To determine the management results in a cohort of patients with rheumatoid arthritis in a specialized integral healthcare institution for this disease in Colombia.
Materials and methodsDescriptive cross-sectional study based on a cohort of rheumatoid arthritis patients according to ACR/EULAR 2010 criteria. The information was analysed based on consolidated data from clinical records and national reports in the period 2015–2018. Administrative records related to medication authorizations and prescriptions were considered. Sociodemographic variables, outcome indicators related to disease activity status and medication use percentage were evaluated.
ResultsAs of June 30th 2018, 698 patients were identified, of which the female sex represented 83.8%, the general average age was 55.47 years, and the highest number of cases were in the 60–64 year age group. Of the patients, 68.3% were between remission and low disease activity. Seventy-three point one percent were managed with conventional disease-modifying antirheumatic drugs and a reduction in the use of biological therapy was recorded from 27.2% in 2016 to 17.8% at the end of the period.
ConclusionsThis study presents the management results of a comprehensive care model for patients with rheumatoid arthritis in Colombia, which managed to maintain the highest proportion of patients in low activity and remission as they had a longer follow-up time, to decrease the percentage of biological DMARDs use, and establish conventional DMARDs as the main therapeutic alternative.
Conocer los resultados de gestión en una cohorte de pacientes con artritis reumatoide en una institución de atención integral especializada en esta enfermedad en Colombia.
Materiales y métodosEstudio descriptivo de corte transversal, a partir de una cohorte de pacientes de artritis reumatoide, según criterios ACR/EULAR 2010. La información se analizó con base en los datos consolidados de historia clínica y reportes nacionales en el periodo 2015–2018. Se tuvieron en cuenta los registros administrativos relacionados con autorizaciones y prescripciones de medicamentos. Se evaluaron variables sociodemográficas, indicadores de resultado relacionados con el estado de actividad de la enfermedad y porcentaje de uso de medicamentos.
ResultadosA 30 de junio de 2018, se identificaron 698 pacientes, de los cuales el 83,8% correspondió a sexo femenino; el promedio general de edad fue de 55,47 años y el grupo de edad de 60 a 64 años concentró el mayor número de casos. El 68,3% se ubicó entre remisión y actividad baja de la enfermedad. El 73,1% se encontró manejado con fármacos antirreumáticos modificadores de enfermedad convencionales y se registró una reducción de uso de terapia biológica desde el 27,2% en 2016 al 17,8% al final del periodo.
ConclusionesEste estudio presenta los resultados de gestión de un modelo de atención integral para pacientes con artritis reumatoide en Colombia, que logró mantener la mayor proporción de pacientes en actividad baja y remisión a medida que estos contaban con mayor tiempo de seguimiento, también logró disminuir el porcentaje de uso de FARME biológicos y establecer los FARME convencionales como la principal alternativa terapéutica.
Rheumatoid arthritis (RA) is a chronic and autoimmune disorder characterized by pain, inflammation, and synovial membrane involvement. Although the etiology is unknown, possibly environmental, endocrine, and genetic factors associated with its onset, development, and progression have been elucidated1,2. It can be accompanied by cardiovascular, pulmonary, bone, dermatological, and psychological manifestations, which can lead to disability and, consequently, generate a negative impact on individual work productivity, social function, and quality of life3–5.
In developed countries, a prevalence of RA has been estimated in the range of 0.5%–1.1% in North America and Northern Europe, respectively6; while in Latin America, there are records between 0.4% and 1,6%2. In Colombia, epidemiological studies that have been conducted indicate a general prevalence between 0.52 and 0.9%. Women have the highest proportion of registered cases and adults aged >40 years are the most affected. The foregoing poses a public health problem given the prospects of chronicity of the disease and population aging2–4.
Scientific progress and knowledge of the pathophysiological factors of the disease have led to the development of new technologies that have significantly reduced morbidity, premature mortality, disability, and related comorbidities; however, they also bring with them an increase in the direct costs of medical care, mainly correspondent with the use of new medications in a setting of disease chronicity5,7.
