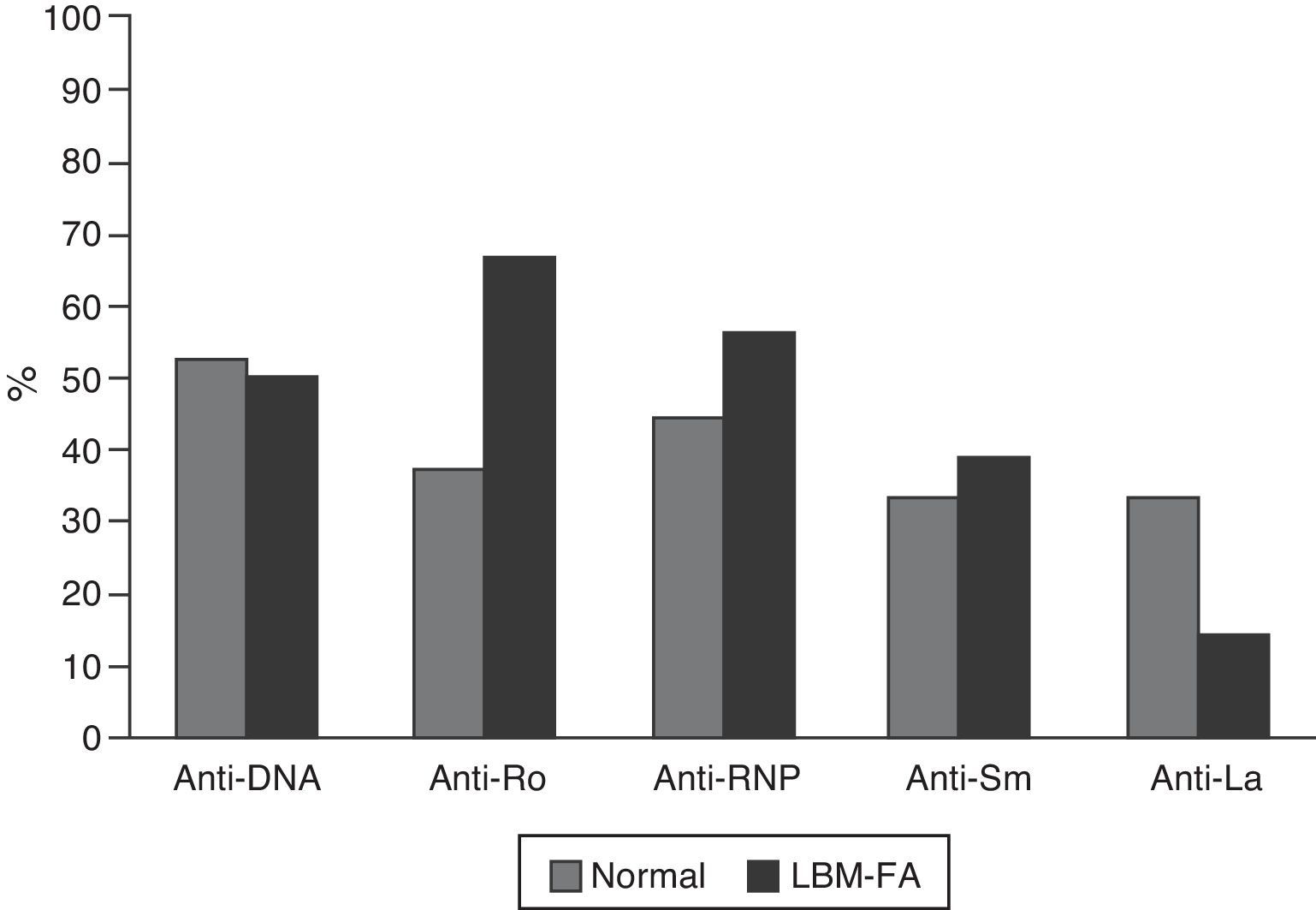
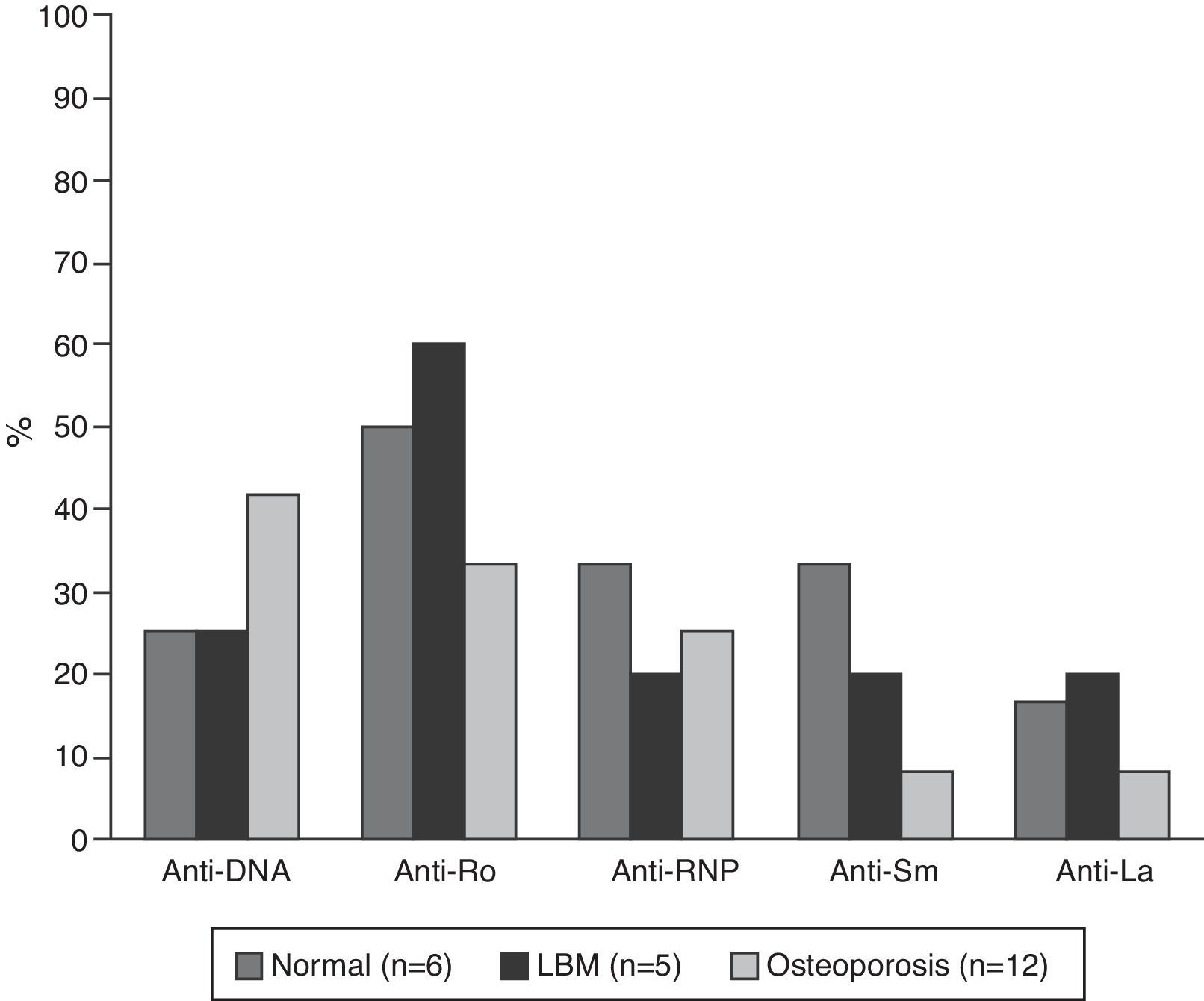
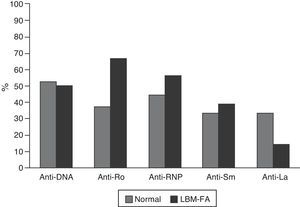
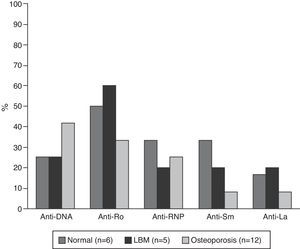
The serological characteristics of the patients by age groups are described in Figs. 2 and 3. The presence of anti-Ro and anti-La antibodies was determined in 46 subjects; 12 (26%) individuals had only anti-Ro antibodies and of them, 8 had alterations in their BD; 3 were younger than 50 years with LBM-FA and 5 were of the group of patients older than 50 years or postmenopausal (2 with LBM and 3 with osteoporosis). In addition, 8 patients had both types of antibodies; of them, 3 had LBM-FA, 1 had LBM and another had osteoporosis. No patient presented only anti-La antibodies.
