To identify the clinical characteristics of patients with gout, and the prescription patterns of anti-gout medications in Colombia.
MethodsCross-sectional study, that analyzed the data from 310 medical records of patients treated in the last quarter of 2016, and who received an anti-gout medication. Sociodemographic, clinical, pharmacological, comorbidities, and paraclinical variables were identified. For each anti-gout drug used, it was determined whether the use was in accordance with Federal Drug Administration (FDA) approved recommendations. Descriptive, bivariate and multivariate analyses were performed.
ResultsPatients from 14 different cities in Colombia were evaluated, with a male predominance of 70.3% (n = 218) and a median age of 64 years (RIC: 26–94 years). The most frequently used anti-gout medication was allopurinol (n = 255; 82.3%), followed by colchicine (n = 54; 17.4%). The main diagnoses found as an indication were: hyperuricaemia (n = 181, 58.4%), gout (n = 34; 11.0%), and gouty arthritis (n = 28; 9.0%). Almost three-quarters (74.5%; n = 231) of the prescriptions had an approved use according to the FDA, especially allopurinol in the management of gout and hyperuricaemia, while colchicine was found to be used in unapproved indications (n = 44, 81.4%). The most frequent comorbidities were hypertension (68.4%) and dyslipidaemia (55.8%).
ConclusionsPatients with gout and on pharmacological treatment have a high frequency of cardiovascular comorbidities. They were being treated with allopurinol for long-term prevention, while a smaller proportion received colchicine, which is often used for indications not approved by regulatory agencies.
Identificar las características clínicas de los pacientes con gota y la forma de utilización de los medicamentos antigotosos en Colombia.
MétodosEstudio de corte transversal en el que se analizaron 310 historias clínicas de pacientes atendidos en el último trimestre del 2016 y que recibieron un medicamento antigotoso. Se identificaron variables sociodemográficas, clínicas, farmacológicas, comorbilidades y paraclínicas. Para cada medicamento antigotoso se determinó si el uso fue según las recomendaciones aprobadas por Federal Drug Administration (FDA). Se realizaron análisis descriptivos, bivariados y multivariados.
ResultadosSe evaluaron pacientes de 14 diferentes ciudades de Colombia, con un predominio masculino 70,3% (n = 218) y una mediana de edad de 64 años (RIC: 26–94 años. El antigotoso más frecuentemente utilizado fue alopurinol (n = 255; 82,3%), seguido de colchicina (n = 54; 17,4%). Los diagnósticos hallados como indicación fueron: hiperuricemia (n = 181, 58,4%), gota (n = 34, 11%), artritis gotosa (n = 28, 9%). El 74,5% (n = 231) de las prescripciones tenía un uso aprobado según la FDA, especialmente alopurinol en el manejo de gota e hiperuricemias, mientras que colchicina se encontró siendo utilizada en indicaciones no aprobadas (n = 44, 81,4%). Las comorbilidades más frecuentes fueron hipertensión (68,4%) y dislipidemia (55,8%).
ConclusionesLos pacientes con gota en tratamiento farmacológico tienen una elevada frecuencia de comorbilidades cardiovasculares, y están siendo tratados con alopurinol para la prevención a largo plazo, mientras que una menor proporción recibe colchicina que comúnmente es utilizada para indicaciones no aprobadas por las agencias reguladoras.
Gout is a chronic disease resulting from monosodium urate crystal deposition. These crystals develop as a result of a very high concentration of uric acid.1 It is estimated that 4% of the adults in the United States develop the condition, and it is more prevalent among elderly males, with a peak at 70 years,1–5 and in patients with comorbidities such as hypertension, chronic kidney disease, obesity, diabetes mellitus and a history of myocardial infarction and cerebrovascular disease.1,4,6–9
The treatment approach for gout is based on prompt treatment of the acute episode, and effective long-term management.10–12 Acute episodes require control of the inflammatory response and the guidelines of the American Colleague of Rheumatologist recommend starting with an NSAID and colchicine during the first 36 h (until the complete resolution of the gout crisis) or corticosteroids.11
Long term hyperuricemia control is intended to prevent complications and the progression of the disease, in patients with recurrent gout (more than one episode per year), tophus, associated chronic kidney disease stage 2 or higher, and urolithiasis. Management is based on xanthine oxidase inhibitors such as alopurinol or febuxostat, which are not recommended in patients with asymptomatic hyperuricemia.8,10,12–17
Presently in Colombia there is no information about the real-life use of anti-gout medications, or a description of the patients with a diagnosis of gout who are undergoing long-term control therapy. It is therefore appropriate to do an observational study to establish the clinical characteristics and the use of these medications in the country.