The aforementioned considerations set a context for the different healthcare actors in which it is necessary to establish an early diagnosis, timely treatment, and continuous monitoring in an articulated manner, to reduce the inflammatory process and delay disease progression and its respective functional implications1,8,9. In this scenario, a comprehensive care approach becomes important to promote the rational use of technologies following scientific evidence, adherence to treatment, and disease control10,11. However, the information on management results in care programs, medication consumption, and performance of indicators correspondingly with the population to be served is still scarce; thus, the objective of this study is to acknowledge the management results in a cohort of patients with RA in a specialized comprehensive care institution in Colombia.
Materials and methodsA descriptive cross-sectional study was carried out, from a cohort of patients diagnosed with RA, in a comprehensive care clinic with a presence in six Colombian cities. These patients were included, treated, and followed up within a standard comprehensive care model that included periodic care in rheumatology, internal medicine, general medicine with RA training, nursing, and psychology, all led by a specialist in rheumatology, in addition to diagnostic support (clinical laboratory and imaging) and medications, under the national recommendations of the clinical practice guideline for the early detection, diagnosis, and treatment of RA10.
Information was analyzed based on the data consolidated by the provider between 2015 and 2018 for the report to the high-cost account (CAC), with a cutoff date of June 30 of each year, in agreement with the provisions of current regulations12. These records were built from information available in the clinical chart and diagnoses related to the International Classification of Diseases tenth version (ICD-10)13 codes: J990, M051, M052, M053, M058, M059, M060, M062, M063, M068 and M069, and compliance with the ACR/EULAR 2010 criteria14. Patients diagnosed with juvenile RA or other musculoskeletal diseases were not included. Similarly, records of synthetic and biológic disease-modifying anti-rheumatic drug (DMARD) prescriptions were taken into account, as well as authorization records of this type of medication with the respective insurers with a current contract. The construction of the database was carried out in each annual period, based on the information systems of the medical history of the service provider, for which a team of health professionals and technicians verified each case, to guarantee the quality of the information subject to audit by the CAC.