C3 hypocomplementemia was present in 18/40 (45%) patients, 14 cases in individuals younger than 50 years, occurring in 10/17 (58.8%) and 4/7 (42.9%) of the patients with normal densitometry and with LBM-FA, respectively. Four cases occurred in the group older than 50 years or postmenopausal, including 2 patients with LBM and one patient with osteoporosis. Regarding the C4 levels, 9/41 (22%) had hypocomplementemia; 8 patients of the group younger than 50 years, of whom 5/18 (27.8%) were within the normal range and 3/7 (42.9%) had LBM-FA. In the group of patients older than 50 years or postmenopausal there was only one case and it was found in the range of LBM-FA.
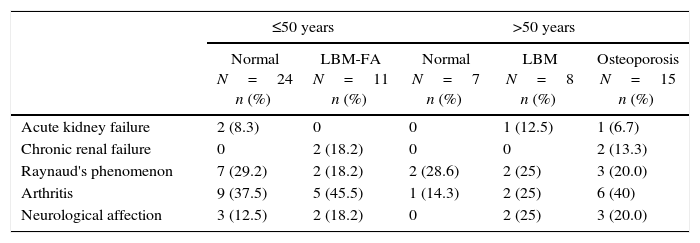
Clinical manifestations and complicationsAs for the disease activity and chronicity indexes, the median SLEDAI score (n=59) was 0 (IQR=0–4) and the median SLICC score (n=64) was 0.5 (IQR=0–1.75). The most frequent clinical manifestations were arthritis and Raynaud's phenomenon in 23 (35.4%) and 16 (24.6%) patients, respectively; followed by the neurological affection observed in 10 (15.4%) cases (2 patients with LBM-FA, 2 with LBM and 3 with osteoporosis). Chronic renal failure was observed in 4 patients (6.2%), 2 of whom had LBM-FA and 2 had osteoporosis. These manifestations, according with the description by subgroups, are shown in Table 2.
Clinical complications associated with alterations in bone mass in patients with SLE.