MethodologyCross-sectional retrospective trial reviewing the medical records of patients over 14 years old, both males and females, affiliated to the contributory social security system in Colombia, who were seen by a doctor between October 1st and December 31st, 2016, and who were prescribed an anti-gout medication.
The total population identified was 5055 patients over that period, with 80% power; finally, a total of 310 patients were assessed.
The medical records of each patient were reviewed, with the purpose of identifying the variables of interest, according to the following groups of variables:
- •
Sociodemographic: age, gender, place of residence, body weight (kilos), and body mass index.
- •
Clinical: clinical diagnosis leading to the prescription of the anti-gout agent; date of diagnosis, time of use of the medication (days).
- •
Pharmacological: type of anti-gout medicine used, dosing interval, time of use (from the first prescription according to the patient’s medical record and the number of days of uninterrupted use of the medication).
- •
Comorbidities: a) diabetes mellitus; b) hypertension; c) cardiac arrhythmia; d) ischemic heart disease; e) cerebrovascular disease; f) dyslipidemia; g) peripheral arterial disease; h) smoking; i) other rheumatic diseases.
- •
Paraclinical tests: serum uric acid; CBC, C-reactive protein, blood pressure, and glycemia. Verification to determine whether the drug was being used according to the Federal Drugs Administration (FDA) approved indications, based on the medical records:
- •
Alopurinol: for the prevention and chronic management of gout, hyperuricemia secondary to tumors or leukemia, and for the prevention of relapsing calcium oxalate stones.
- •
Febuxostat: for the chronic management of hyperuricemia in patients with gout.
- •
Colchicine: management and prevention of acute gout episodes, treatment of familial Mediterranean fever. Off-label uses: primary biliary cirrhosis, pericarditis.
The data were analyzed using SPSS version 23.0 (IBM USA). The means and medians for the qualitative variables were established, as well as frequencies and proportions for the categorical variables. A binary logistic regression model was used to determine the variables associated with the use of the anti-gout medications in non-FDA approved indications. The level of P < .05 was statistically significant.
This research was approved by the Bioethics Committee of the Universidad Tecnológica de Pereira, as a risk-free category, in compliance with the principles established under the Declaration of Helsinki. No personal data of the patients were used.
ResultsA total of 310 medical records of patients prescribed anti-gout medications within the established period of time were reviewed, of which 70.3% (n = 218) were males, with a mean age of 64 years (IQR: 26–94 years) and a mean body mass index of 27.8 ± 4.5 kg/m2.
The trial included patients from 14 cities in Colombia, mostly from Bogotá (37.4%; n = 116), Medellín (21.3%; n = 66) and Bucaramanga (8.4%; n = 26).
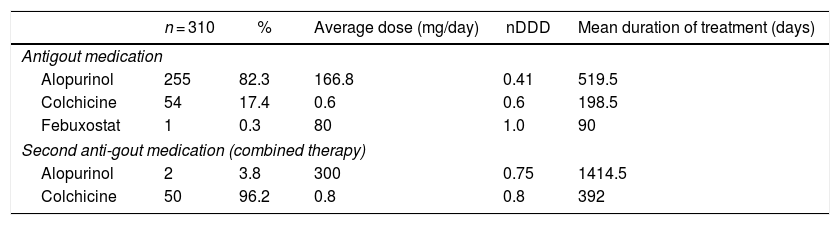
Use of anti-gout medications81.6% (n = 253) of the prescriptions were issued by general practitioners, and alopurinol was the drug most frequently used (82.3%); furthermore, combined therapy with more than one anti-gout agent was prescribed in 52 cases (16.8%). Table 1 shows the drugs prescribed for the management of gout and the doses. The mean time of use of these drugs was 394 days, considering the initial date of the prescription as reported in the medical record, up to the review date (IQR: 92−1039 days).