Sociodemographic variables such as gender, city of service provision, age group, type of pharmacological treatment, and follow-up time were analyzed. In the same way, outcome indicators related to the current disease activity status measured with DAS28 in each annual period were assessed, along with the percentage of medication use and disease activity by type of DMARD.
The general processing of the data was done in Microsoft Excel® for descriptive statistics such as frequencies and measures of central tendency. Evaluations of normality, comparisons of proportions with the χ² statistic, and age averages were carried out using the Student’s t-distribution in the IBM SPSS 22® software. This evaluation was considered without risk since it was done based on clinical history records, no intervention was made in the biological, physiological, or social variables of the individuals, and the confidentiality of the information was strictly preserved. Likewise, it was conducted following Colombian and international regulations on health research15,16 and was approved by the Ethics and Research Committee of the institution providing health services in which the study was conducted (approval code: MI-CEI-2019-01).
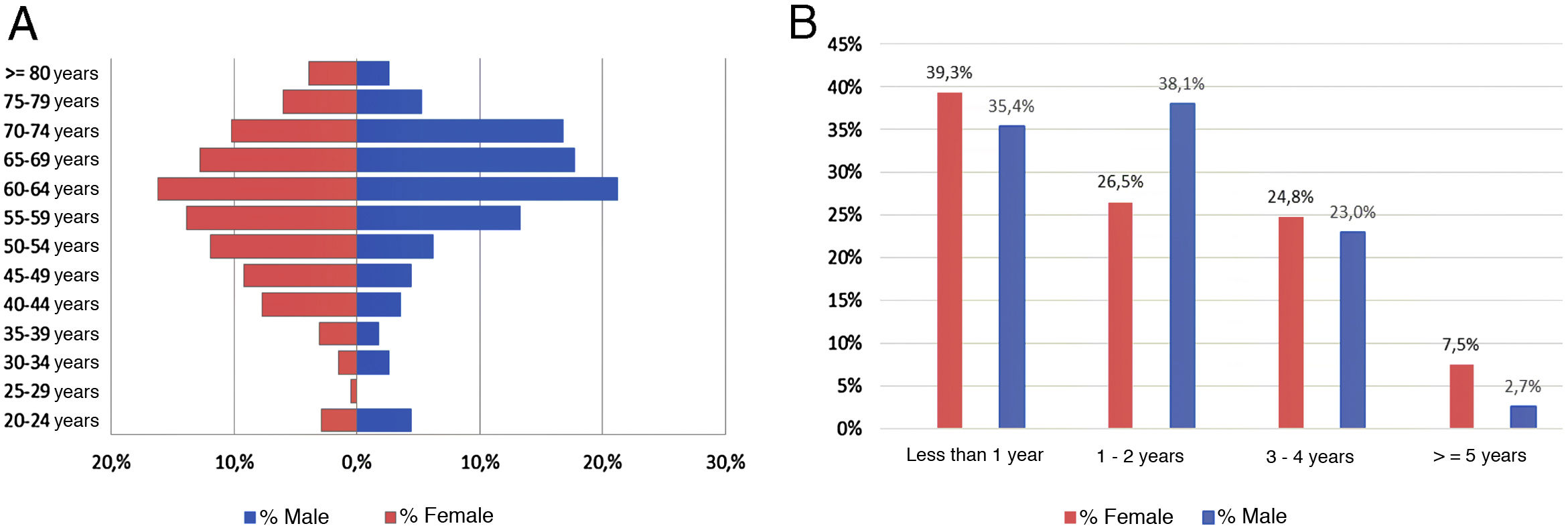
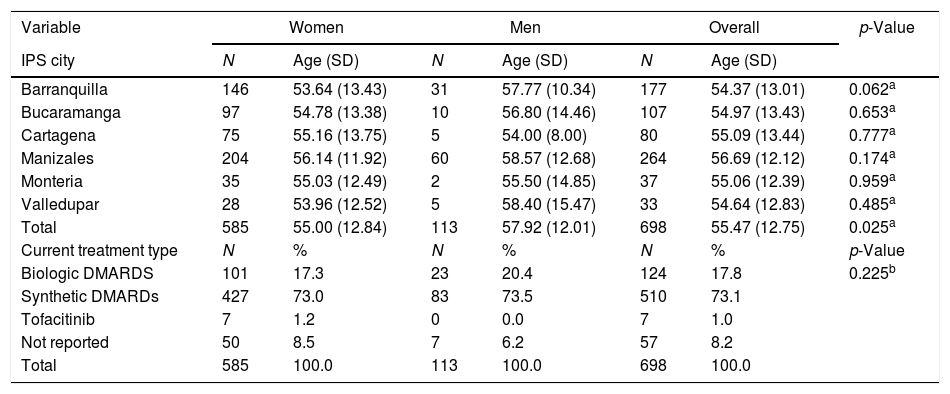
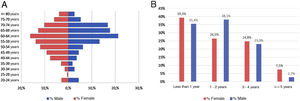
ResultsAs of June 30, 2018, 698 individuals with a diagnosis of RA were identified nationwide, of which the female sex represented 83.8%, for a female: male ratio of 5.2:1. The average (standard deviation) of the general age of the population was 55.47 years (12.75), which was higher for men: 57.92 years (12.01) (p<0.05). The age group that recorded the highest concentration of cases was 60–64 years (17%), while the cities with the highest number of cases were Manizales (37.8%), Barranquilla (25.4%) and Bucaramanga (15.3%). Concerning therapeutic management, 73.1% were treated with conventional DMARDs, while biologic therapy was used in 17.8%. Regarding the follow-up time in the cohort, most of the patients had a recent enrollment: 38, 7% were admitted during the last year before the cut-off date, while 28.4% had been followed up in the care program between 1 and 2 years. The details of these data are presented in Table 1 and Fig. 1.
Characteristics of the population with rheumatoid arthritis by sex as of June 30, 2018.