| ≤50 years | >50 years | ||||
|---|---|---|---|---|---|
| Normal N=24 n (%) | LBM-FA N=11 n (%) | Normal N=7 n (%) | LBM N=8 n (%) | Osteoporosis N=15 n (%) | |
| Acute kidney failure | 2 (8.3) | 0 | 0 | 1 (12.5) | 1 (6.7) |
| Chronic renal failure | 0 | 2 (18.2) | 0 | 0 | 2 (13.3) |
| Raynaud's phenomenon | 7 (29.2) | 2 (18.2) | 2 (28.6) | 2 (25) | 3 (20.0) |
| Arthritis | 9 (37.5) | 5 (45.5) | 1 (14.3) | 2 (25) | 6 (40) |
| Neurological affection | 3 (12.5) | 2 (18.2) | 0 | 2 (25) | 3 (20.0) |
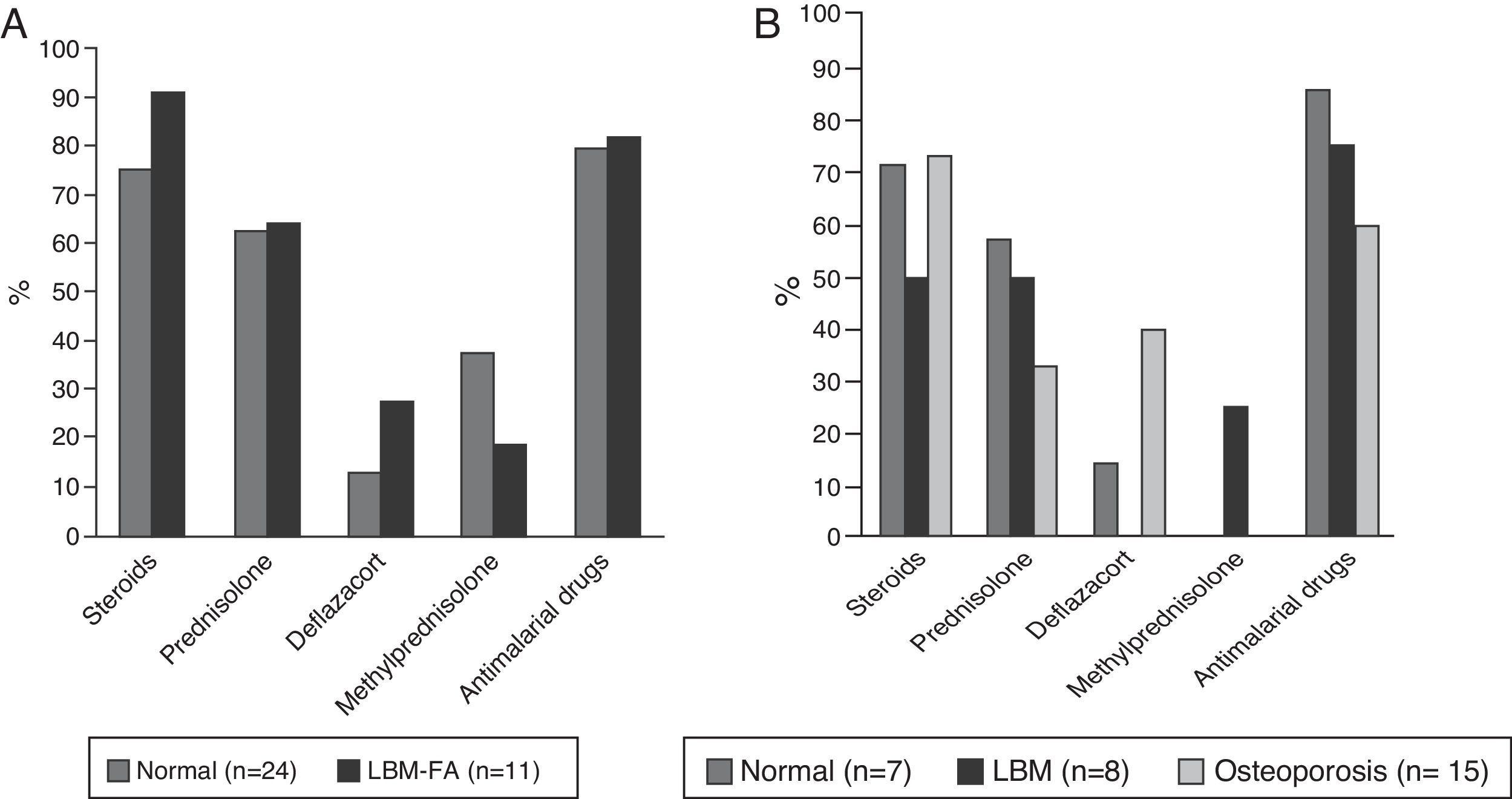
Forty-eight patients (73.8%) used glucocorticoids; 35 (53.8%) were taking prednisolone with a median daily dose of 10mg (IQR 5–52) and 49 (75.4%) received some type of antimalarial agent, of which the most used was chloroquine in 31 (63.2%). The description of the treatment by age groups is shown in Fig. 4.