Frequency of use of anti-gout medications in a sample of 310 patients affiliated to the Colombian Healthcare System.
| n = 310 | % | Average dose (mg/day) | nDDD | Mean duration of treatment (days) | |
|---|---|---|---|---|---|
| Antigout medication | |||||
| Alopurinol | 255 | 82.3 | 166.8 | 0.41 | 519.5 |
| Colchicine | 54 | 17.4 | 0.6 | 0.6 | 198.5 |
| Febuxostat | 1 | 0.3 | 80 | 1.0 | 90 |
| Second anti-gout medication (combined therapy) | |||||
| Alopurinol | 2 | 3.8 | 300 | 0.75 | 1414.5 |
| Colchicine | 50 | 96.2 | 0.8 | 0.8 | 392 |
nDDD: relationship between the defined daily dose and the dose used.
The most frequent indications for which these drugs were being used were hyperuricemia with no signs of inflammatory arthritis and tophus disease (n = 181; 58.4%), gout (n = 34; 11%), gout arthritis (n = 28; 9%), failure to report (n = 22; 7.1%), arthrosis (n = 14; 4.5%), joint pain (n = 14; 4.5%), cardiovascular problems (n = 10; 3.2%), and urolithiasis (n = 7; 2.3%).
74.5% (n = 231) of all the prescriptions were for an FDA-approved use, particularly alopurinol for the management of gout and hyperuricemias (86.3% of uses according to the approved indications). Colchicine was being used for other off-label indications, such as pain and inflammation in 81.5% (n = 44) of the cases.
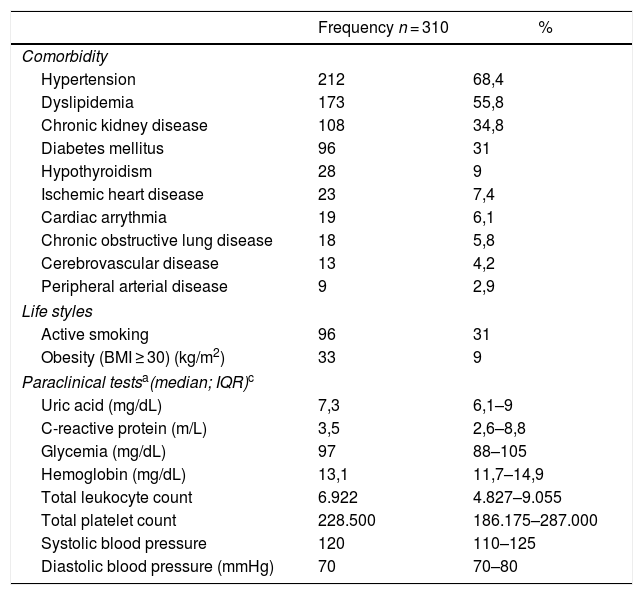
95.5% (n = 296) of the patients had other comorbidities, and the most frequent ones were hypertension and dyslipidemia (Table 2). The mean blood levels of uric acid and the most recent paraclinical tests are shown in Table 2.
Variables associated with comorbidities, life styles, and paraclinical tests in patients affiliated to the Colombian Healthcare System, treated with anti-gout medications.
| Frequency n = 310 | % | |
|---|---|---|
| Comorbidity | ||
| Hypertension | 212 | 68,4 |
| Dyslipidemia | 173 | 55,8 |
| Chronic kidney disease | 108 | 34,8 |
| Diabetes mellitus | 96 | 31 |
| Hypothyroidism | 28 | 9 |
| Ischemic heart disease | 23 | 7,4 |
| Cardiac arrythmia | 19 | 6,1 |
| Chronic obstructive lung disease | 18 | 5,8 |
| Cerebrovascular disease | 13 | 4,2 |
| Peripheral arterial disease | 9 | 2,9 |
| Life styles | ||
| Active smoking | 96 | 31 |
| Obesity (BMI ≥ 30) (kg/m2) | 33 | 9 |
| Paraclinical testsa(median; IQR)c | ||
| Uric acid (mg/dL) | 7,3 | 6,1–9 |
| C-reactive protein (m/L) | 3,5 | 2,6–8,8 |
| Glycemia (mg/dL) | 97 | 88–105 |
| Hemoglobin (mg/dL) | 13,1 | 11,7–14,9 |
| Total leukocyte count | 6.922 | 4.827–9.055 |
| Total platelet count | 228.500 | 186.175–287.000 |
| Systolic blood pressure | 120 | 110–125 |
| Diastolic blood pressure (mmHg) | 70 | 70–80 |
BMI: Body mass index; IQR: Interquartile range.