| Variable | Women | Men | Overall | p-Value | |||
|---|---|---|---|---|---|---|---|
| IPS city | N | Age (SD) | N | Age (SD) | N | Age (SD) | |
| Barranquilla | 146 | 53.64 (13.43) | 31 | 57.77 (10.34) | 177 | 54.37 (13.01) | 0.062a |
| Bucaramanga | 97 | 54.78 (13.38) | 10 | 56.80 (14.46) | 107 | 54.97 (13.43) | 0.653a |
| Cartagena | 75 | 55.16 (13.75) | 5 | 54.00 (8.00) | 80 | 55.09 (13.44) | 0.777a |
| Manizales | 204 | 56.14 (11.92) | 60 | 58.57 (12.68) | 264 | 56.69 (12.12) | 0.174a |
| Monteria | 35 | 55.03 (12.49) | 2 | 55.50 (14.85) | 37 | 55.06 (12.39) | 0.959a |
| Valledupar | 28 | 53.96 (12.52) | 5 | 58.40 (15.47) | 33 | 54.64 (12.83) | 0.485a |
| Total | 585 | 55.00 (12.84) | 113 | 57.92 (12.01) | 698 | 55.47 (12.75) | 0.025a |
| Current treatment type | N | % | N | % | N | % | p-Value |
| Biologic DMARDS | 101 | 17.3 | 23 | 20.4 | 124 | 17.8 | 0.225b |
| Synthetic DMARDs | 427 | 73.0 | 83 | 73.5 | 510 | 73.1 | |
| Tofacitinib | 7 | 1.2 | 0 | 0.0 | 7 | 1.0 | |
| Not reported | 50 | 8.5 | 7 | 6.2 | 57 | 8.2 | |
| Total | 585 | 100.0 | 113 | 100.0 | 698 | 100.0 | |
SD: standard deviation; DMARDs: disease-modifying anti-rheumatic drugs; IPS: health provider institution.
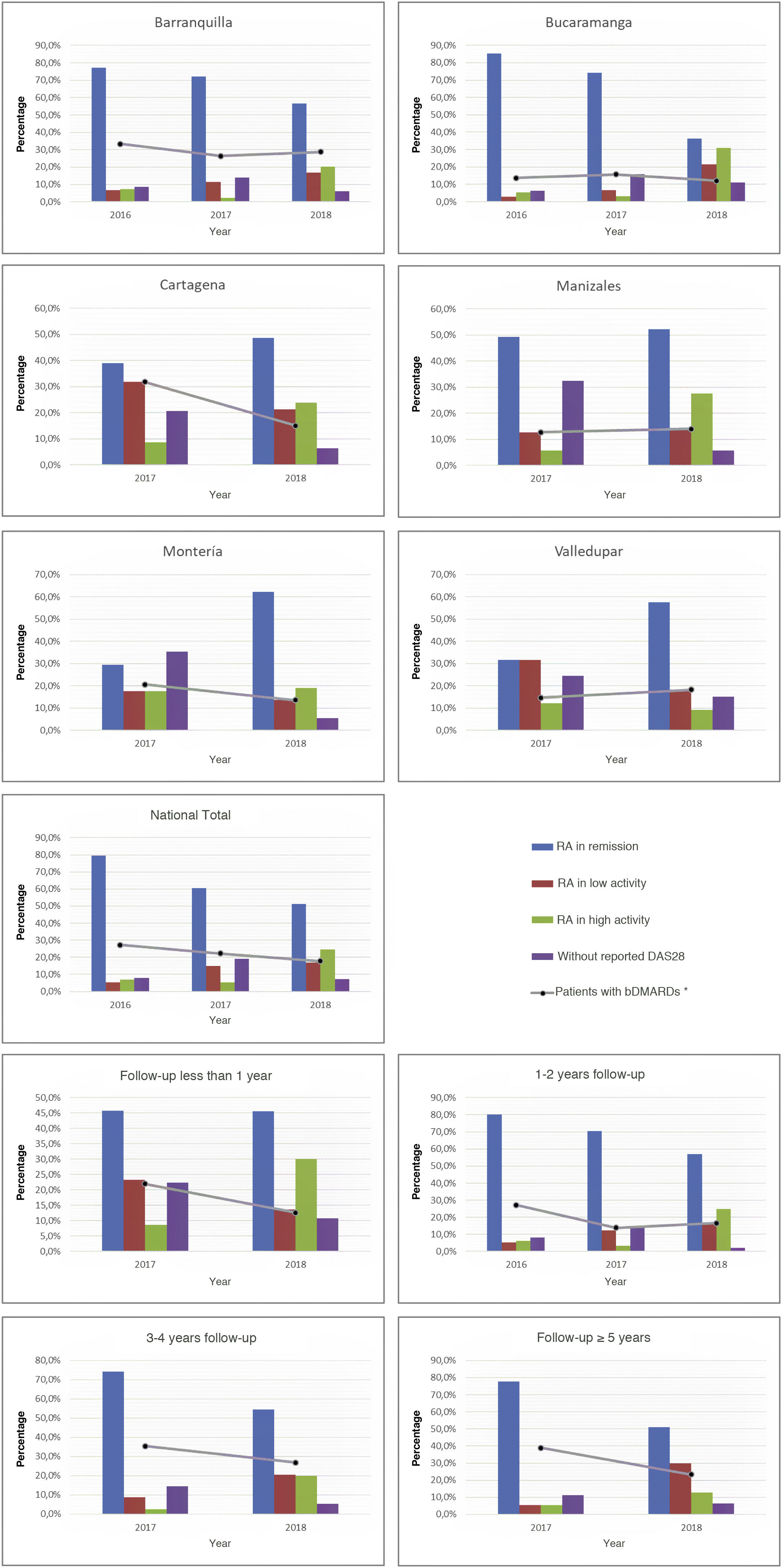
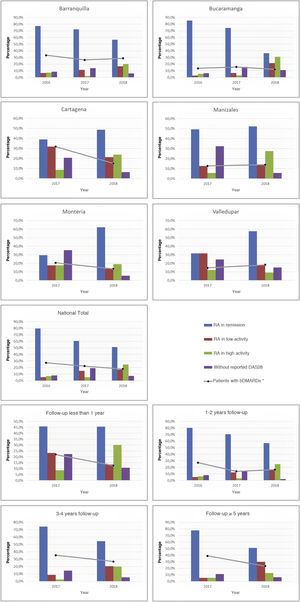