DiscussionIn the present study were found several findings of interest: the first is derived from its retrospective nature, which allows to evaluate the usual clinical practice, specifically the use of BMD for the screening of the alterations in bone mass in patients with SLE. Although there have been published precise indications for BD in individuals with this disease, such as the use of glucocorticoids (use for more than 3 months at doses higher than 7.5mg day), the age (women older than 65 years or men older than 70 years) and the presence of additional risk factors for osteoporosis,3,15–17 in this study only 21.5% of the clinical records evaluated had a record of DXA. This, even though is a limitation because of the few data available for analysis, reveals the need to reinforce the search for these alterations by rheumatologists and non-rheumatologist physicians, and thus provide important and relevant information to local knowledge.
Regarding the alterations in BMD, a significant frequency both of LBM-FA and LBM/osteoporosis was found in the respective age groups. This result is difficult to compare, since the studies published previously have used the T-score lower than −2.5 as diagnostic in patients of all ages. This is susceptible to debate, since in healthy population the classification criteria vary according to the age group.2 Another approach to this issue is to assume the alterations in BMD as derived primarily from the use of steroids, but even so, the determination to initiate therapy with bisphosphonates depends predominantly on the dose and the duration of the therapy with steroids; the guidelines that recommend the measurement of the bone mass use it as part of the FRAX score to calculate the fracture risk.15 In addition, the FRAX is designed for a minimum age of 45 years and dichotomously evaluates the use of steroids, being difficult to adjust the effect according to the dose and although some strategies to correct this fact are available, these are based on recommendation of experts.5 Finally, the effect on BMD in SLE is multifactorial, so this approach would only be considering a single aspect of this problem.
In the present study were found frequencies of LBM-FA, osteoporosis and LBM of 31, 50 and 31%, respectively. These values are similar to those reported by Mok et al.,18 who evaluated 34 patients with an average age of 52.9 years and found a frequency of osteoporosis in the lumbar spine of 48% and of LBM of 33%. Jacobs et al.,19 compared 126 patients with an average age of 39 years and with 18% of menopausal patients, finding 39.7% of LBM and 6.3% of osteoporosis, for a total of 46% of alterations in BMD. It is important to emphasize that the results are not directly comparable due to the methodology used in the present study; however, our value of LBM-FA and the results of Jacobs are similar (31 vs. 46%) reiterating the importance of screening for this type of musculoskeletal complications in this group of patients.
Menopause has been clearly described as an independent risk factor for developing alterations in bone mass3,20; however, in these patients it is not the only factor that determines the appearance of such alterations, which somehow is evidenced in this study, since in the group of patients younger than 50 years of age or premenopausal, 31% had LBM-FA. However, more than 90% of the premenopausal women who used steroids had LBM-FA and, of the postmenopausal patients, 73% had osteoporosis. In this subgroup of patients the effect of steroids is independent of menopause, as demonstrated by Zhu et al.,21 who included 75 premenopausal women and 44 postmenopausal patients, finding that the volumetric bone density was reduced to 2.66% after 2 years of using glucocorticoids, outcome that is independent of the menopausal status. Tang et al.,22 found a decrease in BMD by area and volume of 5.3 and 5.7%, respectively, after one year of intake of prednisolone at an average dose of 5mg daily.