The logistic regression that analyzed the variables associated with the use of anti-gout agents in the off-label indications was adjusted for age, gender, and the significant variables in the bivariate analyses. The findings indicated that colchicine had a higher probability of being used for off-label indications (OR:35; 95% CI: 14.29–85.79; P < .001), whilst patients being treated in Bogotá (OR:0.43; 95% CI: 0.19−0.92; P = .031) were associated with a lower probability of off-label uses.
DiscussionIt was possible to identify the anti-gout prescription patterns for a group of patients in Colombia, together with their respective indications for use. This information could be the basis for further studies and potential interventions aimed at improving and rationalizing the prescription practices for this group of drugs.
The prevalence of male, adult patients over 60 years old and with multiple comorbidities, particularly overweight, hypertension, and dyslipidemias, is consistent with the studies conducted in different countries.1,3 These comorbidities are of particular interest due to their consequences on cardiovascular risk, and increased mortality of patients with gout, which deserve special care to reduce such mortality.18 Considering the evidence that patients with high uric acid levels have worse cardiovascular outcomes, due to the inflammatory process that is characteristic of gout, which further increases the cardiovascular risk in these patients, it is necessary to strictly manage the levels of uric acid, develop healthy life styles, and adequately treat any additional comorbidities.19,20
The most frequently used medication in this group of patients was alopurinol, particularly for gout-associated hyperuricemia control. However, it should be highlighted that the median of uric acid was 7.3 mg/dL, which is above the under 6 mg/dL goal defined in several clinical practice guidelines11,21; this evidences the lack of proper control and the need to optimize therapy to accomplish the therapeutic goals, but keeping in mind the inherent risks of alopurinol use, such as agranulocytosis and hypersensitivity reactions.22
When assessing the use of anti-gout medications, in general 74.5% (n = 231) of the prescriptions were for an FDA-approved use. In contrast to the results of the multivariate analysis, it is more likely that colchicine be used for off-label indications; in almost every case, the indications identified were for acute pain or chronic hyperuricemia (use for extended periods of time – median of 198 days), which is outside the recommendation of use exclusively during the acute inflammatory episode.12,21 It should be noted that the inadequate use of colchicine may place the patients at risk of serious adverse reactions, including agranulocytosis, aplastic anemia, and neuropathies.22,23 Consequently, based on the identification of this problem relating to the use of medications, there is an opportunity to intervene and optimize the treatment approach to gout.
The limitations of this study are the typical limitations of observational studies, with information collected from medical records and possibly a lack of information because of failure of the treating physicians to record all of the information, such as the clinical diagnosis, the status of the disease and all of the medications prescribed. However, there are some strengths such as the rigorous search of information, the comprehensive record of the anti-gout agents dispensed and delivered.
The conclusion is that the patients with gout in Colombia are mostly elderly males, with overweight and comorbidities such as high blood pressure and dyslipidemia; these patients are being treated with alopurinol for long term prevention, while a smaller proportion receives colchicine, which is frequently used for off-label indications. Moreover, the mean uric acid levels in these patients shows that they are not properly controlled and therefore further studies are required to establish the effectiveness of the treatment, its safety and compliance, in addition to estimating the cardiovascular risk.
FinancingThis research project had no sources of funding.
Conflict of interestsThe authors have no conflict of interest to disclose.
We want to acknowledge Soffy López for her contribution in generating the initial database.
Please cite this article as: Machado-Duque ME, Montes-Montoya MC, Serna-Echeverri LS, Manrique-Castaño S, Machado-Alba JE. Estudio de utilización de fármacos antigotosos en población colombiana, 2016. Rev Colomb Reumatol. 2021;28:11–15.