As part of the management results, it was possible to show during the last period, in terms of the current activity status of the disease on a national scale, that the highest proportion of individuals was between remission and low activity (68.3%), while this proportion in the cities with the highest number of cases, such as Manizales, Barranquilla, and Bucaramanga, was 66.7, 73.4, and 57.9%, respectively. About the relationship between follow-up time and disease activity, it was observed that as patients were older in the care model, a higher proportion of low activity and remission was recorded: 80.9% among those with more than 5 years in the program, 74.9% between 3 and 4 years, and 73.2% between 1 and 2 years. These data can be seen in Fig. 2.
About the use of medicines, on a national scale, a reduction in the percentage of biological DMARDs could be determined in the last 3 years, which had gone from 27.2% in 2016 to 17.8% in 2018, with similar behavior for each of the cities. Similarly, and due to follow-up time for these patients, a reduction in the use of this type of drug was observed both among older individuals, which had gone from 38.9% in 2017 to 23.4% in 2018 in those with more than 5 years in the program, such as those who were recruited in the last year at the cut-off date, in which it went from 22% in 2017 to 12.6% in 2018 (Fig. 2). On the other hand, the conventional DMARDs with the highest proportion of use in the cohort are leflunomide and methotrexate; the first has experienced an increase as the therapy of choice (has passed from 31.3% in 2015 to 48.7% in 2018), while the second went from 55.7% to 46.8% (Table 2).
Annual distribution of percentages of use of DMARDs 2015–2018.