With respect to the traditional risk factors in relation to the decrease in BMD and SLE, our data are similar to those found by other authors.6,18,23,24 However, some studies have found a higher frequency of consumption of tobacco (22%)24 and alcohol (33.8%), specifically in African-American population.25 These variations can be explained by 2 factors: the first is that being our study based on the review of medical records, a significant underreporting of these variables could exist; and the second are the socio-cultural variations in different populations, especially in the aspect of smoking.
When analyzing the manifestations derived from the disease, in this study all patients with chronic kidney failure had osteoporosis or LBM-FA. This result is expectable, since in these subjects it has been described the presence of other independent factors for the development of osteoporosis, such as secondary hyperparathyroidism, vitamin D deficiency and alterations in calcium absorption3; as far as is known, there are no other studies that evaluate specifically the triad osteoporosis, renal failure and lupus. In the same way, 7 of the 10 patients with neurological affection due to SLE had some alteration in the bone mass. One of the possible explanations for this finding is the presence of a prolonged immobilization secondary to this organ commitment; in these conditions it has been demonstrated, in humans, an increased bone resorption and, in animals, an alteration in the regulation of osteoclasts26; nevertheless, neuropsychiatric lupus is considered a serious manifestation of the disease which requires aggressive immunosuppression with high doses of steroids for an extended period of time, and therefore, it could be simply a marker of disease activity.
Another relevant result of the present study was the higher frequency of anti-Ro antibodies in patients with LBM; this finding coincides with what was reported by Mok et al., who suggest that, in these patients, the special restriction of sunlight exposure to prevent relapses of the disease could be associated with a lower absorption of vitamin D and this would contribute to the presence of LBM, taking into account that the presence of anti-Ro antibodies is associated with photosensitivity manifestations of SLE.18
In relation to the pharmacological aspect, our patients received antimalarial drugs in a high proportion (75.4%), factor that has been described as a protector of BMD.19 If the use of steroids for the treatment of SLE is analyzed, in our study is of 73%; in this regard, a Spanish multicenter study which included 3658 lupus patients found that their use at any time of the disease or at the entry into this cohort was between 52.4 and 84.6%, respectively.27 However, our median dose of prednisolone was 10mg, higher than the described in other studies: Bultink: 7mg, Mok: 4mg; Jacobs: 6.5mg,18,19,24 being a high dose taking into account that our patients had a median SLEDAI score of 0, reflecting disease remission. This finding obliges to consider the importance of tending to adjust the dose of steroids in each evaluation of the patient, whenever the situation allows it, in order to avoid the unnecessary and unjustified exposure of patients to these drugs. Finally, in the patients with alterations in BMD it was found a greater use of deflazacort, which was possibly established as a reactive measure to the densitometric findings due to its better bone safety profile; however, being a cross-sectional study, is impossible to establish the temporality between the alteration in BMD and the use of this drug and this affirmation is speculative.
The present study has several limitations, most of which are inherent to its design, since being based on the collection of medical records, it presents a high probability of underreporting of the information. It is also recognized that because of the methodology of the work itself, other aspects that according to the literature could influence the presence of osteoporosis in patients with SLE, such as the body mass index and the serum levels of vitamin D, among others, were not assessed.24 Finally, being a descriptive cross-sectional study, its scope is limited to the description of the variables found in the patients with alterations in BMD and it can generate hypothesis about the possible factors involved in the development of the densitometric alterations, but it does not demonstrate any type of association.
ConclusionIn a cohort of patients with SLE of the Colombian Northwest, there is a high frequency of alterations in BMD; it was remarkable the low frequency of request of this diagnostic aid as part of the clinical control of these individuals as well as the low percentage of related traditional factors, except the use of glucocorticoids.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflict of interestThe authors declare they do not have any conflict of interest.
Please cite this article as: Velásquez Franco CJ, Zuluaga Henao MP, Lozano Pineda F, Pulgarín Montoya S, Vallejo EO, Rodríguez Padilla LM, et al. Baja masa ósea y osteoporosis en pacientes con lupus eritematoso sistémico. Rev Colomb Reumatol. 2017;24:4–10.