| Medicine | 2015 | 2016 | 2017 | 2018 | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| Azathioprine | 1 | 0.3 | 1 | 0.3 | 1 | 0.2 | 22 | 3.2 |
| Chloroquine | 21 | 6.6 | 3 | 0.9 | 44 | 7.1 | 85 | 12.2 |
| Hydroxychloroquine | 1 | 0.3 | 3 | 0.9 | 4 | 0.6 | 10 | 1.4 |
| Leflunomide | 99 | 31.3 | 114 | 32.7 | 220 | 35.6 | 340 | 48.7 |
| Methotrexate | 176 | 55.7 | 193 | 55.3 | 282 | 45.6 | 327 | 46.8 |
| Sulfasalazine | 23 | 7.3 | 30 | 8.6 | 65 | 10.5 | 220 | 31.5 |
| Tofacitinib | 1 | 0.3 | 1 | 0.3 | 2 | 0.3 | 7 | 1.0 |
| Other treatments | 0 | 0.0 | 1 | 0.3 | 0 | 0.0 | 0 | 0.0 |
| Abatacept | 0 | 0.0 | 9 | 2.6 | 22 | 3.6 | 17 | 2.4 |
| Adalimumab | 7 | 2.2 | 8 | 23 | 14 | 23 | 14 | 2.0 |
| Certolizumab | 11 | 3.5 | 12 | 3.4 | 11 | 1.8 | 9 | 1.3 |
| Etanercept | 20 | 6.3 | 29 | 8.3 | 37 | 6.0 | 41 | 5.9 |
| Golimumab | 1 | 0.3 | 0 | 0.0 | 4 | 0.6 | 4 | 0.6 |
| Infliximab | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 1 | 0.1 |
| Rituximab | 19 | 6.0 | 19 | 5.4 | 21 | 3.4 | 16 | 23 |
| Tocilizumab | 22 | 7.0 | 26 | 7.4 | 29 | 4.7 | 22 | 3.2 |
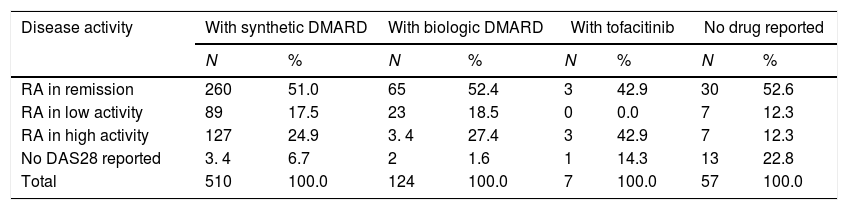
Regarding the relationship between the type of medication and disease activity, it was possible to note that conventional DMARDs are established as the main therapeutic alternative in each of the groups and that patients in remission and low activity are the ones with the greatest number of individuals and proportion of use registered: 260 (72.6%) and 89 (74.8%), respectively, while of the total number of patients with biologic DMARDs, 71% presented low activity and remission (Table 3).
Distribution of patients with rheumatoid arthritis by type of medication and disease activity.
| Disease activity | With synthetic DMARD | With biologic DMARD | With tofacitinib | No drug reported | ||||
|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |
| RA in remission | 260 | 51.0 | 65 | 52.4 | 3 | 42.9 | 30 | 52.6 |
| RA in low activity | 89 | 17.5 | 23 | 18.5 | 0 | 0.0 | 7 | 12.3 |
| RA in high activity | 127 | 24.9 | 3. 4 | 27.4 | 3 | 42.9 | 7 | 12.3 |
| No DAS28 reported | 3. 4 | 6.7 | 2 | 1.6 | 1 | 14.3 | 13 | 22.8 |
| Total | 510 | 100.0 | 124 | 100.0 | 7 | 100.0 | 57 | 100.0 |
DMARDs: disease-modifying anti-rheumatic drugs.
The annual report of RA to the CAC has contributed to the improvement of the availability of information on the disease in recent years, because it has been settled as a condition of interest for the country, due to the direct costs generated as a consequence of the use of pharmacological therapy, support services, and care for associated complications. This initiative has enabled each one of the healthcare actors to make significant efforts for adherence to therapeutic management guidelines for patients, the consignment of data in the clinical history, the report, and the continuous improvement of quality of data10,12,17.
In terms of population, the results of this study were consistent with national studies that show a female predominance, with a female: male ratio of between 6:1 and 4:11–4. Regarding the average age, only the studies by Machado-Alba et al. and Bautista-Molano et al. recorded values of 53.2 and 58 years, respectively; the first developed in 5 cities and the second concentrated in a single city1,3. Concerning age groups, the results also coincided with what was revealed by the CAC for 2018 and by other previous national studies, which place the group between 50 and 64 years as the one with the highest proportion of cases1,4,17.
Regarding the management of results of the care program in these patients, following standardized measurements of the CAC and the Ministry of Health and Social Protection18, this study was able to show that the proportion of patients with RA who attained remission of the disease and obtained low activity (measured by DAS28 in the last 6 months), in general, was higher (51.3% and 17%, respectively) than the general results of the country for the same year (8.6% and 2.2%) and the national goal (20% and 40%)17. This same behavior was observed for each of the cities in which the specialized institution operates, of which Monteria (62.2% and 13.5%), Valledupar (57.6% and 18.2%), and Barranquilla (56.5% and 16.9%) stand out. Similarly, in this study, it was found that patients who had a follow-up of more than one year had a higher proportion of remission and low activity (between 51.1% and 57.1%, and in the range of 16.2% and 29.8%, respectively), which generally reflects the results of the program in terms of care processes, adequate therapy, and follow-up, once the individuals completed the minimum time between the initiation of treatment and the achievement of objectives, as well as compliance with the conditions of continuity and adherence to the care model.
Concerning therapeutic management, the results of the study reveal a proportion of use of synthetic DMARDs (73.1%) lower than that reported by the CAC on a national scale for the same year (79.4%), while the use of biologic DMARDs was higher (17.8%) than that reported by the national entity (4.7%)17. However, when analyzing the follow-up time in those who use biologic DMARDs, it was observed in the last 2 years that more than half of the patients (74.4% in 2017 and 50.3% in 2018) had more than 2 years in the care program. These results were consistent with the findings of Bautista-Molano et al., who recorded a percentage of the use of synthetic and biologic DMARDs in monotherapy and combined that was 74.8% and 20.9%, respectively1. Since both studies come from institutions specializing in RA care, patients were likely admitted with high-severity criteria or with state-of-the-art treatments established, as a consequence of a therapeutic approach that did not follow the recommendations of the scientific evidence at the first levels of care; nonetheless, this aspect becomes a topic of interest for future research10.
On the other hand, it was possible to show, in terms of disease activity measured by DAS 28, that in 2018 half of the patients were on synthetic DMARDs and that, regardless of the type of DMARD used, the proportion of patients who attained or remained in remission and low activity was 64.9%–71%, which is considered an achievement of the program when comparing the proportion of use of synthetic and biologic DMARDs in previous years. At the time of this analysis, no similar studies evaluating this aspect were identified.
The use of pharmacological therapy is a cause for concern since it represents up to 86% of the costs of RA care and can increase up to 40 times with the use of biologic DMARDs5. This type of medication has been considered the successive therapy to conventional DMARDs when these do not achieve a therapeutic response to control symptoms and disease progression; therefore, an expert specialist and adherence to the recommendations of the scientific evidence that evaluate both the clinical characteristics of the development of the disease and those of the individual are key in the indication of these therapies, and also when reducing the dose is considered, maintaining the balance between efficacy and safety, or suspending the medication when he determines that its use is inappropriate10,19–21.
The chronic and progressive course of the disease, as well as the progress in the development of increasingly expensive new technologies, in a context of finite resources and the need to maintain the financial sustainability of health systems, mean that results management in this type of patients is key to early diagnosis, timely access to consultation with a rheumatologist, evaluation of comorbidities, relevance and adherence to treatment, and identification of potential opportunities for improvement, to prevent structural damage, associated complications, and improve the quality of life of individuals with the disorder.
In conclusion, this study constitutes an approach to assess the results in a population with RA in a specialized institution in Colombia, in which it has been possible to present an experience through the management of a comprehensive care model. This model is based on follow-up by an interdisciplinary team and is aligned with the recommendations of scientific evidence regarding therapeutic and rehabilitation approach strategies. In this way, it was possible to maintain the highest proportion of patients in low activity and remission as they had longer follow-up time, in addition to reducing the percentage of use of biologic DMARDs and, therefore, establishing conventional DMARDs as the main alternative therapy, mostly in patients in low activity and remission. Thus, it will be useful in comparing results and as a baseline for planning new studies in the field of disease care.
It is important to point out that this work was developed with a population captured in a single comprehensive care institution, concentrated in large cities, and affiliated with a single insurer, mainly from the contributory regime, so the findings must be considered within that context. Second, it should be noted that the information analyzed is part of the mandatory reports to the CAC and was not designed for academic or health research purposes.
FinancingThis study was financed by Medicina Integral IPS, SA, Barranquilla, Colombia.
Conflict of interestsThe authors declare that they have no conflicts of interest for the development of this study.
Please cite this article as: Piñeros Castillo JA, Arévalo Roa HO, Muñoz-Galindo IM. Resultados de gestión en una cohorte de pacientes con artritis reumatoide en una institución de atención integral especializada en Colombia. Rev Colomb Reumatol. 2022;29:93–100.